Ceftriaxone Inhibits Conditioned Fear and Compulsive-like Repetitive Marble Digging without Central Nervous System Side Effects Typical of Diazepam—A Study on DBA2/J Mice and a High-5HT Subline of Wistar–Zagreb 5HT Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs
2.2. Animals
2.3. Platelet 5HT Level and Thrombocyte Transporter (Uptake) Activity
2.4. Marble-Burying (MB) Test
2.5. The Contextual Fear-Conditioning (CFC) Test
2.6. Western Blot Analysis
2.7. Open-Field Test, Beam-Walking Test and Wire-Hanging Test
2.8. Statistical Analysis
3. Results
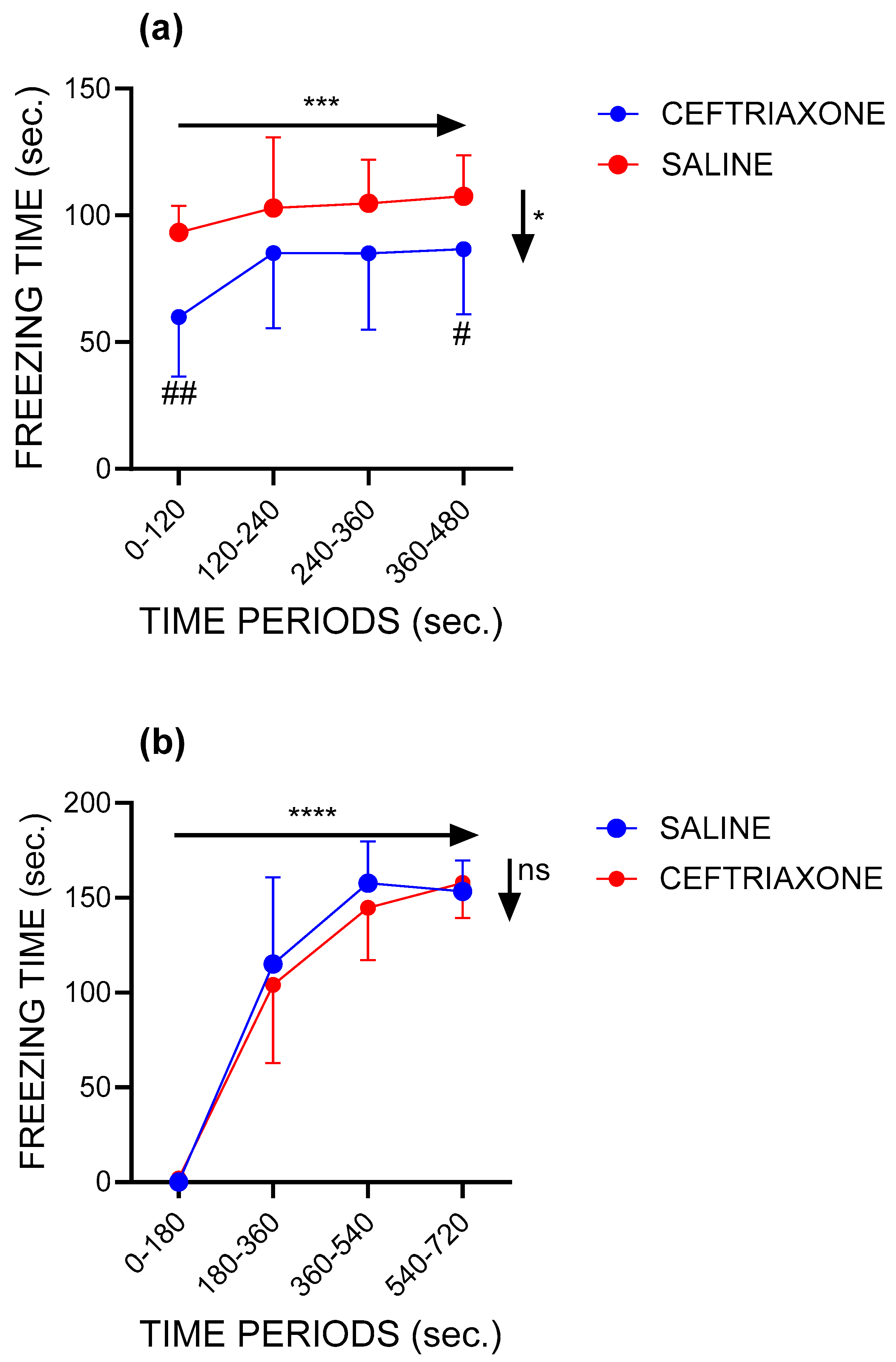
3.1. Contextual Fear Conditioning
3.1.1. Context Session
3.1.2. Conditioning Session
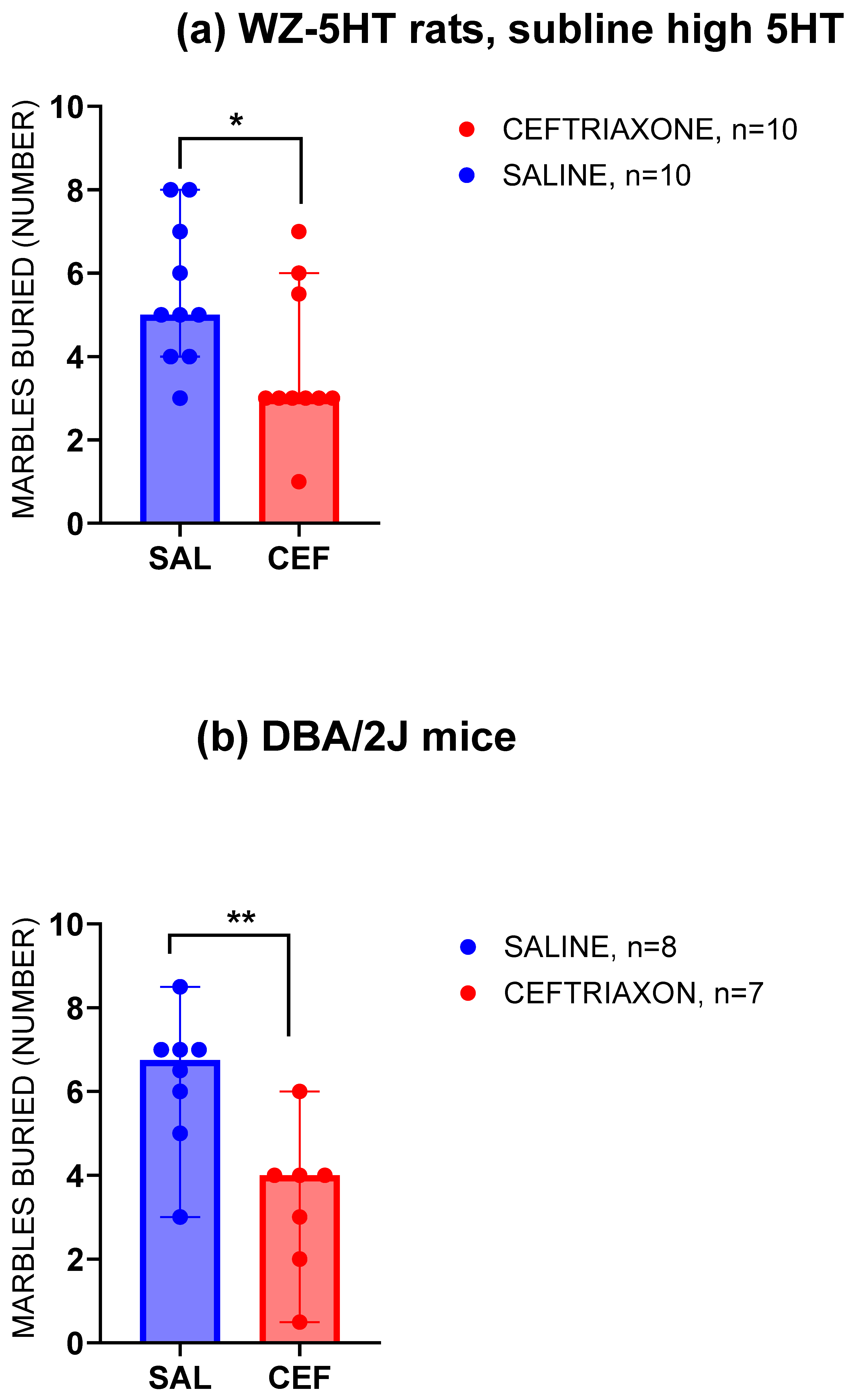
3.2. Marble-Burying Test
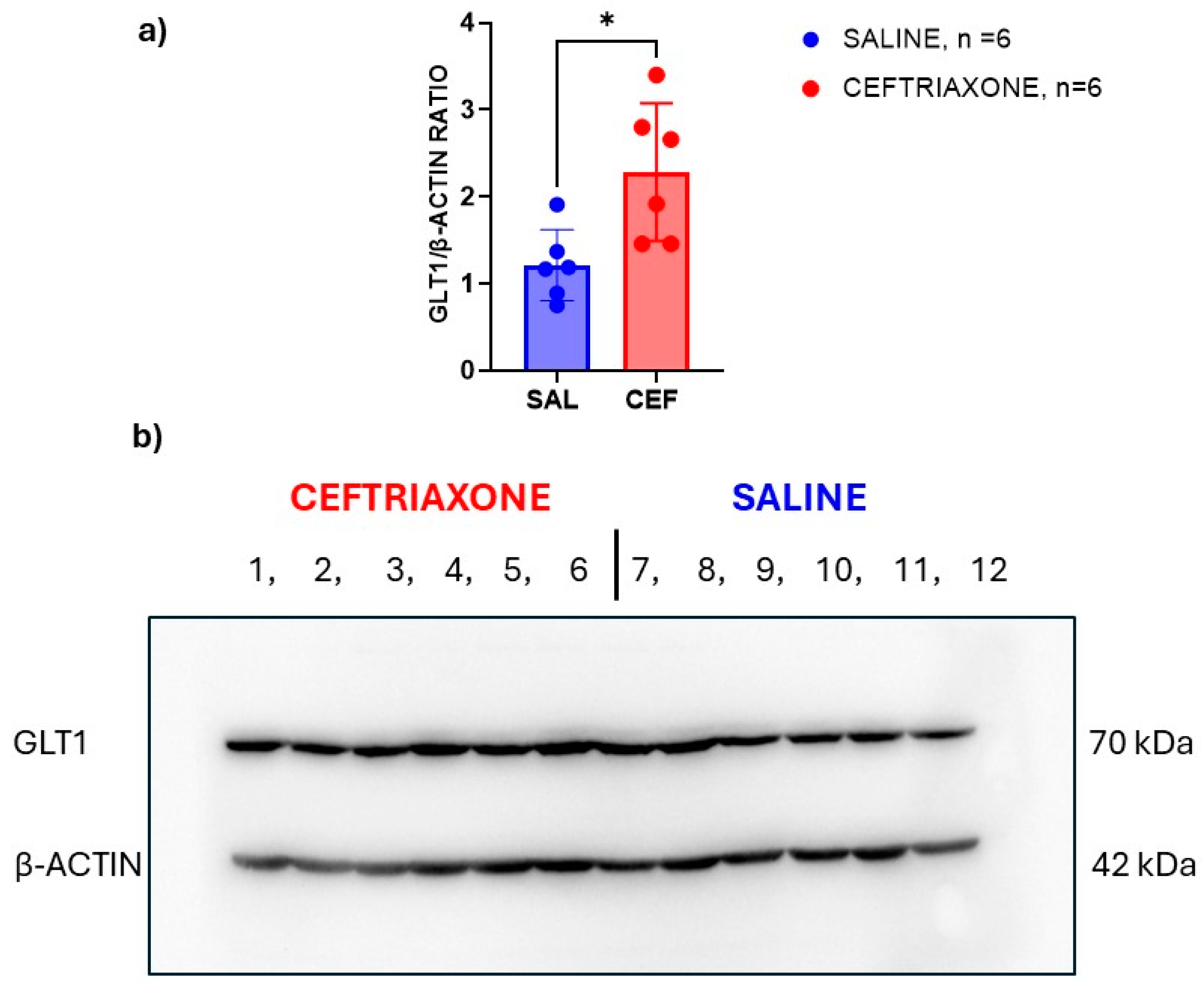
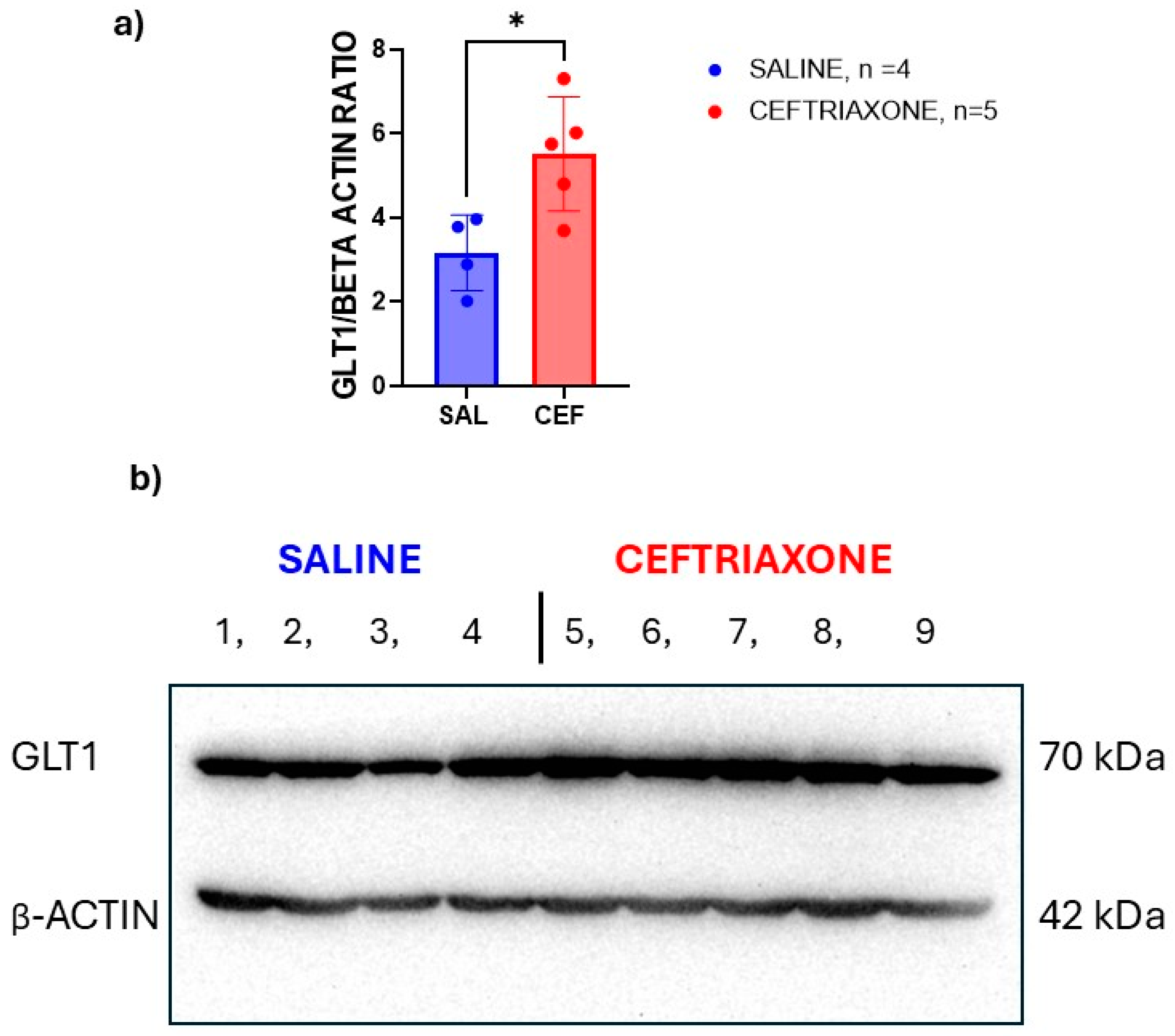
3.3. Western Blot Analysis of GLT1 Protein
3.4. Side Effects of Ceftriaxone—Comparison with Diazepam
3.5. Platelet 5HT Level and Thrombocyte Transporter (Uptake) Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Chin, N.E.; Strawbridge, S.A.; et al. DrugBank 6.0: The DrugBank Knowledgebase for 2024. Nucleic Acids Res. 2024, 52, D1265–D1275. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Campuzano, A.G.; Ortega, A. Glutamate Transporters: Critical Components of Glutamatergic Transmission. Neuropharmacology 2021, 192, 108602. [Google Scholar] [CrossRef] [PubMed]
- Beart, P.M. SLC1 Family of Amino Acid Transporters in GtoPdb v.2023.1. IUPHARBPS Guide Pharmacol. CITE 2023, S386–S388. [Google Scholar] [CrossRef]
- Martinez-Lozada, Z.; Guillem, A.M.; Robinson, M.B. Transcriptional Regulation of Glutamate Transporters. Adv. Pharmacol. 2016, 76, 103–145. [Google Scholar] [CrossRef] [PubMed]
