Cellular and Molecular Mechanisms Underlying Altered Excitability of Cardiac Efferent Neurons in Cirrhotic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of Cirrhosis
2.3. Measurement of Arterial Blood Pressure, Heart Rate, and Portal Pressure
2.4. Power Spectral Analysis of HRV
2.5. Dissociation of Single Cardiac Efferent Neurons
2.6. Single-Cell Real-Time PCR Analysis
2.7. Patch-Clamp Recordings
2.8. Data Analysis and Statistics
3. Results
3.1. Evaluation of Blood Pressure, Heart Rate, Portal Pressure, and Myocardial Mass in Normal and Cirrhotic Rats
3.2. Decrease in HRV in Cirrhotic Rats
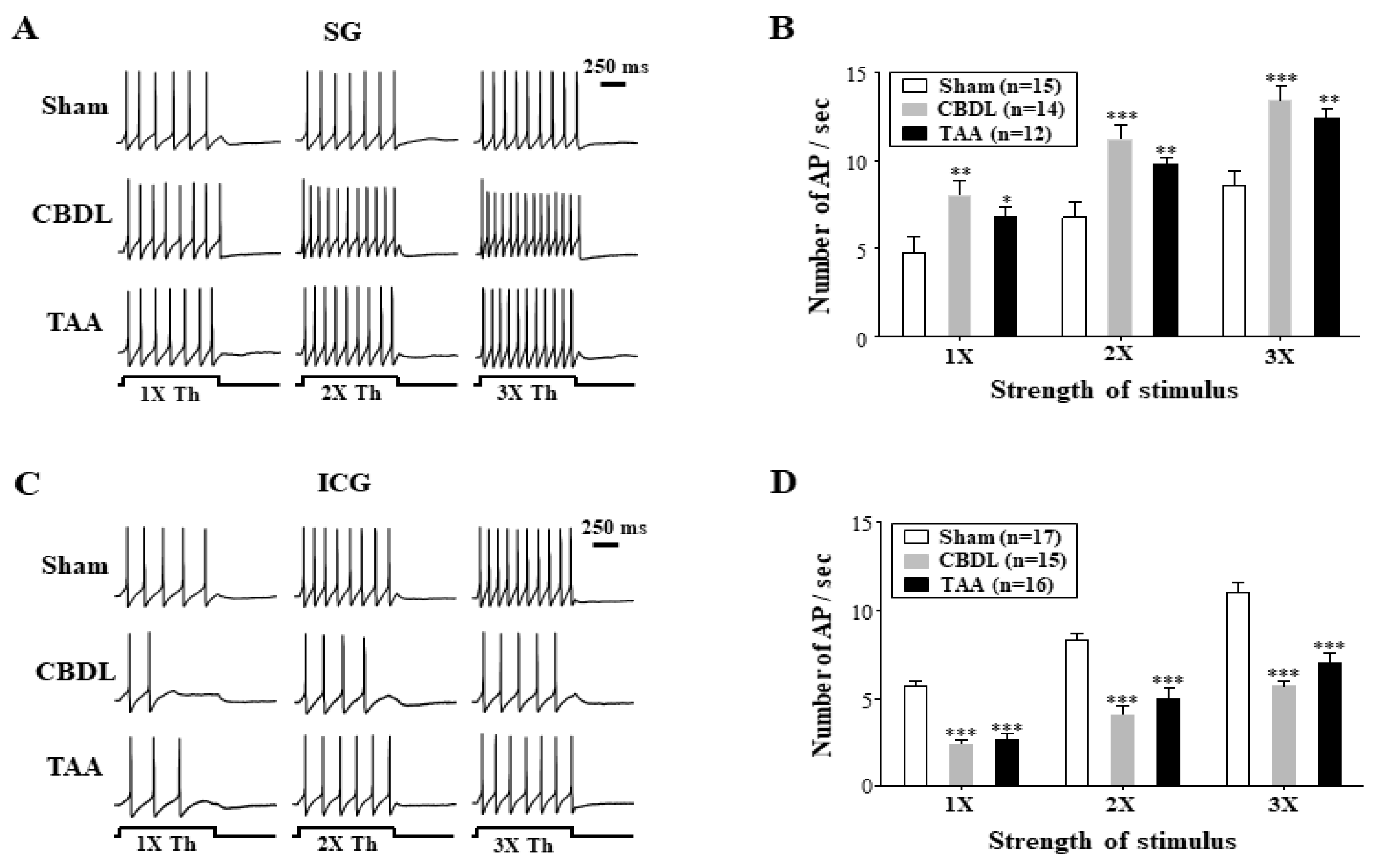
3.3. Cirrhosis-Induced Alterations in the Excitability of Cardiac Efferent Neurons
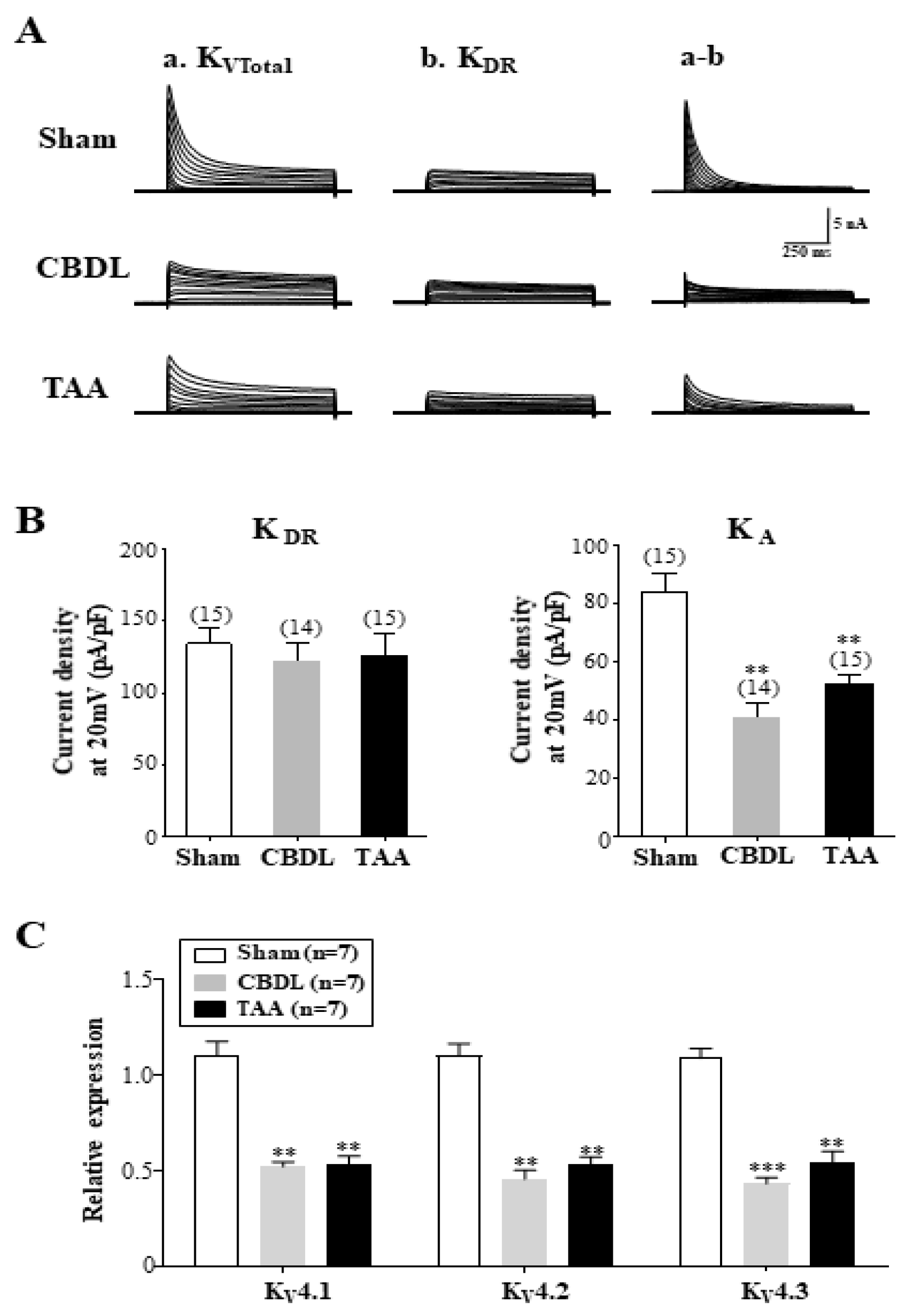
3.4. Downregulation of A-Type K+ Currents in SG Neurons of Cirrhotic Rats
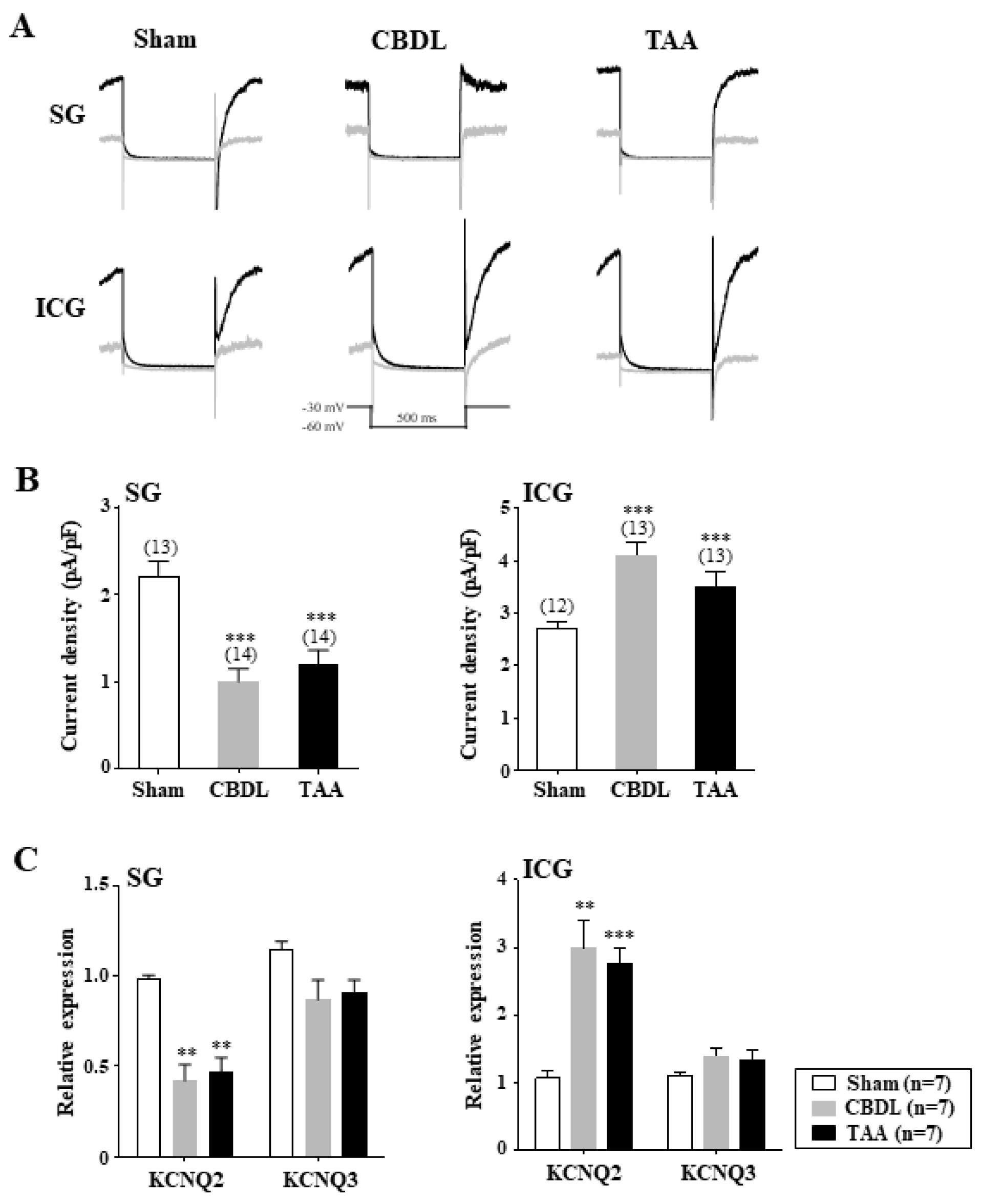
3.5. Differential Regulation of M-Type K+ Channels in Cardiac Efferent Neurons of Cirrhotic Rats
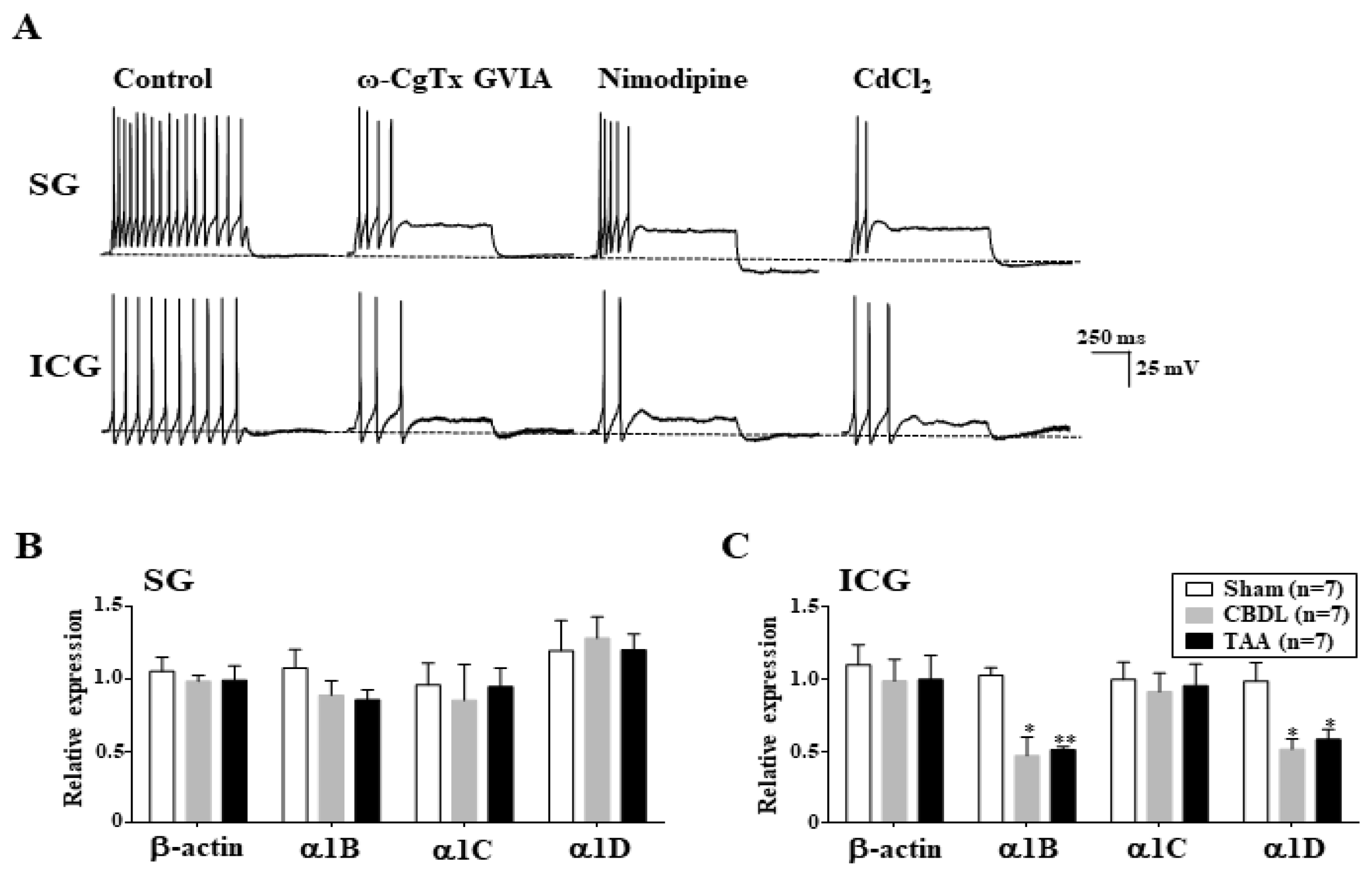
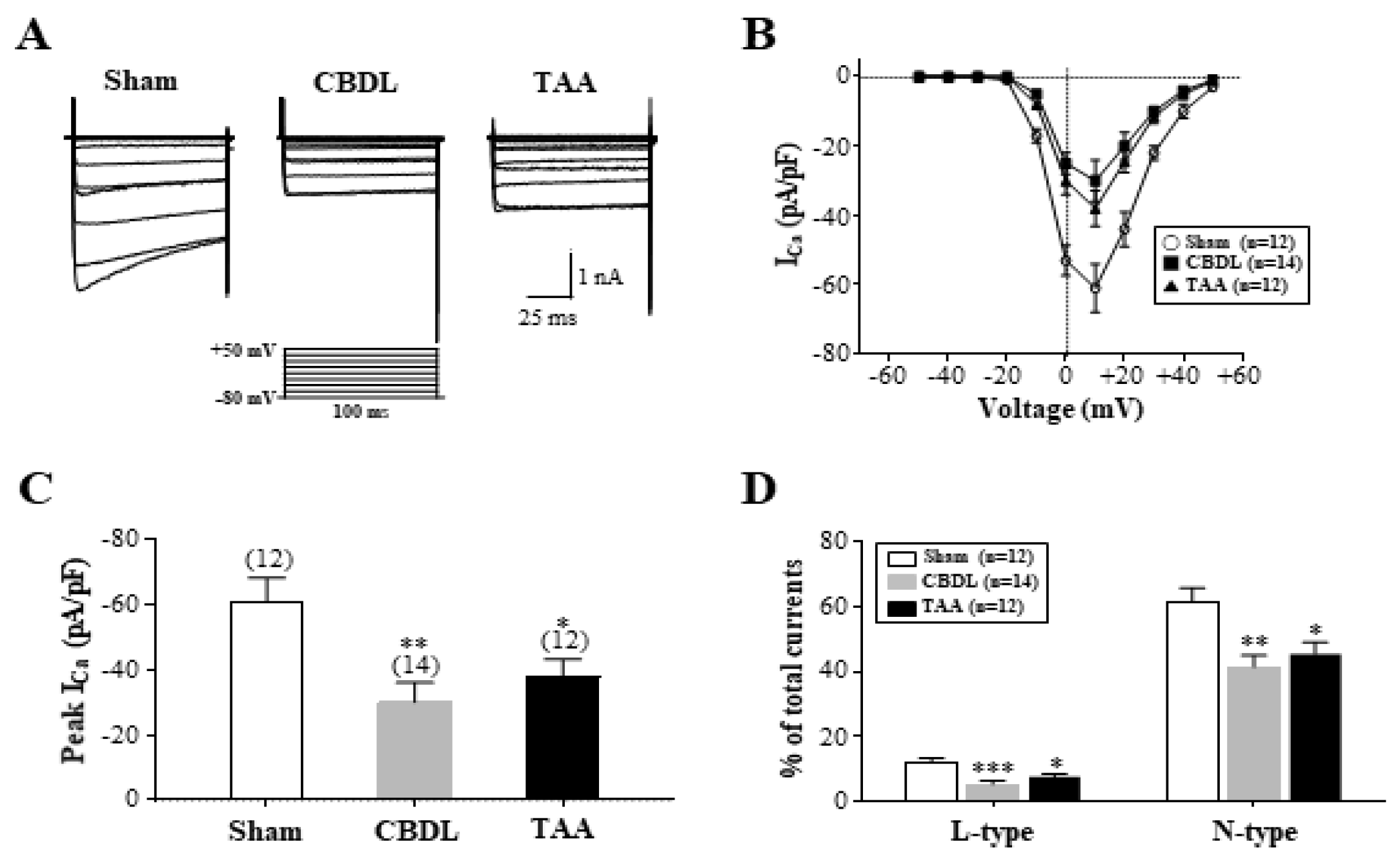
3.6. Downregulation of L- and N-Type Ca2+ Channels in ICG Neurons of Cirrhotic Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dillon, J.; Plevris, J.; Nolan, J.; Ewing, D.; Neilson, J.; Bouchier, I.; Hayes, P. Autonomic function in cirrhosis assessed by cardiovascular reflex tests and 24-hour heart rate variability. Am. J. Gastroenterol. 1994, 89, 1544. [Google Scholar] [PubMed]
- Hendrickse, M.; Triger, D. Autonomic dysfunction in chronic liver disease. Clin. Auton. Res. 1993, 3, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.L.; Allen, J.; Kerr, S.; Jones, D.E. Reduced heart rate variability and baroreflex sensitivity in primary biliary cirrhosis. Liver Int. 2006, 26, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Trevisani, F.; Sica, G.; Bernardi, M. Autonomic neuropathy in advanced liver disease. Hepatology 1996, 24, 1549. [Google Scholar] [CrossRef] [PubMed]
- Barron, H.V.; Alam, I.; Lesh, M.D.; Strunk, A.; Bass, N.M. Autonomic nervous system tone measured by baroreflex sensitivity is depressed in patients with end-stage liver disease. Am. J. Gastroenterol. 1999, 94, 986–989. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, J.F. Presence of autonomic neuropathy is a poor prognostic indicator in patients with advanced liver disease. Hepatology 1996, 23, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Fornalè, L.; Di Marco, C.; Trevisani, F.; Baraldini, M.; Gasbarrini, A.; De Collibus, C.; Zacà, F.; Ligabue, A.; Colantoni, A. Hyperdynamic circulation of advanced cirrhosis: A re-appraisal based on posture-induced changes in hemodynamics. J. Hepatol. 1995, 22, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Møller, S.; Iversen, J.S.; Henriksen, J.H.; Bendtsen, F. Reduced baroreflex sensitivity in alcoholic cirrhosis: Relations to hemodynamics and humoral systems. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H2966–H2972. [Google Scholar] [CrossRef]
