The Potential Mechanisms Involved in the Disruption of Spermatogenesis in Mice by Nanoplastics and Microplastics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microplastic Particle
2.2. Animals and Treatment
2.3. Cell Culture and Treatment
2.4. Transcriptome Sequencing
2.5. DEG Screening Analysis
2.6. Functional Analysis of the DEGs
2.7. Real-Time qPCR
2.8. Statistical Analysis
3. Results
3.1. PS-MP and PS-NP Exposure Reduced Spermatocytes In Vivo
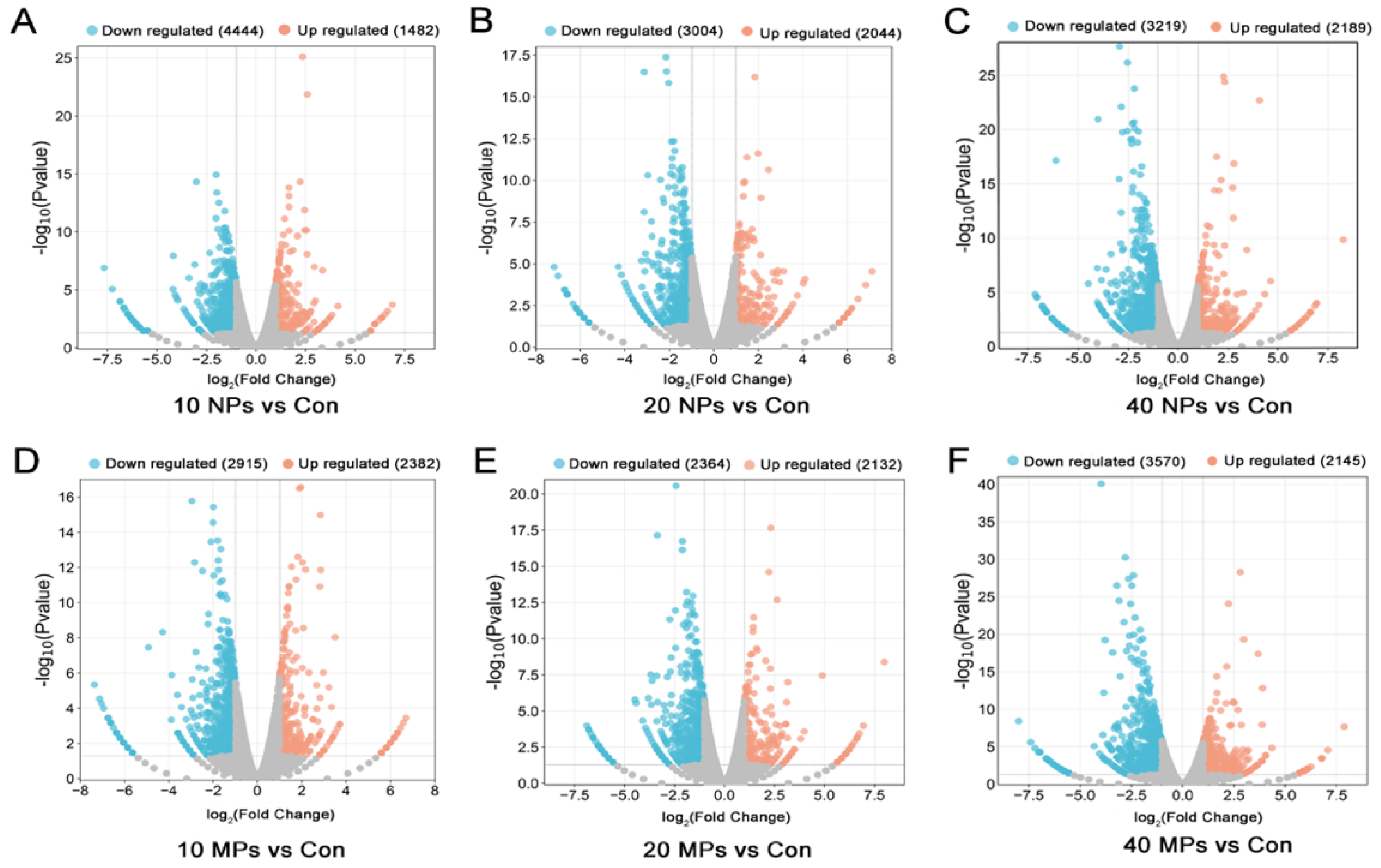
3.2. PS-NP and PS-MP Exposure Altered the Transcriptome of Spermatocytes
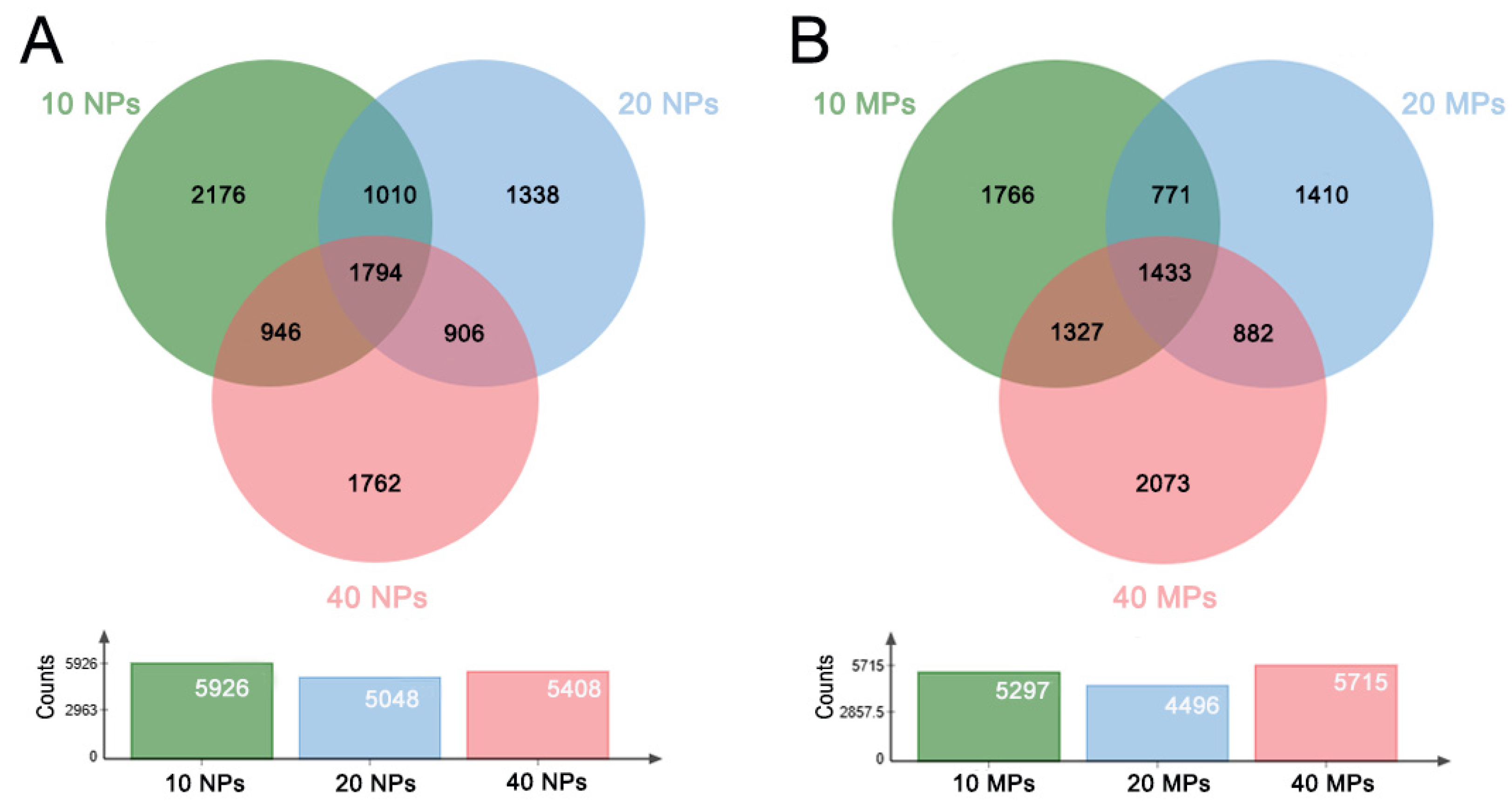
3.3. The Common and Specific GO Entries and KEGG Pathways in the PS-NP and PS-MP Groups
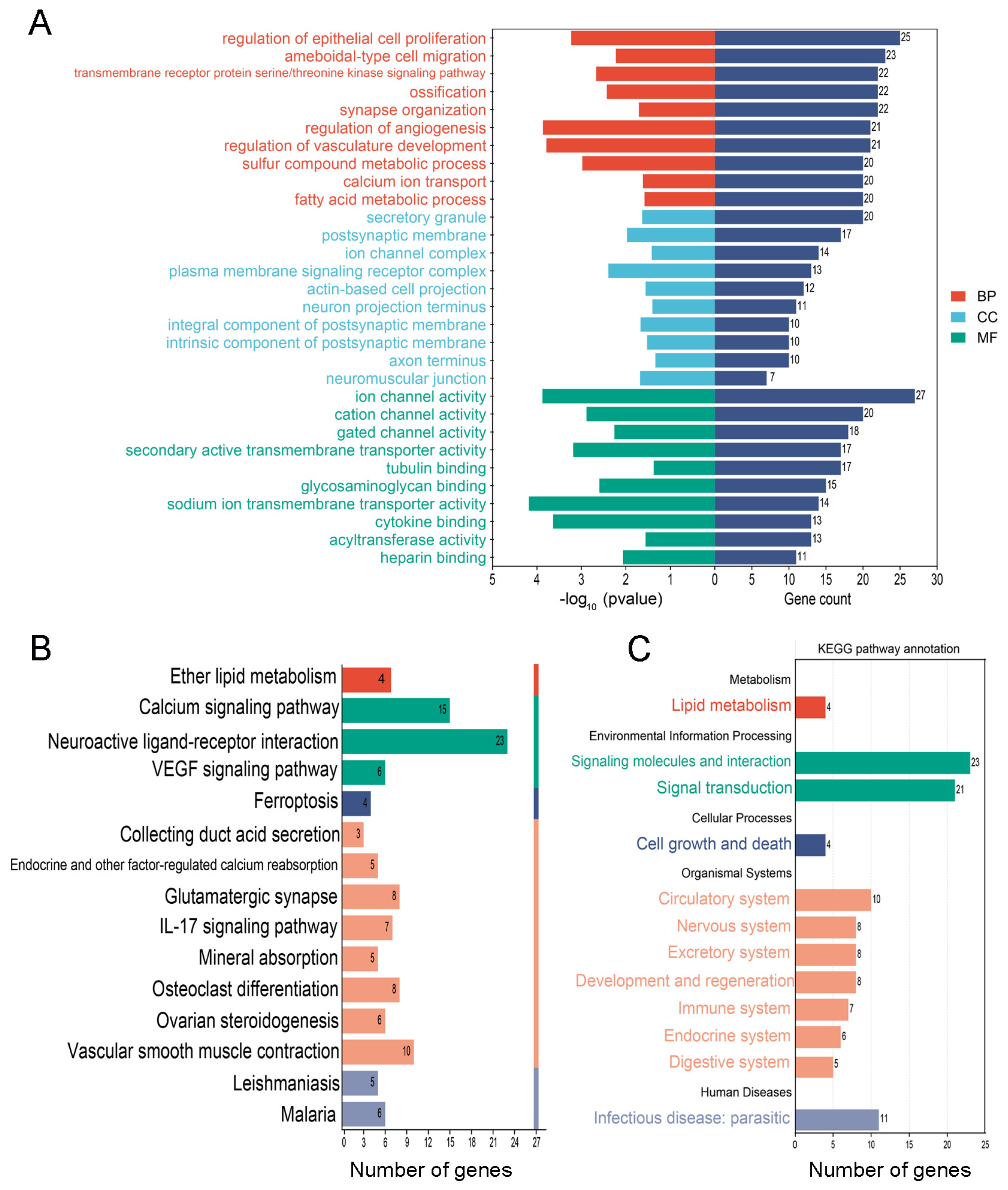
3.4. The Special GO Entries and KEGG Pathways in the PS-NP Group
3.5. The Special GO Entries and KEGG Pathways in the PS-MP Group
3.6. Screening and Validation of the Key Genes from the DEGs in the PS-NP and PS-MP Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satti, S.M.; Shah, A.A. Polyester-based biodegradable plastics: An approach towards sustainable development. Lett. Appl. Microbiol. 2020, 70, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Petersen, F.; Hubbart, J.A. The occurrence and transport of microplastics: The state of the science. Sci. Total Environ. 2021, 758, 143936. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lan, R.; Wang, Z.; Su, W.; Song, D.; Xue, R.; Liu, Z.; Liu, X.; Dai, Y.; Yue, T.; et al. Microplastic fragmentation by rotifers in aquatic ecosystems contributes to global nanoplastic pollution. Nat. Nanotechnol. 2023, 19, 406–414. [Google Scholar] [CrossRef]
- Ivar Do Sul, J.A.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.C.; Monnolo, A.; Del Piano, F.; Raso, G.M.; Meli, R. The Pressing Issue of Micro- and Nanoplastic Contamination: Profiling the Reproductive Alterations Mediated by Oxidative Stress. Antioxidants 2022, 11, 193. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Xiong, F.; Xu, K.; Pu, Y.; Huang, J.; Zhang, J.; Pu, Y.; Sun, R.; Cheng, K. Research advances of microplastics and potential health risks of microplastics on terrestrial higher mammals: A bibliometric analysis and literature review. Environ. Geochem. Health 2023, 45, 2803–2838. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Galvão, L.D.S.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Montano, L.; Giorgini, E.; Notarstefano, V.; Notari, T.; Ricciardi, M.; Piscopo, M.; Motta, O. Raman Microspectroscopy evidence of microplastics in human semen. Sci. Total Environ. 2023, 901, 165922. [Google Scholar] [CrossRef]
- Li, B.; Ding, Y.; Cheng, X.; Sheng, D.; Xu, Z.; Rong, Q.; Wu, Y.; Zhao, H.; Ji, X.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244, 125492. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kopatz, V.; Wen, K.; Kovács, T.; Keimowitz, A.S.; Pichler, V.; Widder, J.; Vethaak, A.D.; Hollóczki, O.; Kenner, L. Micro- and Nanoplastics Breach the Blood-Brain Barrier (BBB): Biomolecular Corona’s Role Revealed. Nanomaterials 2023, 13, 1404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sokratian, A.; Duda, A.M.; Xu, E.; Stanhope, C.; Fu, A.; Strader, S.; Li, H.; Yuan, Y.; Bobay, B.G.; et al. Anionic nanoplastic contaminants promote Parkinson’s disease-associated α-synuclein aggregation. Sci. Adv. 2023, 9, eadi8716. [Google Scholar] [CrossRef]
- Jin, H.; Ma, T.; Sha, X.; Liu, Z.; Zhou, Y.; Meng, X.; Chen, Y.; Han, X.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater. 2021, 401, 123430. [Google Scholar] [CrossRef]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Sui, A.; Yao, C.; Chen, Y.; Li, Y.; Yu, S.; Qu, J.; Wei, H.; Tang, J.; Chen, G. Polystyrene nanoplastics inhibit StAR expression by activating HIF-1α via ERK1/2 MAPK and AKT pathways in TM3 Leydig cells and testicular tissues of mice. Food Chem. Toxicol. 2023, 173, 113634. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Qin, X.; Zhang, J.; Zhu, Y.; Zeng, W.; Lin, Y.; Liu, X. Polystyrene microplastics disturb maternal-fetal immune balance and cause reproductive toxicity in pregnant mice. Reprod. Toxicol. 2021, 106, 42–50. [Google Scholar] [CrossRef]
- Huang, C.; Li, B.; Xu, K.; Liu, D.; Hu, J.; Yang, Y.; Nie, H.; Fan, L.; Zhu, W. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil. Steril. 2017, 107, 83–88.e2. [Google Scholar] [CrossRef]