- Karki, P.; Smith, K.; Johnson, J.; Aschner, M.; Lee, E.Y. Genetic Dys-Regulation of Astrocytic Glutamate Transporter EAAT2 and Its Implications in Neurological Disorders and Manganese Toxicity. Neurochem. Res. 2015, 40, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Lalo, U.; Koh, W.; Lee, C.J.; Pankratov, Y. The Tripartite Glutamatergic Synapse. Neuropharmacology 2021, 199, 108758. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hassel, B.; Eid, T.; Danbolt, N.C. Axon-Terminals Expressing EAAT2 (GLT-1; Slc1a2) Are Common in the Forebrain and Not Limited to the Hippocampus. Neurochem. Int. 2019, 123, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.K.; Storch, E.A.; Sheth, S.A. Harmonizing the Neurobiology and Treatment of Obsessive-Compulsive Disorder. Am. J. Psychiatry 2021, 178, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lu, Y.; Pan, B.-X.; Zhang, W.-H. New Insights into the Pivotal Role of the Amygdala in Inflammation-Related Depression and Anxiety Disorder. Int. J. Mol. Sci. 2022, 23, 11076. [Google Scholar] [CrossRef]
- Peterson, A.R.; Binder, D.K. Post-Translational Regulation of GLT-1 in Neurological Diseases and Its Potential as an Effective Therapeutic Target. Front. Mol. Neurosci. 2019, 12, 164. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Sullivan, C.R.; McCullumsmith, R.E. The Role of Glutamate Transporters in the Pathophysiology of Neuropsychiatric Disorders. Npj Schizophr. 2017, 3, 32. [Google Scholar] [CrossRef]
- Parkin, G.M.; Udawela, M.; Gibbons, A.; Dean, B. Glutamate Transporters, EAAT1 and EAAT2, Are Potentially Important in the Pathophysiology and Treatment of Schizophrenia and Affective Disorders. World J. Psychiatry 2018, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.C.K. Current Approaches to Enhance Glutamate Transporter Function and Expression. J. Neurochem. 2015, 134, 982–1007. [Google Scholar] [CrossRef] [PubMed]