- Trevisani, F.; Sica, G.; Mainquà, P.; Santese, G.; De Notariis, S.; Caraceni, P.; Domenicali, M.; Zacà, F.; Grazi, G.L.; Mazziotti, A. Autonomic dysfunction and hyperdynamic circulation in cirrhosis with ascites. Hepatology 1999, 30, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Bichet, D.G.; Putten, V.J.V.; Schrier, R.W. Potential role of increased sympathetic activity in impaired sodium and water excretion in cirrhosis. N. Engl. J. Med. 1982, 307, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, M.; Grassi, G.; Redaelli, E.; Dell’Oro, R.; Ratti, L.; Redaelli, A.; Foglia, G.; Di Lelio, A.; Mancia, G. Patterns of regional sympathetic nerve traffic in preascitic and ascitic cirrhosis. Hepatology 2001, 34, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Macgilchrist, A.; Howes, L.; Hawksby, C.; Reid, J. Plasma noradrenaline in cirrhosis: A study of kinetics and temporal relationship to ascites formation. Eur. J. Clin. Investig. 1991, 21, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.; Newton, J.L. Autonomic dysfunction in chronic liver disease. Liver Int. 2009, 29, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Thuluvath, P.; Triger, D. Autonomic neuropathy and chronic liver disease. QJM Int. J. Med. 1989, 72, 737–747. [Google Scholar]
- Breitman, D.R.; Lee, S.S. Blunted responsiveness of the neuronal activation marker Fos in brainstem cardiovascular nuclei of cirrhotic rats. Hepatology 1997, 26, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-K.; Park, K.-H.; Baik, S.-K.; Jeong, S.-W. Decreased excitability and voltage-gated sodium currents in aortic baroreceptor neurons contribute to the impairment of arterial baroreflex in cirrhotic rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R1088–R1101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, B.; Liu, L.; Tang, L.; Zhang, J.; Ling, Y.; Fallon, M.B. ET-1 and TNF-α in HPS: Analysis in prehepatic portal hypertension and biliary and nonbiliary cirrhosis in rats. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 286, G294–G303. [Google Scholar] [CrossRef]
- Dekel, R.; Zvibel, I.; Brill, S.; Brazovsky, E.; Halpern, Z.; Oren, R. Gliotoxin ameliorates development of fibrosis and cirrhosis in a thioacetamide rat model. Dig. Dis. Sci. 2003, 48, 1642–1647. [Google Scholar] [CrossRef]
- Groszmann, R.J.; Vorobioff, J.; Riley, E. Splanchnic hemodynamics in portal-hypertensive rats: Measurement with gamma-labeled microspheres. Am. J. Physiol.-Gastrointest. Liver Physiol. 1982, 242, G156–G160. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Niwa, H.; Kushikata, T.; Watanabe, H.; Hirota, K.; Ono, K.; Ohba, T. Inhalation anesthesia is preferable for recording rat cardiac function using an electrocardiogram. Biol. Pharm. Bull. 2014, 37, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-W.; Lee, C.-K.; Whang, K.; Jeong, S.-W. Functional plasticity of cardiac efferent neurons contributes to traumatic brain injury-induced cardiac autonomic dysfunction. Brain Res. 2021, 1753, 147257. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, M.; Yayou, K.-I.; Ishii, K.; Hashimoto, S.