Magnesium Ion: A New Switch in Tumor Treatment
Abstract
:1. Introduction
2. Methods
3. Magnesium Ion Transport Proteins and Their Implications in Tumor Development
3.1. TRPM Family
3.2. SLC Family
3.3. MagT Family
3.4. CNNM Family
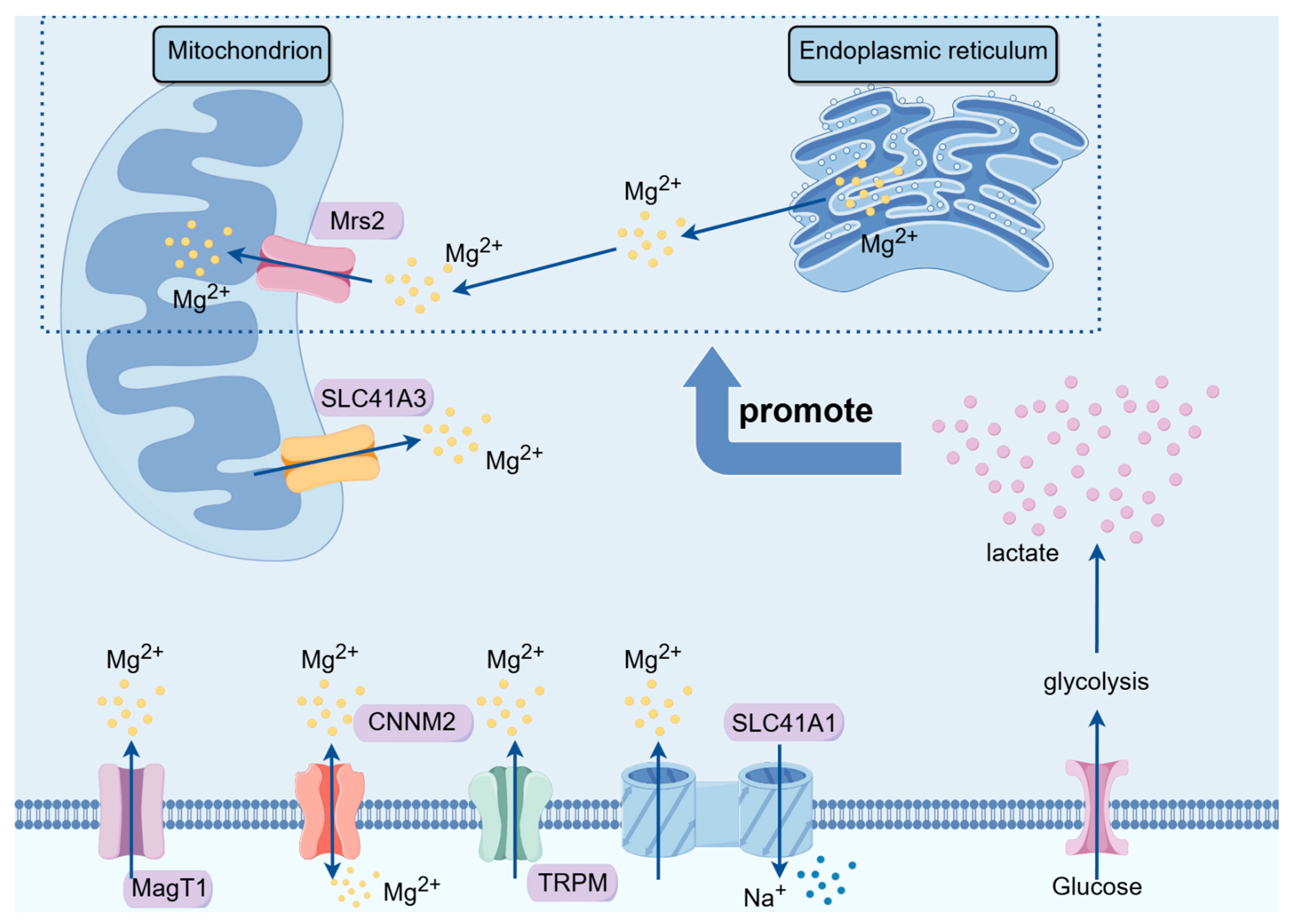
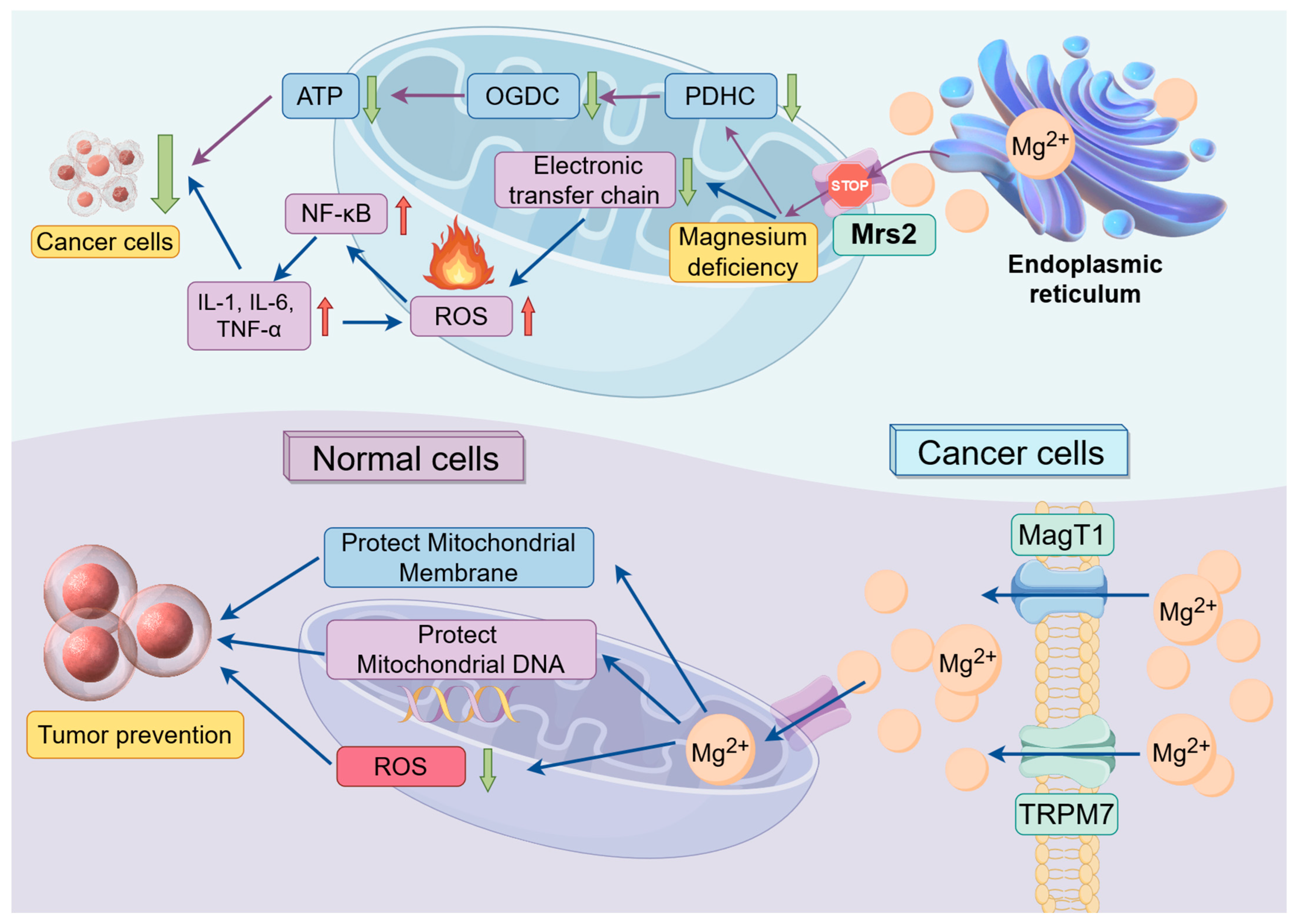
3.5. MRS Family
4. The Impact of Magnesium Ions on Tumor Development
4.1. Breast Cancer
4.2. Colorectal Cancer
4.3. Other Cancers
5. Mechanisms of Magnesium Ion Therapy in Tumor Treatment
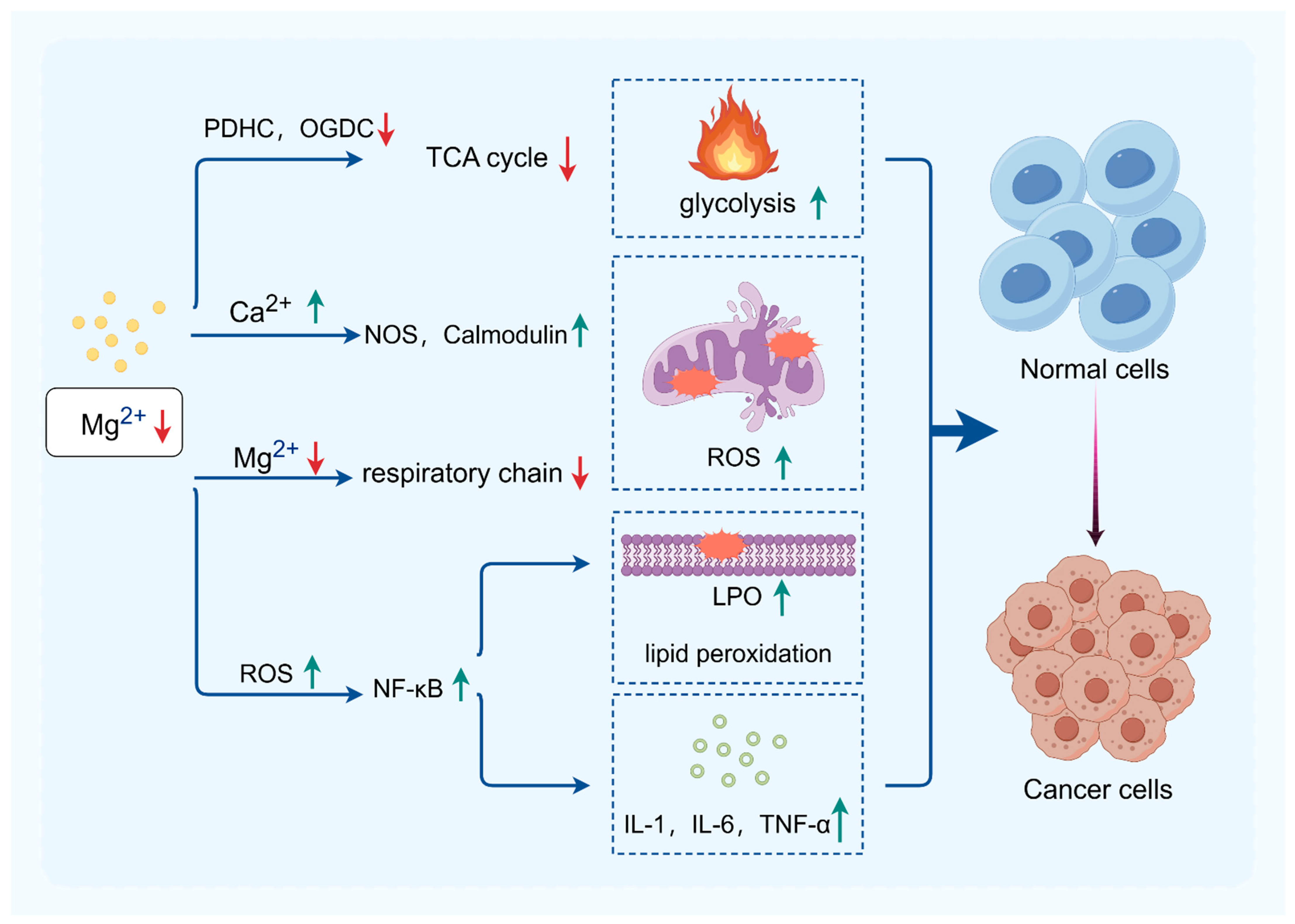
5.1. The Impact of Magnesium Ions on Mitochondrial Function
5.1.1. The Impact of Magnesium Ions on Mitochondrial Energy Metabolism
5.1.2. The Impact of Magnesium Ions on Oxidative Stress
5.1.3. Relationships between Mitochondrial Function and Cancer
5.2. Magnesium Ions and Inflammation
5.3. Magnesium Regulates Apoptosis in Tumor Cells
6. Prospects of Magnesium Ions in Tumor Therapy
6.1. Clinical Applications of Magnesium in Cancer Therapy
6.2. Other Roles of Magnesium Ions in Cancer Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| TRPM | Transient receptor potential melastatin channel protein family |
| SLC | Human solute carrier superfamily |
| MagT | Magnesium transporter proteins |
| CNNM | Cyclin M family proteins |
| Mrs2 | Mitochondrial RNA splicing 2 family genes |
| PDHC | Pyruvate dehydrogenase complex |
| OGDC | Oxoglutarate dehydrogenase complex |
| NOS | Nitric oxide synthase |
| LPO | Lipid hydroperoxide |
| NF-κB | Nuclear factor kappa-B |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| HE | Hematoxylin–eosin staining |
References
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Al Alawi, A.M.; Al Badi, A.; Al Huraizi, A.; Falhammar, H. Magnesium: The recent research and developments. Adv. Food Nutr. Res. 2021, 96, 193–218. [Google Scholar] [PubMed]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Huang, L. Study on the Relationship between the Changes of Retinal Dopamine Neurons and Magnesium Ionsin 6-OHDA-Induced Parkinsonian Rats; Fujian Medical University: Fuzhou, China, 2021. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, C.; Zou, X.; Wu, W.; Di, Y.; Li, N.; Fu, A. Iron promotes ovarian cancer malignancy and advances platinum resistance by enhancing DNA repair via FTH1/FTL/POLQ/RAD51 axis. Cell Death Dis. 2024, 15, 329. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Fan, H.; Zou, Y.; Chen, S. Research progress of copper and its compounds in anti-tumor therapy. Chin. J. Oncol. Prev. Treat. 2023, 15, 328–334. [Google Scholar]
- Bois, P.; Sandborn, E.; Messier, P. A study of thymic lymphosarcoma developing in magnesium-deficient rats. Cancer Res. 1969, 29, 763–775. [Google Scholar] [PubMed]