- Yimer, E.M.; Hishe, H.Z.; Tuem, K.B. Repurposing of the β-Lactam Antibiotic, Ceftriaxone for Neurological Disorders: A Review. Front. Neurosci. 2019, 13, 236. [Google Scholar] [CrossRef]
- Smaga, I.; Fierro, D.; Mesa, J.; Filip, M.; Knackstedt, L.A. Molecular Changes Evoked by the Beta-Lactam Antibiotic Ceftriaxone across Rodent Models of Substance Use Disorder and Neurological Disease. Neurosci. Biobehav. Rev. 2020, 115, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Egashira, N.; Okuno, R.; Harada, S.; Matsushita, M.; Mishima, K.; Iwasaki, K.; Nishimura, R.; Oishi, R.; Fujiwara, M. Effects of Glutamate-Related Drugs on Marble-Burying Behavior in Mice: Implications for Obsessive-Compulsive Disorder. Eur. J. Pharmacol. 2008, 586, 164–170. [Google Scholar] [CrossRef]
- Cicin-Sain, L.; Jernej, B. Wistar–Zagreb 5HT Rats: A Rodent Model with Constitutional Upregulation/Downregulation of Serotonin Transporter. In Experimental Models in Serotonin Transporter Research; Kalueff, A.V., LaPorte, J.L., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 214–243. [Google Scholar] [CrossRef]
- Hranilovic, D.; Cicinsain, L.; Bordukaloniksic, T.; Jernej, B. Rats with Constitutionally Upregulated/Downregulated Platelet 5HT Transporter: Differences in Anxiety-Related Behavior. Behav. Brain Res. 2005, 165, 271–277. [Google Scholar] [CrossRef]
- Bordukalo-Niksic, T.; Mokrovic, G.; Stefulj, J.; Zivin, M.; Jernej, B.; Cicin-Sain, L. 5HT-1A Receptors and Anxiety-like Behaviours: Studies in Rats with Constitutionally Upregulated/Downregulated Serotonin Transporter. Behav. Brain Res. 2010, 213, 238–245. [Google Scholar] [CrossRef]