- de Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13 (Suppl. 1), 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; La Vignera, S.; Calogero, A.E. Molecular Biology of Spermatogenesis: Novel Targets of Apparently Idiopathic Male Infertility. Int. J. Mol. Sci. 2020, 21, 1728. [Google Scholar] [CrossRef]
- Lu, C.; Liang, Y.; Cheng, Y.; Peng, C.; Sun, Y.; Liu, K.; Li, Y.; Lou, Y.; Jiang, X.; Zhang, A.; et al. Microplastics cause reproductive toxicity in male mice through inducing apoptosis of spermatogenic cells via p53 signaling. Food Chem. Toxicol. 2023, 179, 113970. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Siregar, P.; Malhotra, N.; Lai, Y.-H.; Liang, S.-T.; Chen, J.-R.; Chen, K.H.-C.; Hsiao, C.-D. Nanoplastics Cause Neurobehavioral Impairments, Reproductive and Oxidative Damages, and Biomarker Responses in Zebrafish: Throwing up Alarms of Wide Spread Health Risk of Exposure. Int. J. Mol. Sci. 2020, 21, 1410. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Shelver, W.L. Micro- and nanoplastic induced cellular toxicity in mammals: A review. Sci. Total Environ. 2021, 755, 142518. [Google Scholar] [CrossRef] [PubMed]
- Zarus, G.M.; Muianga, C.; Hunter, C.M.; Pappas, R.S. A review of data for quantifying human exposures to micro and nanoplastics and potential health risks. Sci. Total Environ. 2021, 756, 144010. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, X.; Zhang, X.; Duan, X.; Lin, H.; Liu, S.; Sui, G. Assessment of cancer-related signaling pathways in responses to polystyrene nanoplastics via a kidney-testis microfluidic platform (KTP). Sci. Total Environ. 2023, 857, 159306. [Google Scholar] [CrossRef]
- Gao, L.; Xiong, X.; Chen, C.; Luo, P.; Li, J.; Gao, X.; Huang, L.; Li, L. The male reproductive toxicity after nanoplastics and microplastics exposure: Sperm quality and changes of different cells in testis. Ecotoxicol. Environ. Saf. 2023, 267, 115618. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Boyko, A.I.; Karlina, I.S.; Zavileyskiy, L.G.; Aleshin, V.A.; Artiukhov, A.V.; Kaehne, T.; Ksenofontov, A.L.; Ryabov, S.I.; Graf, A.V.; Tramonti, A.; et al. Delayed Impact of 2-Oxoadipate Dehydrogenase Inhibition on the Rat Brain Metabolism Is Linked to Protein Glutarylation. Front. Med. 2022, 9, 896263. [Google Scholar] [CrossRef] [PubMed]