- Auwercx, J.; Rybarczyk, P.; Kischel, P.; Dhennin-Duthille, I.; Chatelain, D.; Sevestre, H.; Van Seuningen, I.; Ouadid-Ahidouch, H.; Jonckheere, N.; Gautier, M. Mg Transporters in Digestive Cancers. Nutrients 2021, 13, 210. [Google Scholar] [CrossRef]
- Folsom, A.R.; Hong, C.P. Magnesium Intake and Reduced Risk of Colon Cancer in a Prospective Study of Women. Am. J. Epidemiol. 2006, 163, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Lötscher, J.; Martí I Líndez, A.; Kirchhammer, N.; Cribioli, E.; Giordano Attianese, G.; Trefny, M.; Lenz, M.; Rothschild, S.; Strati, P.; Künzli, M.; et al. Magnesium sensing via LFA-1 regulates CD8 T cell effector function. Cell 2022, 185, 585–602.e529. [Google Scholar] [CrossRef]
- Castiglioni, S.; Maier, J. Magnesium and cancer: A dangerous liason. Magnes. Res. 2011, 24, S92–S100. [Google Scholar] [CrossRef]
- Blaszczyk, U.; Duda-Chodak, A. Magnesium: Its role in nutrition and carcinogenesis. Rocz. Panstw. Zakl. Hig. 2013, 64, 165–171. [Google Scholar] [PubMed]
- Liu, Y.; Li, X.; Zou, Q.; Liu, L.; Zhu, X.; Jia, Q.; Wang, L.; Yan, R. Inhibitory effect of magnesium cantharidate on human hepatoma SMMC-7721 cell proliferation by blocking MAPK signaling pathway. Xi Bao Yu Fen. Zi Mian Yi Xue Za Zhi=Chin. J. Cell. Mol. Immunol. 2017, 33, 347–351. [Google Scholar]
- Liu, Y.; Xu, Y.; Ma, H.; Wang, B.; Xu, L.; Zhang, H.; Song, X.; Gao, L.; Liang, X.; Ma, C. Hepatitis B virus X protein amplifies TGF-β promotion on HCC motility through down-regulating PPM1a. Oncotarget 2016, 7, 33125–33135. [Google Scholar] [CrossRef] [PubMed]
- Koning, G.; Leverin, A.; Nair, S.; Schwendimann, L.; Ek, J.; Carlsson, Y.; Gressens, P.; Thornton, C.; Wang, X.; Mallard, C.; et al. Magnesium induces preconditioning of the neonatal brain via profound mitochondrial protection. J. Cereb. Blood Flow. Metab. Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2019, 39, 1038–1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, H.; Hu, J.; Li, H.; Guo, S.; Chen, B.; Liu, C.; Wang, G.; Zhou, F. Magnesium Isoglycyrrhizinate Reduces the Target-Binding Amount of Cisplatin to Mitochondrial DNA and Renal Injury through SIRT3. Int. J. Mol. Sci. 2022, 23, 13093. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.; Chaturvedi, M.; Aggarwal, B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Ciaglia, T.; Vestuto, V.; Bertamino, A.; González-Muñiz, R.; Gómez-Monterrey, I. On the modulation of TRPM channels: Current perspectives and anticancer therapeutic implications. Front. Oncol. 2023, 12, 1065935. [Google Scholar] [CrossRef]
- Arancibia-Hernández, Y.; Hernández-Cruz, E.; Pedraza-Chaverri, J. Magnesium (Mg) Deficiency, Not Well-Recognized Non-Infectious Pandemic: Origin and Consequence of Chronic Inflammatory and Oxidative Stress-Associated Diseases. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2023, 57, 1–23. [Google Scholar] [CrossRef]