- Kesić, M.; Štefulj, J.; Mokrović, G.; Čičin-Šain, L. The Effect of Chronic Fluoxetine Administration on Anxiety-like Behavior and Expression of 5-HT-Related Proteins in Rats with Constitutively Altered 5-HT Homeostasis. BMC Pharmacol. Toxicol. 2012, 13, A30. [Google Scholar] [CrossRef]
- Filiou, M.D.; Nussbaumer, M.; Teplytska, L.; Turck, C.W. Behavioral and Metabolome Differences between C57BL/6 and DBA/2 Mouse Strains: Implications for Their Use as Models for Depression- and Anxiety-like Phenotypes. Metabolites 2021, 11, 128. [Google Scholar] [CrossRef]
- Pittenger, C.; Bloch, M.H.; Wasylink, S.; Billingslea, E.; Simpson, R.; Jakubovski, E.; Kelmendi, B.; Sanacora, G.; Coric, V. Riluzole Augmentation in Treatment-Refractory Obsessive-Compulsive Disorder: A Pilot Placebo-Controlled Trial. J. Clin. Psychiatry 2015, 76, 1075–1084. [Google Scholar] [CrossRef]
- Bloch, M.H.; Wasylink, S.; Landeros-Weisenberger, A.; Panza, K.E.; Billingslea, E.; Leckman, J.F.; Krystal, J.H.; Bhagwagar, Z.; Sanacora, G.; Pittenger, C. Effects of Ketamine in Treatment-Refractory Obsessive-Compulsive Disorder. Biol. Psychiatry 2012, 72, 964–970. [Google Scholar] [CrossRef]
- Saitoh, A.; Soda, A.; Kayashima, S.; Yoshizawa, K.; Oka, J.-I.; Nagase, H.; Yamada, M. A Delta Opioid Receptor Agonist, KNT-127, in the Prelimbic Medial Prefrontal Cortex Attenuates Glial Glutamate Transporter Blocker-Induced Anxiety-like Behavior in Mice. J. Pharmacol. Sci. 2018, 138, 176–183. [Google Scholar] [CrossRef]
- van Zutphen, L.F.M.; Baumans, V.; Beynen, A.C. (Eds.) Principles of Laboratory Animal Science, Revised Edition; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Jernej, B.; Cicin-Sain, L.; Iskrić, S. A Simple and Reliable Method for Monitoring Platelet Serotonin Levels in Rats. Life Sci. 1988, 43, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Llaneza, D.C.; Frye, C.A. Progestogens and Estrogen Influence Impulsive Burying and Avoidant Freezing Behavior of Naturally Cycling and Ovariectomized Rats. Pharmacol. Biochem. Behav. 2009, 93, 337–342. [Google Scholar] [CrossRef]