-I.; Tsubone, H.; Sugano, S. Power spectral analysis of heart rate variability as a new method for assessing autonomic activity in the rat. J. Electrocardiol. 1994, 27, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Kuwahara, M.; Tsubone, H.; Sugano, S. Diurnal variation of autonomic nervous activity in the rat: Investigation by power spectral analysis of heart rate variability. J. Electrocardiol. 1999, 32, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Velasco, V.; Puhl, H.L.; Fuller, B.C.; Sumner, A.D. Modulation of Ca2+ channels by opioid receptor-like 1 receptors natively expressed in rat stellate ganglion neurons innervating cardiac muscle. J. Pharmacol. Exp. Ther. 2005, 314, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-W.; Ikeda, S.R.; Wurster, R.D. Activation of various G-protein coupled receptors modulates Ca2+ channel currents via PTX-sensitive and voltage-dependent pathways in rat intracardiac neurons. J. Auton. Nerv. Syst. 1999, 76, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L.; Zheng, H. Angiotensin II-NADPH oxidase-derived superoxide mediates diabetes-attenuated cell excitability of aortic baroreceptor neurons. Am. J. Physiol.-Cell Physiol. 2011, 301, C1368–C1377. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.-J.; Whang, K.; Kong, I.D.; Park, K.-S.; Lee, J.-W.; Jeong, S.-W. Expression profiles of high voltage-activated calcium channels in sympathetic and parasympathetic pelvic ganglion neurons innervating the urogenital system. J. Pharmacol. Exp. Ther. 2006, 317, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Nagula, S.; Jain, D.; Groszmann, R.J.; Garcia-Tsao, G. Histological-hemodynamic correlation in cirrhosis—A histological classification of the severity of cirrhosis. J. Hepatol. 2006, 44, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Girod, C.; Braillon, A.; Hadengue, A.; Lebrec, D. Hemodynamic characterization of chronic bile duct-ligated rats: Effect of pentobarbital sodium. Am. J. Physiol. -Gastrointest. Liver Physiol. 1986, 251, G176–G180. [Google Scholar] [CrossRef] [PubMed]
- Neef, M.; Ledermann, M.; Saegesser, H.; Schneider, V.; Reichen, J. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J. Hepatol. 2006, 45, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Pardini, B.; Patel, K.; Schmid, P.; Lund, D. Location, distribution and projections of intracardiac ganglion cells in the rat. J. Auton. Nerv. Syst. 1987, 20, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Pardini, B.J.; Lund, D.D.; Schmid, P.G. Organization of the sympathetic postganglionic innervation of the rat heart. J. Auton. Nerv. Syst. 1989, 28, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tu, H.; Zheng, H.; Zhang, L.; Tran, T.P.; Muelleman, R.L.; Li, Y.-L. Alterations of calcium channels and cell excitability in intracardiac ganglion neurons from type 2 diabetic rats. Am. J. Physiol.-Cell Physiol. 2012, 302, C1119–C1127. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Liu, J.; Zhang, D.; Zheng, H.; Patel, K.P.; Cornish, K.G.; Wang, W.-Z.; Muelleman, R.L.; Li, Y.-L. Heart failure-induced changes of voltage-gated Ca2+ channels and cell excitability in rat cardiac postganglionic neurons. Am. J. Physiol.-Cell Physiol. 2014, 306, C132–C142. [Google Scholar] [CrossRef] [PubMed]