- Gao, Y.; Liao, P. TRPM4 channel and cancer. Cancer Lett. 2019, 454, 66–69. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Xia, L.; Wu, N.; Wang, Y.; Li, H.; Chen, X.; Zhang, X.; Liu, Z.; Zhu, M.; et al. TRPM7 silencing modulates glucose metabolic reprogramming to inhibit the growth of ovarian cancer by enhancing AMPK activation to promote HIF-1α degradation. J. Exp. Clin. Cancer Res. CR 2022, 41, 44. [Google Scholar] [CrossRef]
- Miller, B. TRPM2 in Cancer. Cell Calcium 2019, 80, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hantute-Ghesquier, A.; Haustrate, A.; Prevarskaya, N.; Lehen’kyi, V.y. TRPM Family Channels in Cancer. Pharmaceuticals 2018, 11, 58. [Google Scholar] [CrossRef]
- Schweigel-Röntgen, M.; Kolisek, M. SLC41 transporters—Molecular identification and functional role. Curr. Top. Membr. 2014, 73, 383–410. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.T.K.; Ha, H.T.T.; Nguyen, T.H.; Nguyen, L.N. The role of SLC transporters for brain health and disease. Cell Mol. Life Sci. 2021, 79, 20. [Google Scholar] [CrossRef]
- Nemoto, T.; Tagashira, H.; Kita, T.; Kita, S.; Iwamoto, T. Functional characteristics and therapeutic potential of SLC41 transporters. J. Pharmacol. Sci. 2023, 151, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Sahni, J.; Scharenberg, A. The SLC41 family of MgtE-like magnesium transporters. Mol. Asp. Med. 2013, 34, 620–628. [Google Scholar] [CrossRef]
- Njiaju, U.; Gamazon, E.; Gorsic, L.; Delaney, S.; Wheeler, H.; Im, H.; Dolan, M. Whole-genome studies identify solute carrier transporters in cellular susceptibility to paclitaxel. Pharmacogenetics Genom. 2012, 22, 498–507. [Google Scholar] [CrossRef]
- Li, Q.; Xiong, D.; Wang, H.; Jin, W.; Ma, Y.; Fan, X. High Expression of SLC41A3 Correlates with Poor Prognosis in Hepatocellular Carcinoma. OncoTargets Ther. 2021, 14, 2975–2988. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Fang, M.; Chen, J.; Liu, L.; Lu, Z.; Hou, J.; Luo, M. Epigenetic activation of the TUSC3 gene as a potential therapy for XMEN disease. J. Allergy Clin. Immunol. 2023, 151, 1622–1633. [Google Scholar] [CrossRef]
- Gotru, S.; Mammadova-Bach, E.; Sogkas, G.; Schuhmann, M.; Schmitt, K.; Kraft, P.; Herterich, S.; Mamtimin, M.; Pinarci, A.; Beck, S.; et al. MAGT1 Deficiency Dysregulates Platelet Cation Homeostasis and Accelerates Arterial Thrombosis and Ischemic Stroke in Mice. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1494–1509. [Google Scholar] [CrossRef]