- Hoffman, K.L.; Cano-Ramírez, H. Lost in Translation? A Critical Look at the Role That Animal Models of Obsessive Compulsive Disorder Play in Current Drug Discovery Strategies. Expert Opin. Drug Discov. 2018, 13, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.M.; Grubb, M.; Deacon, R.M.J.; Yee, B.K.; Feldon, J.; Rawlins, J.N.P. Ventral Hippocampal Lesions Affect Anxiety but Not Spatial Learning. Behav. Brain Res. 2003, 139, 197–213. [Google Scholar] [CrossRef]
- Navarro-Sánchez, M.; Gil-Miravet, I.; Montero-Caballero, D.; Castillo-Gómez, E.; Gundlach, A.L.; Olucha-Bordonau, F.E. Some Key Parameters in Contextual Fear Conditioning and Extinction in Adult Rats. Behav. Brain Res. 2024, 462, 114874. [Google Scholar] [CrossRef]
- Andolina, D.; Di Segni, M.; Bisicchia, E.; D’Alessandro, F.; Cestari, V.; Ventura, A.; Concepcion, C.; Puglisi-Allegra, S.; Ventura, R. Effects of Lack of microRNA-34 on the Neural Circuitry Underlying the Stress Response and Anxiety. Neuropharmacology 2016, 107, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Spijker, S. Dissection of Rodent Brain Regions. In Neuroproteomics; Li, K.W., Ed.; Neuromethods; Humana Press: Totowa, NJ, USA, 2011; Volume 57, pp. 13–26. [Google Scholar] [CrossRef]
- Gilbert, K.; Godbout, R.; Rousseau, G. Caspase-3 Activity in the Rat Amygdala Measured by Spectrofluorometry after Myocardial Infarction. J. Vis. Exp. 2016, 107, e53207. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-like Behavior. In Pre-Clinical Models; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; Volume 1916, pp. 99–103. [Google Scholar] [CrossRef]