- Galvan, M.; Sedlmeir, C. Outward currents in voltage-clamped rat sympathetic neurones. J. Physiol. 1984, 356, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Belluzzi, O.; Sacchi, O.; Wanke, E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J. Physiol. 1985, 358, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Schofield, G.G.; Ikeda, S.R. Potassium currents of acutely isolated adult rat superior cervical ganglion neurons. Brain Res. 1989, 485, 205–214. [Google Scholar] [CrossRef]
- Liu, P.W.; Bean, B.P. Kv2 channel regulation of action potential repolarization and firing patterns in superior cervical ganglion neurons and hippocampal CA1 pyramidal neurons. J. Neurosci. 2014, 34, 4991–5002. [Google Scholar] [CrossRef] [PubMed]
- Zemel, B.M.; Ritter, D.M.; Covarrubias, M.; Muqeem, T. A-type KV channels in dorsal root ganglion neurons: Diversity, function, and dysfunction. Front. Mol. Neurosci. 2018, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Na Phuket, T.R.; Covarrubias, M.L. Kv4 channels underlie the subthreshold-operating A-type K+-current in nociceptive dorsal root ganglion neurons. Front. Mol. Neurosci. 2009, 2, 651. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.A.; Nerbonne, J.M. Elimination of the Fast Transient in Superior Cervical Ganglion Neurons with Expression of KV4. 2W362F: Molecular Dissection ofIA. J. Neurosci. 2000, 20, 5191–5199. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.A.; Nerbonne, J.M. Molecular heterogeneity of the voltage-gated fast transient outward K+ current, IAf, in mammalian neurons. J. Neurosci. 2001, 21, 8004–8014. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-J.; Adams, D.J. Resting membrane potential and potassium currents in cultured parasympathetic neurones from rat intracardiac ganglia. J. Physiol. 1992, 456, 405–424. [Google Scholar] [CrossRef]
- Cuevas, J.; Harper, A.A.; Trequattrini, C.; Adams, D.J. Passive and active membrane properties of isolated rat intracardiac neurons: Regulation by H-and M-currents. J. Neurophysiol. 1997, 78, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-S.; McKinnon, D. Potassium currents in rat prevertebral and paravertebral sympathetic neurones: Control of firing properties. J. Physiol. 1995, 485, 319–335. [Google Scholar] [CrossRef]
- Xi-Moy, S.X.; Dun, N. Potassium currents in adult rat intracardiac neurones. J. Physiol. 1995, 486, 15–31. [Google Scholar] [CrossRef]
- Wang, H.-S.; Pan, Z.; Shi, W.; Brown, B.S.; Wymore, R.S.; Cohen, I.S.; Dixon, J.E.; McKinnon, D. KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-channel. Science 1998, 282, 1890–1893. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.S.; Roche, J.P.; Kaftan, E.J.; Cruzblanca, H.; Mackie, K.; Hille, B. Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3 K+ channels that underlie the neuronal M current. J. Neurosci. 2000, 20, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Vandael, D.H.; Marcantoni, A.; Mahapatra, S.; Caro, A.; Ruth, P.; Zuccotti, A.; Knipper, M.; Carbone, E. Cav1. 