- Artiukhov, A.V.; Kazantsev, A.V.; Lukashev, N.V.; Bellinzoni, M.; Bunik, V.I. Selective Inhibition of 2-Oxoglutarate and 2-Oxoadipate Dehydrogenases by the Phosphonate Analogs of Their 2-Oxo Acid Substrates. Front. Chem. 2020, 8, 596187. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Gao, Y.; Trappetti, V.; Hertig, D.; Karatkevich, D.; Losmanova, T.; Urzi, C.; Ge, H.; Geest, G.A.; Bruggmann, R.; et al. Targeting lactate dehydrogenase B-dependent mitochondrial metabolism affects tumor initiating cells and inhibits tumorigenesis of non-small cell lung cancer by inducing mtDNA damage. Cell. Mol. Life Sci. 2022, 79, 445. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, S.; van Geest, F.S.; Peeters, R.P.; Heuer, H.; Visser, W.E. Thyroid Hormone Transporters. Endocr. Rev. 2020, 41, bnz008. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in take-out food containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.P.D.C.; Gomes, A.R.; Malafaia, G. Hepatotoxicity of pristine polyethylene microplastics in neotropical physalaemus cuvieri tadpoles (Fitzinger, 1826). J. Hazard. Mater. 2020, 386, 121992. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-D.; Chen, C.-W.; Chen, Y.-C.; Chen, H.-H.; Lee, J.-S.; Lin, C.-H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef]
- Amereh, F.; Babaei, M.; Eslami, A.; Fazelipour, S.; Rafiee, M. The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge. Environ. Pollut. 2020, 261, 114158. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Qiao, B.; Zhong, G.; Li, T.; Su, Q.; Wu, S.; Tang, Z.; Hu, L. Arsenic and polystyrene-nano plastics co-exposure induced testicular toxicity: Triggers oxidative stress and promotes apoptosis and inflammation in mice. Environ. Toxicol. 2024, 39, 264–276. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Madrid-Gambin, F.; Llavanera, M.; Gomez-Gomez, A.; Haro, N.; Pozo, O.J.; Yeste, M. Sperm physiology and in vitro fertilising ability rely on basal metabolic activity: Insights from the pig model. Commun. Biol. 2023, 6, 344. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, G.; Sheng, C.; Zheng, Y.; Tang, D.; Zhang, Y.; Hou, X.; Yao, M.; Zong, Q.; Zhou, Z. Intracellular Protein Adsorption Behavior and Biological Effects of Polystyrene Nanoplastics in THP-1 Cells. Environ. Sci. Technol. 2024, 58, 2652–2661. [Google Scholar] [CrossRef]