- Carter, R.J.; Morton, J.; Dunnett, S.B. Motor Coordination and Balance in Rodents. Curr. Protoc. Neurosci. 2001, 15, 8.12.1–8.12.14. [Google Scholar] [CrossRef] [PubMed]
- Glynn, D. Complexin II Is Essential for Normal Neurological Function in Mice. Hum. Mol. Genet. 2003, 12, 2431–2448. [Google Scholar] [CrossRef] [PubMed]
- Lotan, D.; Cunningham, M.; Joel, D. Antibiotic Treatment Attenuates Behavioral and Neurochemical Changes Induced by Exposure of Rats to Group A Streptococcal Antigen. PLoS ONE 2014, 9, e101257. [Google Scholar] [CrossRef]
- Kim, D.J.; King, J.A.; Zuccarelli, L.; Ferris, C.F.; Koppel, G.A.; Snowdon, C.T.; Ahn, C.H. Clavulanic Acid: A Competitive Inhibitor of Beta-Lactamases with Novel Anxiolytic-like Activity and Minimal Side Effects. Pharmacol. Biochem. Behav. 2009, 93, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.O.; Alasmari, F.; Albaker, A.B.; Alhazmi, H.A.; Alameen, A.A.; Alagail, N.M.; Alwaeli, S.A.; Rizwan Ahamad, S.; AlAsmari, A.F.; AlSharari, S.D. Clavulanic Acid Improves Memory Dysfunction and Anxiety Behaviors through Upregulating Glutamatergic Transporters in the Nucleus Accumbens of Mice Repeatedly Exposed to Khat Extract. Int. J. Mol. Sci. 2023, 24, 15657. [Google Scholar] [CrossRef] [PubMed]
- Matos-Ocasio, F.; Hernández-López, A.; Thompson, K.J. Ceftriaxone, a GLT-1 Transporter Activator, Disrupts Hippocampal Learning in Rats. Pharmacol. Biochem. Behav. 2014, 122, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Hakimizadeh, E.; Kaeidi, A.; Taghipour, Z.; Mehrzadi, S.; Allahtavakoli, M.; Shamsizadeh, A.; Bazmandegan, G.; Hassanshahi, J.; Reza, M.; Fatemi, I. Ceftriaxone Improves Senile Neurocognition Damages Induced by D-Galactose in Mice. Iran. J. Basic Med. Sci. 2020, 23, 368–375. [Google Scholar]