3 and BK channels for timing and regulating cell firing. Mol. Neurobiol. 2010, 42, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Sharkey, K.A.; Breitman, D.R.; Zhang, Y.; Lee, S.S. Disordered central cardiovascular regulation in portal hypertensive and cirrhotic rats. Am. J. Physiol.-Gastrointest. Liver Physiol. 2001, 280, G420–G430. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Epstein, P.N.; Li, L.; Wurster, R.D.; Cheng, Z.J. Functional changes in baroreceptor afferent, central and efferent components of the baroreflex circuitry in type 1 diabetic mice (OVE26). Neuroscience 2008, 152, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Minisi, A.J.; Quinn, M.S.; Jeong, H. Aortic baroreceptor function and depressed baroreflex sensitivity following myocardial infarction. Auton. Neurosci. 2009, 150, 33–37. [Google Scholar] [CrossRef]
- Laffi, G.; Lagi, A.; Cipriani, M.; Barletta, G.; Bernardi, L.; Fattorini, L.; Melani, L.; Riccardi, D.; Bandinelli, G.; Mannelli, M. Impaired cardiovascular autonomic response to passive tilting in cirrhosis with ascites. Hepatology 1996, 24, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Malliani, A.; Pagani, M.; Cerutti, S. Heart rate variability and its sympatho-vagal modulation. Cardiovasc. Res. 1996, 32, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Malliani, A.; Pagani, M.; Lombardi, F.; Furlan, R.; Guzzetti, S.; Cerutti, S. Spectral analysis to assess increased sympathetic tone in arterial hypertension. Hypertension 1991, 17, III36. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Pinna, G.D.; Raczak, G. Baroreflex sensitivity: Measurement and clinical implications. Ann. Noninvasive Electrocardiol. 2008, 13, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Mo, N.; Wallis, D.; Watson, A. Properties of putative cardiac and non-cardiac neurones in the rat stellate ganglion. J. Auton. Nerv. Syst. 1994, 47, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Selyanko, A. Membrane properties and firing characteristics of rat cardiac neurones in vitro. J. Auton. Nerv. Syst. 1992, 39, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Pongs, O. Voltage-gated potassium channels: From hyperexcitability to excitement. FEBS Lett. 1999, 452, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Rudy, B. Diversity and ubiquity of K channels. Neuroscience 1988, 25, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Malin, S.A.; Nerbonne, J.M. Delayed rectifier K+ currents, IK, are encoded by Kv2 α-subunits and regulate tonic firing in mammalian sympathetic neurons. J. Neurosci. 2002, 22, 10094–10105. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Nakahira, K.; Shibasaki, K.; Wakazono, Y.; Imoto, K.; Ikenaka, K. A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. J. Neurosci. 2000, 20, 4145–4155. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, S.G.; Varga, A.W.; Yuan, L.-L.; Anderson, A.E.; Sweatt, J.D.; Schrader, L.A. Structure and function of Kv4-family transient potassium channels. Physiol. Rev. 2004, 84, 803–833. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Kovacs, I.; Zhang, Z. Differential distribution of KChIPs mRNAs in adult mouse brain. Mol. Brain Res. 2004, 128, 103–111. [Google Scholar] [CrossRef]