- Aghaei, Z.; Mercer, G.V.; Schneider, C.M.; Sled, J.G.; Macgowan, C.K.; Baschat, A.A.; Kingdom, J.C.; Helm, P.A.; Simpson, A.J.; Simpson, M.J.; et al. Maternal exposure to polystyrene microplastics alters placental metabolism in mice. Metabolomics 2022, 19, 1. [Google Scholar] [CrossRef]
- Goodman, K.E.; Hare, J.T.; Khamis, Z.I.; Hua, T.; Sang, Q.-X.A. Exposure of Human Lung Cells to Polystyrene Microplastics Significantly Retards Cell Proliferation and Triggers Morphological Changes. Chem. Res. Toxicol. 2021, 34, 1069–1081. [Google Scholar] [CrossRef]
- Pompura, S.L.; Dominguez-Villar, M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J. Leukoc. Biol. 2018, 103, 1065–1076. [Google Scholar] [CrossRef]
- Wang, S.; Wu, H.; Shi, X.; Wang, Y.; Xu, S. Polystyrene microplastics with different sizes induce the apoptosis and necroptosis in liver through the PTEN/PI3K/AKT/autophagy axis. Sci. Total Environ. 2023, 899, 165461. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, X.; Qi, X.; Liu, X.; Zhang, Y.; Qiao, S.; Lin, H. Di-(2-Ethylhexyl) Phthalate and Microplastics Induced Neuronal Apoptosis through the PI3K/AKT Pathway and Mitochondrial Dysfunction. J. Agric. Food Chem. 2022, 70, 10771–10781. [Google Scholar] [CrossRef]
- Feng, L.; Chen, C.; Xiong, X.; Wang, X.; Li, X.; Kuang, Q.; Wei, X.; Gao, L.; Niu, X.; Li, Q.; et al. PS-MPs promotes the progression of inflammation and fibrosis in diabetic nephropathy through NLRP3/Caspase-1 and TGF-β1/Smad2/3 signaling pathways. Ecotoxicol. Environ. Saf. 2024, 273, 116102. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Vercauteren, M.; Grootaert, C.; Rajkovic, A.; Boon, N.; Janssen, C.; Asselman, J. Cellular and bioenergetic effects of polystyrene microplastic in function of cell type, differentiation status and post-exposure time. Environ. Pollut. 2023, 337, 122550. [Google Scholar] [CrossRef]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies, and future prospects. Environ. Sci. Pollut. Res. Int. 2015, 22, 4122–4143. [Google Scholar] [CrossRef]