- Kang, S.; Li, J.; Bekker, A.; Ye, J.-H. Rescue of Glutamate Transport in the Lateral Habenula Alleviates Depression- and Anxiety-like Behaviors in Ethanol-Withdrawn Rats. Neuropharmacology 2018, 129, 47–56. [Google Scholar] [CrossRef]
- Gargano, S.P.; Santos, M.G.; Taylor, S.M.; Pastis, I. A Closer Look to Neural Pathways and Psychopharmacology of Obsessive Compulsive Disorder. Front. Behav. Neurosci. 2023, 17, 1282246. [Google Scholar] [CrossRef]
- Ratti, E.; Berry, J.D.; Greenblatt, D.J.; Loci, L.; Ellrodt, A.S.; Shefner, J.M.; Cudkowicz, M.E. Preclinical Rodent Toxicity Studies for Long Term Use of Ceftriaxone. Toxicol. Rep. 2015, 2, 1396–1403. [Google Scholar] [CrossRef]
- Tai, C.-H.; Bellesi, M.; Chen, A.-C.; Lin, C.-L.; Li, H.-H.; Lin, P.-J.; Liao, W.-C.; Hung, C.-S.; Schwarting, R.K.; Ho, Y.-J. A New Avenue for Treating Neuronal Diseases: Ceftriaxone, an Old Antibiotic Demonstrating Behavioral Neuronal Effects. Behav. Brain Res. 2019, 364, 149–156. [Google Scholar] [CrossRef]
- Suthard, R.L.; Senne, R.A.; Buzharsky, M.D.; Pyo, A.Y.; Dorst, K.E.; Diep, A.H.; Cole, R.H.; Ramirez, S. Basolateral Amygdala Astrocytes Are Engaged by the Acquisition and Expression of a Contextual Fear Memory. J. Neurosci. 2023, 43, 4997–5013. [Google Scholar] [CrossRef]
- Chaaya, N.; Battle, A.R.; Johnson, L.R. An Update on Contextual Fear Memory Mechanisms: Transition between Amygdala and Hippocampus. Neurosci. Biobehav. Rev. 2018, 92, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Dahlmanns, M.; Dahlmanns, J.K.; Savaskan, N.; Steiner, H.-H.; Yakubov, E. Glial Glutamate Transporter-Mediated Plasticity: System Xc-/xCT/SLC7A11 and EAAT1/2 in Brain Diseases. Front. Biosci.-Landmark 2023, 28, 57. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Saline | Ceftriaxone | Diazepam |
|---|---|---|---|
| Horizontal locomotion (m) | 12.4 ± 1.04 (10) | 10.8 ± 1.27 (10) | 5.99 ± 1.20 **; # (10) |