- Oh, M.; Jang, J.; Lee, J. Polarization of THP-1-Derived Macrophage by Magnesium and MAGT1 Inhibition in Wound Healing. Arch. Plast. Surg. 2023, 50, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Yang, Q.; Xie, L.; Qiu, Z.; Huang, Y.; Lin, Y.; Tu, L.; Cui, C. Overexpression of MAGT1 is associated with aggressiveness and poor prognosis of colorectal cancer. Oncol. Lett. 2019, 18, 3857–3862. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Li, Y.; Xiao, B.; Pei, S.; Jiang, H.; Zhang, X. Transcription factor KLF16 activates MAGT1 to regulate the tumorigenesis and progression of breast cancer. Int. J. Mol. Med. 2022, 50, 115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, X.; Ni, H. Long non-coding RNA FLVCR1-AS1 functions as a ceRNA to aggravate cervical cancer cell growth by the miR-381-3p/MAGT1 axis. Arch. Gynecol. Obstet. 2022, 306, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Zhang, X.; Chen, Y.; Dong, Y.; Shi, Y.; Lei, Y.; Lv, D.; Cao, X.; Li, W.; Shi, H. MAGT1 is required for HeLa cell proliferation through regulating p21 expression, S-phase progress, and ERK/p38 MAPK MYC axis. Cell Cycle (Georget. Tex.) 2021, 20, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Funato, Y.; Miki, H. Molecular function and biological importance of CNNM family Mg2+ transporters. J. Biochem. 2019, 165, 219–225. [Google Scholar] [CrossRef]
- Bai, Z.; Feng, J.; Franken, G.A.C.; Al’Saadi, N.; Cai, N.; Yu, A.S.; Lou, L.; Komiya, Y.; Hoenderop, J.G.J.; de Baaij, J.H.F.; et al. CNNM proteins selectively bind to the TRPM7 channel to stimulate divalent cation entry into cells. PLoS Biol. 2021, 19, e3001496. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Funato, Y.; Takano, Y.; Miki, H. Mg2+-dependent interactions of ATP with the cystathionine-β-synthase (CBS) domains of a magnesium transporter. J. Biol. Chem. 2014, 289, 14731–14739. [Google Scholar] [CrossRef]
- Funato, Y.; Yamazaki, D.; Mizukami, S.; Du, L.; Kikuchi, K.; Miki, H. Membrane protein CNNM4-dependent Mg2+ efflux suppresses tumor progression. J. Clin. Investig. 2014, 124, 5398–5410. [Google Scholar] [CrossRef]
- Daw, C.; Ramachandran, K.; Enslow, B.; Maity, S.; Bursic, B.; Novello, M.; Rubannelsonkumar, C.; Mashal, A.; Ravichandran, J.; Bakewell, T.; et al. Lactate Elicits ER-Mitochondrial Mg Dynamics to Integrate Cellular Metabolism. Cell 2020, 183, 474–489.e417. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Lu, Y.; Li, J.; Lu, X.; Ren, Y.; Wen, T.; Wang, Y.; Chang, S.; Zhang, X.; et al. Molecular basis of Mg permeation through the human mitochondrial Mrs2 channel. Nat. Commun. 2023, 14, 4713. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, Q.; He, W.; Cao, J.; Yao, S.; Huang, Q.; Zheng, Y. Hepatocellular carcinoma subtypes based on metabolic pathways reveals potential therapeutic targets. Front. Oncol. 2023, 13, 1086604. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.; Locasale, J. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Madaris, T.; Venkatesan, M.; Maity, S.; Stein, M.; Vishnu, N.; Venkateswaran, M.; Davis, J.; Ramachandran, K.; Uthayabalan, S.; Allen, C.; et al. Limiting Mrs2-dependent mitochondrial Mg uptake induces metabolic programming in prolonged dietary stress. Cell Rep. 2023, 42, 112155. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.H.; Dai, Q.; Millen, A.E.; Nie, J.; Edge, S.B.; Trevisan, M.; Shields, P.G.; Freudenheim, J.L. Associations of intakes of magnesium and calcium and survival among women with breast cancer: Results from Western New York Exposures and Breast Cancer (WEB) Study. Am. J. Cancer Res. 2016, 6, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Role of magnesium in genomic stability. Mutat. Res. 2001, 475, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Wolf, F.I.; Maier, J.A.; Nasulewicz, A.; Feillet-Coudray, C.; Simonacci, M.; Mazur, A.; Cittadini, A. Magnesium and neoplasia: From carcinogenesis to tumor growth and progression or treatment. Arch. Biochem. Biophys. 2007, 458, 24–32. [Google Scholar] [CrossRef]
- Yang, D.K.; Tungalag, T.; Lee, S.J.; Kim, S.J. Methyl Jasmonate-induced Increase in Intracellular Magnesium Promotes Apoptosis in Breast Cancer Cells. Anticancer. Res. 2024, 44, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, D.L.C.; Mendes, P.M.V.; Melo, S.R.S.; Dos Santos, L.R.; Santos, R.O.; Vieira, S.C.; Henriques, G.S.; Freitas, B.; Marreiro, D.D.N. Hypomagnesemia and Its Relationship with Oxidative Stress Markers in Women with Breast Cancer. Biol. Trace Elem. Res. 2021, 199, 4466–4474. [Google Scholar] [CrossRef]
- Gorczyca, A.M.; He, K.; Xun, P.; Margolis, K.L.; Wallace, J.P.; Lane, D.; Thomson, C.; Ho, G.Y.; Shikany, J.M.; Luo, J. Association between magnesium intake and risk of colorectal cancer among postmenopausal women. Cancer Causes Control 2015, 26, 1761–1769. [Google Scholar] [CrossRef]
- Polter, E.J.; Onyeaghala, G.; Lutsey, P.L.; Folsom, A.R.; Joshu, C.E.; Platz, E.A.; Prizment, A.E. Prospective Association of Serum and Dietary Magnesium with Colorectal Cancer Incidence. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, E.; Winkels, R.M.; van Baar, H.; Geijsen, A.; van Zutphen, M.; van Halteren, H.K.; Hansson, B.M.E.; Radema, S.A.; de Wilt, J.H.W.; Kampman, E.; et al. Dietary Intake of Magnesium or Calcium and Chemotherapy-Induced Peripheral Neuropathy in Colorectal Cancer Patients. Nutrients 2018, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, E.; Kok, D.E.; Bours, M.J.L.; de Wilt, J.H.W.; van Baar, H.; van Zutphen, M.; Geijsen, A.; Keulen, E.T.P.; Hansson, B.M.E.; van den Ouweland, J.; et al. Vitamin D, magnesium, calcium, and their interaction in relation to colorectal cancer recurrence and all-cause mortality. Am. J. Clin. Nutr. 2020, 111, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Feldman, D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 311–336. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Feng, X.; Li, H.; Ma, S.; Song, W.; Yang, B.; Jiang, T.; Yang, C. The Supplement of Magnesium Element to Inhibit Colorectal Tumor Cells. Biol. Trace Elem. Res. 2023, 201, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Zhu, X.; Dai, Q.; Peek, R.M.; Shrubsole, M.J. Magnesium intake is associated with a reduced risk of incident liver cancer, based on an analysis of the NIH-American Association of Retired Persons (NIH-AARP) Diet and Health Study prospective cohort. Am. J. Clin. Nutrition. 2021, 113, 630–638. [Google Scholar] [CrossRef]
- Zan, R.; Ji, W.; Qiao, S.; Wu, H.; Wang, W.; Ji, T.; Yang, B.; Zhang, S.; Luo, C.; Song, Y.; et al. Biodegradable magnesium implants: A potential scaffold for bone tumor patients. Sci. China Mater. 2020, 64, 1007–1020. [Google Scholar] [CrossRef]
- Qiao, S.; Wang, Y.; Zan, R.; Wu, H.; Zhang, X. iodegradable Mg Implants Suppress the Growth of Ovarian Tumor. ACS Biomater. Sci. Eng. 2020, 6, 1755–1763. [Google Scholar] [CrossRef]
- Mastrototaro, L.; Smorodchenko, A.; Aschenbach, J.; Kolisek, M.; Sponder, G. Solute carrier 41A3 encodes for a mitochondrial Mg(2+) efflux system. Sci. Rep. 2016, 6, 27999. [Google Scholar] [CrossRef]
- Pilchova, I.; Klacanova, K.; Tatarkova, Z.; Kaplan, P.; Racay, P. The Involvement of Mg in Regulation of Cellular and Mitochondrial Functions. Oxidative Med. Cell. Longev. 2017, 2017, 6797460. [Google Scholar] [CrossRef] [PubMed]
- Ha, B.; Moon, D.; Kim, H.; Shon, Y. Magnesium and calcium-enriched deep-sea water promotes mitochondrial biogenesis by AMPK-activated signals pathway in 3T3-L1 preadipocytes. Biomed. Pharmacother.=Biomed. Pharmacother. 2016, 83, 477–484. [Google Scholar] [CrossRef]
- Panov, A.; Scarpa, A. Independent modulation of the activity of alpha-ketoglutarate dehydrogenase complex by Ca2+ and Mg2+. Biochemistry 1996, 35, 427–432. [Google Scholar] [CrossRef]
- Gorgoglione, V.; Laraspata, D.; La Piana, G.; Marzulli, D.; Lofrumento, N. Protective effect of magnesium and potassium ions on the permeability of the external mitochondrial membrane. Arch. Biochem. Biophys. 2007, 461, 13–23. [Google Scholar] [CrossRef]
- Liu, M.; Dudley, S. Magnesium, Oxidative Stress, Inflammation, and Cardiovascular Disease. Antioxidants 2020, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Maier, J.; Rock, E.; Gueux, E.; Nowacki, W.; Rayssiguier, Y. Magnesium and the inflammatory response: Potential physiopathological implications. Arch. Biochem. Biophys. 2007, 458, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Li, X.; Zhu, J.; Wu, J.; Geng, S.; Zhong, C. Magnesium isoglycyrrhizinate suppresses LPS-induced inflammation and oxidative stress through inhibiting NF-κB and MAPK pathways in RAW264.7 cells. Bioorganic Med. Chem. 2019, 27, 516–524. [Google Scholar] [CrossRef]