- Endo, T.; Mikedis, M.M.; Nicholls, P.K.; Page, D.C.; de Rooij, D.G. Retinoic Acid and Germ Cell Development in the Ovary and Testis. Biomolecules 2019, 9, 755. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, C.A.; Evans, E.; Onken, J.; Kent, T.; Mitchell, D.; Petkovich, M.; Griswold, M.D. CYP26 Enzymes Are Necessary Within the Postnatal Seminiferous Epithelium for Normal Murine Spermatogenesis. Biol. Reprod. 2015, 93, 19. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Shahzadi, S.; Samad, A.; Ehsan, N.; Ahmed, H.; Tahir, A.; Rehman, H.; Anwar, H. Dose-Dependent Effect of Polystyrene Microplastics on the Testicular Tissues of the Male Sprague Dawley Rats. Dose Response 2021, 19, 15593258211019882. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhou, Y.; Long, C.; Wu, H.; Hong, Y.; Fu, Y.; Wang, J.; Wu, Y.; Shen, L.; Wei, G. Polystyrene microplastics disrupt the blood-testis barrier integrity through ROS-Mediated imbalance of mTORC1 and mTORC2. Environ. Pollut. 2021, 289, 117904. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, Y.; Wang, S.; Xie, J.; Han, Q.; Chen, M. Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice. Toxicology 2022, 465, 153059. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yan, M.; Pan, C.; Liu, Z.; Sha, X.; Jiang, C.; Li, L.; Pan, M.; Li, D.; Han, X.; et al. Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Part. Fibre Toxicol. 2022, 19, 13. [Google Scholar] [CrossRef]
- Liu, T.; Hou, B.; Wang, Z.; Yang, Y. Polystyrene microplastics induce mitochondrial damage in mouse GC-2 cells. Ecotoxicol. Environ. Saf. 2022, 237, 113520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Shen, L.; Ye, X.; Bai, G.; Liao, C.; Chen, Z.; Peng, T.; Li, X.; Kang, X.; An, G. Prenatal and postnatal exposure to polystyrene microplastics induces testis developmental disorder and affects male fertility in mice. J. Hazard. Mater. 2023, 445, 130544. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef]