- Matsuyoshi, H.; Takimoto, K.; Yunoki, T.; Erickson, V.L.; Tyagi, P.; Hirao, Y.; Wanaka, A.; Yoshimura, N. Distinct cellular distributions of Kv4 pore-forming and auxiliary subunits in rat dorsal root ganglion neurons. Life Sci. 2012, 91, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Passmore, G.M.; Selyanko, A.A.; Mistry, M.; Al-Qatari, M.; Marsh, S.J.; Matthews, E.A.; Dickenson, A.H.; Brown, T.A.; Burbidge, S.A.; Main, M. KCNQ/M currents in sensory neurons: Significance for pain therapy. J. Neurosci. 2003, 23, 7227–7236. [Google Scholar] [CrossRef] [PubMed]
- Soh, H.; Pant, R.; LoTurco, J.J.; Tzingounis, A.V. Conditional deletions of epilepsy-associated KCNQ2 and KCNQ3 channels from cerebral cortex cause differential effects on neuronal excitability. J. Neurosci. 2014, 34, 5311–5321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tu, H.; Cao, L.; Zheng, H.; Muelleman, R.L.; Wadman, M.C.; Li, Y.L. Reduced N-Type Ca2+ Channels in Atrioventricular Ganglion Neurons Are Involved in Ventricular Arrhythmogenesis. J. Am. Heart Assoc. 2018, 7, e007457. [Google Scholar] [CrossRef] [PubMed]
- Kukwa, W.; Macioch, T.; Szulczyk, P.J. Stellate neurones innervating the rat heart express N, L and P/Q calcium channels. J. Auton. Nerv. Syst. 1998, 74, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-W.; Wurster, R.D. Calcium channel currents in acutely dissociated intracardiac neurons from adult rats. J. Neurophysiol. 1997, 77, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Gu, N.; Vervaeke, K.; Hu, H.; Storm, J.F. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J. Physiol. 2005, 566, 689–715. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Sasaki, K.; Ma, L.; Ueda, H. Neuron-restrictive silencer factor causes epigenetic silencing of Kv4. 3 gene after peripheral nerve injury. Neuroscience 2010, 166, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rose, K.; Ooi, L.; Dalle, C.; Robertson, B.; Wood, I.C.; Gamper, N. Transcriptional repression of the M channel subunit Kv7. 2 in chronic nerve injury. Pain 2011, 152, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Mucha, M.; Ooi, L.; Linley, J.E.; Mordaka, P.; Dalle, C.; Robertson, B.; Gamper, N.; Wood, I.C. Transcriptional control of KCNQ channel genes and the regulation of neuronal excitability. J. Neurosci. 2010, 30, 13235–13245. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, M.; Di Pascoli, M.; Verardo, A.; Gatta, A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J. Gastroenterol. WJG 2014, 20, 2555. [Google Scholar] [CrossRef]