- Wang, Y.; Patti, G. The Warburg effect: A signature of mitochondrial overload. Trends Cell Biol. 2023, 33, 1014–1020. [Google Scholar] [CrossRef]
- Hasumi, H.; Baba, M.; Hasumi, Y.; Huang, Y.; Oh, H.; Hughes, R.; Klein, M.; Takikita, S.; Nagashima, K.; Schmidt, L.; et al. Regulation of mitochondrial oxidative metabolism by tumor suppressor FLCN. J. Natl. Cancer Inst. 2012, 104, 1750–1764. [Google Scholar] [CrossRef]
- Lang, M.; Vocke, C.; Merino, M.; Schmidt, L.; Linehan, W. Mitochondrial DNA mutations distinguish bilateral multifocal renal oncocytomas from familial Birt-Hogg-Dubé tumors. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2015, 28, 1458–1469. [Google Scholar] [CrossRef] [PubMed]
- Cannino, G.; Ciscato, F.; Masgras, I.; Sánchez-Martín, C.; Rasola, A. Metabolic Plasticity of Tumor Cell Mitochondria. Front. Oncol. 2018, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Yadav, T.; Rutter, J. Stressed to death: Mitochondrial stress responses connect respiration and apoptosis in cancer. Mol. Cell 2022, 82, 3321–3332. [Google Scholar] [CrossRef] [PubMed]
- Jain, I.; Zazzeron, L.; Goli, R.; Alexa, K.; Schatzman-Bone, S.; Dhillon, H.; Goldberger, O.; Peng, J.; Shalem, O.; Sanjana, N.; et al. Hypoxia as a therapy for mitochondrial disease. Science 2016, 352, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Liu, J.; Kang, R.; Klionsky, D.; Tang, D. Mitochondrial DNA stress triggers autophagy-dependent ferroptotic death. Autophagy 2021, 17, 948–960. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yesilkanal, A.; Wynne, J.; Frankenberger, C.; Liu, J.; Yan, J.; Elbaz, M.; Rabe, D.; Rustandy, F.; Tiwari, P.; et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 2019, 568, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Vollmer, J.; Machacek, M.; Kortvely, E. Modeling the activation of the alternative complement pathway and its effects on hemolysis in health and disease. PLoS Comput. Biol. 2020, 16, e1008139. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Tang, Z.; Wu, H.; Zuo, X.; Dong, H.; Tan, L.; Wang, W.; Liu, Y.; Wu, Z.; Shi, L.; et al. Biofunctional magnesium-coated Ti6Al4V scaffolds promote autophagy-dependent apoptosis in osteosarcoma by activating the AMPK/mTOR/ULK1 signaling pathway. Mater. Today Bio 2021, 12, 100147. [Google Scholar] [CrossRef]
- Peng, H.; Fan, K.; Zan, R.; Gong, Z.J.; Sun, W.; Sun, Y.; Wang, W.; Jiang, H.; Lou, J.; Ni, J.; et al. Degradable magnesium implants inhibit gallbladder cancer. Acta Biomater. 2021, 128, 514–522. [Google Scholar] [CrossRef]
- Yuan, Z.; Guo, G.; Sun, G.; Li, Q.; Wang, L.; Qiao, B. Magnesium isoglycyrrhizinate suppresses bladder cancer progression by modulating the miR-26b/Nox4 axis. Bioengineered 2022, 13, 7986–7999. [Google Scholar] [CrossRef] [PubMed]