| Vertical locomotion (number) | 30.1 ± 3.47 (10) | 27.2 ± 2.83 (10) | 3.30 ± 0.817 ****; #### (10) |
| Beam-walking latencies (s) | 18.5 ± 8.67 (6) | 19.7 ± 8.37 (6) | 60.0 ± 0.00 * (5) |
| Latencies to fall (s) | 51.9 ± 6.65 (6) | 54.7 ± 5.33 (6) | 14.0 ± 11.54 # (5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poljak, L.; Miše, B.; Čičin-Šain, L.; Tvrdeić, A. Ceftriaxone Inhibits Conditioned Fear and Compulsive-like Repetitive Marble Digging without Central Nervous System Side Effects Typical of Diazepam—A Study on DBA2/J Mice and a High-5HT Subline of Wistar–Zagreb 5HT Rats. Biomedicines 2024, 12, 1711. https://doi.org/10.3390/biomedicines12081711
Poljak L, Miše B, Čičin-Šain L, Tvrdeić A. Ceftriaxone Inhibits Conditioned Fear and Compulsive-like Repetitive Marble Digging without Central Nervous System Side Effects Typical of Diazepam—A Study on DBA2/J Mice and a High-5HT Subline of Wistar–Zagreb 5HT Rats. Biomedicines. 2024; 12(8):1711. https://doi.org/10.3390/biomedicines12081711
Chicago/Turabian StylePoljak, Ljiljana, Branko Miše, Lipa Čičin-Šain, and Ante Tvrdeić. 2024. "Ceftriaxone Inhibits Conditioned Fear and Compulsive-like Repetitive Marble Digging without Central Nervous System Side Effects Typical of Diazepam—A Study on DBA2/J Mice and a High-5HT Subline of Wistar–Zagreb 5HT Rats" Biomedicines 12, no. 8: 1711. https://doi.org/10.3390/biomedicines12081711
APA StylePoljak, L., Miše, B., Čičin-Šain, L., & Tvrdeić, A. (2024). Ceftriaxone Inhibits Conditioned Fear and Compulsive-like Repetitive Marble Digging without Central Nervous System Side Effects Typical of Diazepam—A Study on DBA2/J Mice and a High-5HT Subline of Wistar–Zagreb 5HT Rats. Biomedicines, 12(8), 1711. https://doi.org/10.3390/biomedicines12081711