- Liu, H.; Gaskari, S.A.; Lee, S.S. Cardiac and vascular changes in cirrhosis: Pathogenic mechanisms. World J. Gastroenterol. WJG 2006, 12, 837. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.; Berry, C. Recent advances in angiotensin II signaling. Braz. J. Med. Biol. Res. 2002, 35, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-X.; Lu, X.-M.; Kimura, S.; Nishiyama, A. Role of mitochondria in angiotensin II-induced reactive oxygen species and mitogen-activated protein kinase activation. Cardiovasc. Res. 2007, 76, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-L. Elevated angiotensin II in rat nodose ganglia primes diabetes-blunted arterial baroreflex sensitivity: Involvement of NADPH oxidase-derived superoxide. J. Diabetes Metab. 2011, 2, 1000135. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Liu, J.; Zhu, Z.; Zhang, L.; Pipinos, I.I.; Li, Y.-L. Mitochondria-derived superoxide and voltage-gated sodium channels in baroreceptor neurons from chronic heart-failure rats. J. Neurophysiol. 2012, 107, 591–602. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Enes, J.; Haburčák, M.; Sona, S.; Gerard, N.; Mitchell, A.C.; Fu, W.; Birren, S.J. Satellite glial cells modulate cholinergic transmission between sympathetic neurons. PLoS ONE 2020, 15, e0218643. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Mapps, A.A.; Boehm, E.; Beier, C.; Keenan, W.T.; Langel, J.; Liu, M.; Thomsen, M.B.; Hattar, S.; Zhao, H.; Tampakakis, E. Satellite glia modulate sympathetic neuron survival, activity, and autonomic function. eLife 2022, 11, e74295. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.S.; Kang, S.J.; Kim, S.; Cha, B.H.; Park, K.-S.; Jeong, S.-W. Changes in the expression of satellite glial cell-specific markers during postnatal development of rat sympathetic ganglia. Brain Res. 2024, 1829, 148809. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Choi, J.Y.; Kim, R.U.; Lee, Y.S.; Cho, H.J.; Kim, D.S. Downregulation of voltage-gated potassium channel α gene expression by axotomy and neurotrophins in rat dorsal root ganglia. Mol. Cells 2003, 16, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, M.; Fukuoka, T.; Takeda, M.; Iwata, K.; Noguchi, K. Spinal glial cell line-derived neurotrophic factor infusion reverses reduction of Kv4. 1-mediated A-type potassium currents of injured myelinated primary afferent neurons in a neuropathic pain model. Mol. Pain 2019, 15, 1744806919841196. [Google Scholar] [CrossRef] [PubMed]


| Target Genes | Accession No. | Sequences (5’-3’) | Size (bp) |
|---|---|---|---|
| β-actin | NM031144.2 | Fex: ACCAGTTCGCCATGGATGAC | 389 |
| Rex: GGTCTCAAACATGATCTGGG | |||
| Fin: ATGGTGGGTATGGGTCAGAA | 119 | ||
| Rin: ACCAACTGGGACGATATGGA | |||
| KV4.1 | NM001105748.1 | Fex: CCGTATATACCACCAGAACC | |
| Rex: GGAAGGTTGACTCTCATCTG | 610 | ||
| Fin: TTGCCAACTCTACTGCGTC | |||
| Rin: TTGGCATTGAGGCTTGAGC | 109 | ||
| KV4.2 | NM031730.2 | Fex: GGTGATGACAGACAATGAGG | |
| Rex: CACAAACTCATGGTTCGTGG | 652 | ||
| Fin: ACAAACGAAGGGCACAGAAG | |||
| Rin: AGTTGGTTGCTCAGTAACCC | 109 | ||
| KV4.3 | NM031739.3 | Fex: CATCATCATCTTTGCCACTG | |
| Rex: ATTAAGGCTGGAGCGACTAG | 733 | ||
| Fin: TATTTGGCTCCATCTGCTCC | |||
| Rin: TTCTTCTGTGCCCTGCGTTT | 127 | ||
| KV7.2 | NM133322 | Fex: ACTGTCCCCATGATCAGCTC | |
| Rex: TCTGATGCTGACTTTGAGGC | 471 | ||
| Fin: GAGTCTCGATGACAGCCCAA | |||
| Rin: AGGGAGGCTTGCTTCTTCTG | 127 | ||