- Zan, R.; Wang, H.; Cai, W.; Ni, J.; Luthringer-Feyerabend, B.; Wang, W.; Peng, H.; Ji, W.; Yan, J.; Xia, J.; et al. Controlled release of hydrogen by implantation of magnesium induces P53-mediated tumor cells apoptosis. Bioact. Mater. 2022, 9, 385–396. [Google Scholar] [CrossRef]
- Xu, B.; Song, Y.; Yang, K.; Li, Y.; Chen, B.; Liao, X.; Jia, Q. Magnesium metal and its corrosion products: Promising materials for tumor interventional therapy. J. Magnes. Alloys 2023, 11, 763–775. [Google Scholar] [CrossRef]
- Na, H.S.; Shin, H.J.; Kang, S.B.; Hwang, J.W.; Do, S.H. Effects of magnesium sulphate on coagulation after laparoscopic colorectal cancer surgery, measured by rotational thromboelastometry (ROTEM(R)). Anaesthesia 2014, 69, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jin, T.; Ma, B.; Ji, Y.; Huang, X.; Wang, P.; Liu, X.; Krylov, B.V.; Liu, X.; Ma, K. Oral application of magnesium-L-threonate enhances analgesia and reduces the dosage of opioids needed in advanced cancer patients-A randomized, double-blind, placebo-controlled trial. Cancer Med. 2023, 12, 4343–4351. [Google Scholar] [CrossRef] [PubMed]
- Hatice Akbudak, I.; Yilmaz, S.; Ilhan, S.; Yuksel Tanriverdi, S.; Erdem, E. The effect of preemptive magnesium sulfate on postoperative pain in patients undergoing mastectomy: A clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 7907–7913. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Hong, J.H.; Kim, D.H.; Yu, J.; Hwang, J.H.; Kim, Y.K. Magnesium and Bladder Discomfort after Transurethral Resection of Bladder Tumor: A Randomized, Double-blind, Placebo-controlled Study. Anesthesiology 2020, 133, 64–77. [Google Scholar] [CrossRef]
- Jiang, W.; Zeng, X.; Zhou, X.; Liao, O.; Ju, F.; Zhao, Z.; Zhang, X. Effect of magnesium sulfate perioperative infusion on postoperative catheter-related bladder discomfort in male patients undergoing laparoscopic radical resection of gastrointestinal cancer: A prospective, randomized and controlled study. BMC Anesth. 2023, 23, 396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Lin, R.; Chen, J.; Qi, Y.; Lin, L. Magnesium Ion: A New Switch in Tumor Treatment. Biomedicines 2024, 12, 1717. https://doi.org/10.3390/biomedicines12081717
Huang L, Lin R, Chen J, Qi Y, Lin L. Magnesium Ion: A New Switch in Tumor Treatment. Biomedicines. 2024; 12(8):1717. https://doi.org/10.3390/biomedicines12081717
Chicago/Turabian StyleHuang, Leyi, Renxi Lin, Jiaxi Chen, Yuanlin Qi, and Ling Lin. 2024. "Magnesium Ion: A New Switch in Tumor Treatment" Biomedicines 12, no. 8: 1717. https://doi.org/10.3390/biomedicines12081717
APA StyleHuang, L., Lin, R., Chen, J., Qi, Y., & Lin, L. (2024). Magnesium Ion: A New Switch in Tumor Treatment. Biomedicines, 12(8), 1717. https://doi.org/10.3390/biomedicines12081717