- Thomas, K.; Del Mazo, J.; Eversole, P.; Bellvé, A.; Hiraoka, Y.; Li, S.S.; Simon, M. Developmental regulation of expression of the lactate dehydrogenase (LDH) multigene family during mouse spermatogenesis. Development 1990, 109, 483–493. [Google Scholar] [CrossRef]
- La Vignera, S.; Vita, R.; Condorelli, R.A.; Mongioì, L.M.; Presti, S.; Benvenga, S.; Calogero, A.E. Impact of thyroid disease on testicular function. Endocrine 2017, 58, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef]
- Lan, Y.; Hu, L.; Feng, X.; Wang, M.; Yuan, H.; Xu, H. Synergistic effect of PS-MPs and Cd on male reproductive toxicity: Ferroptosis via Keap1-Nrf2 pathway. J. Hazard. Mater. 2024, 461, 132584. [Google Scholar] [CrossRef]




| Gene | Sequences (5′–3′) | |
|---|---|---|
| Foreword | Reverse | |
| Dhtkd1 | 5′-CCTCGCTCTCCTTGGTTGTAG-3′ | 5′-CCTCAGTTGACCATGGCCTT-3′ |
| Ldhb | 5′-GGATTCACCCCGTGTCTACC-3′ | 5′-GAAGGACGATGAGGTCGCTC-3′ |
| Slc16a2 | 5′-CCCATTGGTTCTTGGCTCTGA-3′ | 5′-GGGTAGGGGGCAGAATAAGT-3′ |
| Thra | 5′-TTGCGTGCTGTTTCCCCATA-3′ | 5′-AACAAATCGAGGGGCCAGAG-3′ |
| Cyp26a1 | 5′-TCTGGGACCTGTACTGTGTGA-3′ | 5′-AAGCCGTATTTCCTGCGCTT-3′ |
| Cyp26b1 | 5′-TGCCCATACCCCATCGCC-3′ | 5′-GGCTGCGAGGTGATCGAAGA-3′ |
| β-actin | 5′-GTCCCTCACCCTCCCAAAAG-3′ | 5′-GCTGCCTCAACACCTCAACCC-3′ |
| Size | Common | 10 μg/mL | 20 μg/mL | 40 μg/mL |
|---|---|---|---|---|
| NPs | 1794 | 2176 | 1338 | 1762 |
| MPs | 1433 | 1766 | 1410 | 2073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, Y.; Cai, J.; Zhang, H.; Li, Y.; Yu, M.; Liu, J.; Han, F. The Potential Mechanisms Involved in the Disruption of Spermatogenesis in Mice by Nanoplastics and Microplastics. Biomedicines 2024, 12, 1714. https://doi.org/10.3390/biomedicines12081714
Wen Y, Cai J, Zhang H, Li Y, Yu M, Liu J, Han F. The Potential Mechanisms Involved in the Disruption of Spermatogenesis in Mice by Nanoplastics and Microplastics. Biomedicines. 2024; 12(8):1714. https://doi.org/10.3390/biomedicines12081714
Chicago/Turabian StyleWen, Yixian, Jing Cai, Huilian Zhang, Yi Li, Manyao Yu, Jinyi Liu, and Fei Han. 2024. "The Potential Mechanisms Involved in the Disruption of Spermatogenesis in Mice by Nanoplastics and Microplastics" Biomedicines 12, no. 8: 1714. https://doi.org/10.3390/biomedicines12081714