| KV7.3 | NM031597.3 | Fex: GCAATGTCCTGGCTACCTCT | |
| Rex: TTTTGGCTGGCTGCTGCTTC | 613 | ||
| Fin: CAGCAAAGAACTCATCACCG | |||
| Rin: ACAGGGCATCAGCATAGGTC | 152 | ||
| CaV1.2 | NM012517.2 | Fex: AAGATGACTCCAACGCCACC | |
| Rex: GATGATGACGAAGAGCACGAGG | 385 | ||
| Fin: AAGATGACTCCAACGCCACC | |||
| Rin: AGAGTAGTCCGTAGGCAATC | 127 | ||
| CaV1.3 | NM017298.1 | Fex: TGAGACACAGACCAAGCGAAGC | |
| Rex: GTTGTCACTGTTGGCTATCTGG | 376 | ||
| Fin: TCCTGCTGAAGCTCTTCTTG | |||
| Rin: GCTTCCTCTTTCTGAGCAGT | 82 | ||
| CaV2.2 | NM147141.1 | Fex: TGGAGGGCTGGACTGACAT | |
| Rex: GCGTTCTTGTCCTCCTCTGC | 283 | ||
| Fin: TGGAGGGCTGGACTGACAT | |||
| Rin: TTGAGCATGAAGAAGGAGCC | 109 |
| Parameters | Sham-Operated (n = 5) | CBDL (n = 6) | Sham-Saline (n = 5) | TAA (n = 6) |
|---|---|---|---|---|
| Body weight (g) | 343 ± 7 | 325 ± 9 | 406 ± 9 † | 287 ± 8 *** |
| Heart/body weight (%) | 0.25 ± 0.01 | 0.38 ± 0.03 *** | 0.26 ± 0.01 | 0.34 ± 0.02 * |
| MAP (mmHg) | 93 ± 4 | 86 ± 5 | 92 ± 4 | 87 ± 4 |
| Heart rate (beat/min) | 336 ± 6 | 323 ± 12 | 341 ± 7 | 321 ± 11 |
| Portal pressure (mmHg) | 8.2 ± 0.2 | 15.3 ± 0.4 *** | 8.3 ± 0.3 | 14.7 ± 0.5 *** |
| Parameters | SG | ICG | ||||
|---|---|---|---|---|---|---|
| Normal (n = 15) | CBDL (n = 14) | TAA (n = 12) | Normal (n = 17) | CBDL (n = 15) | TAA (n = 16) | |
| Capacitance (pA/pF) | 43 ± 7 | 48 ± 4 | 49 ± 4 | 45 ± 3 | 41 ± 3 | 43 ± 4 |
| RMP (mV) | −56 ± 2 | −53 ± 2 | −51 ± 2 | −53 ± 3 | −55 ± 2 | −53 ± 2 |
| Rheobase (pA) | 61 ± 4 | 32 ± 4 * | 37 ± 3 * | 54 ± 4 | 87 ± 4 * | 90 ± 4 * |
| Threshold (mV) | −30.3 ± 1.3 | −38.3 ± 1.6 * | −37.7 ± 1.3 * | −31.3 ± 1.3 | −21.7 ± 1.1 * | −22 ± 1 * |
| AP amplitude (mV) | 99 ± 4 | 100 ± 3 | 100 ± 3 | 103 ± 1 | 104 ± 2 | 106 ± 2 |
| AP duration (ms) at 0 mV | 2.9 ± 0.1 | 2.1 ± 0.2 * | 2.2 ± 0.2 * | 2.2 ± 0.1 | 3.1 ± 0.2 * | 2.9 ± 0.2 * |
| AHP amplitude (mV) | 18.5 ± 0.5 | 17 ± 0.9 | 17 ± 1 | 16 ± 0.5 | 17.3 ± 1 | 18.2 ± 1 |
| AHP duration (ms) | 287 ± 18 | 221 ± 15 * | 234 ± 17 * | 222 ± 13 | 298 ± 18 * | 303 ± 17 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-K.; Nguyen, H.S.; Kang, S.J.; Jeong, S.-W. Cellular and Molecular Mechanisms Underlying Altered Excitability of Cardiac Efferent Neurons in Cirrhotic Rats. Biomedicines 2024, 12, 1722. https://doi.org/10.3390/biomedicines12081722
Lee C-K, Nguyen HS, Kang SJ, Jeong S-W. Cellular and Molecular Mechanisms Underlying Altered Excitability of Cardiac Efferent Neurons in Cirrhotic Rats. Biomedicines. 2024; 12(8):1722. https://doi.org/10.3390/biomedicines12081722
Chicago/Turabian StyleLee, Choong-Ku, Huu Son Nguyen, Seong Jun Kang, and Seong-Woo Jeong. 2024. "Cellular and Molecular Mechanisms Underlying Altered Excitability of Cardiac Efferent Neurons in Cirrhotic Rats" Biomedicines 12, no. 8: 1722. https://doi.org/10.3390/biomedicines12081722
APA StyleLee, C.-K., Nguyen, H. S., Kang, S. J., & Jeong, S.-W. (2024). Cellular and Molecular Mechanisms Underlying Altered Excitability of Cardiac Efferent Neurons in Cirrhotic Rats. Biomedicines, 12(8), 1722. https://doi.org/10.3390/biomedicines12081722







