The Proton Leak of the Inner Mitochondrial Membrane Is Enlarged in Freshly Isolated Pancreatic Islets
Abstract
1. Introduction
2. Materials and Methods
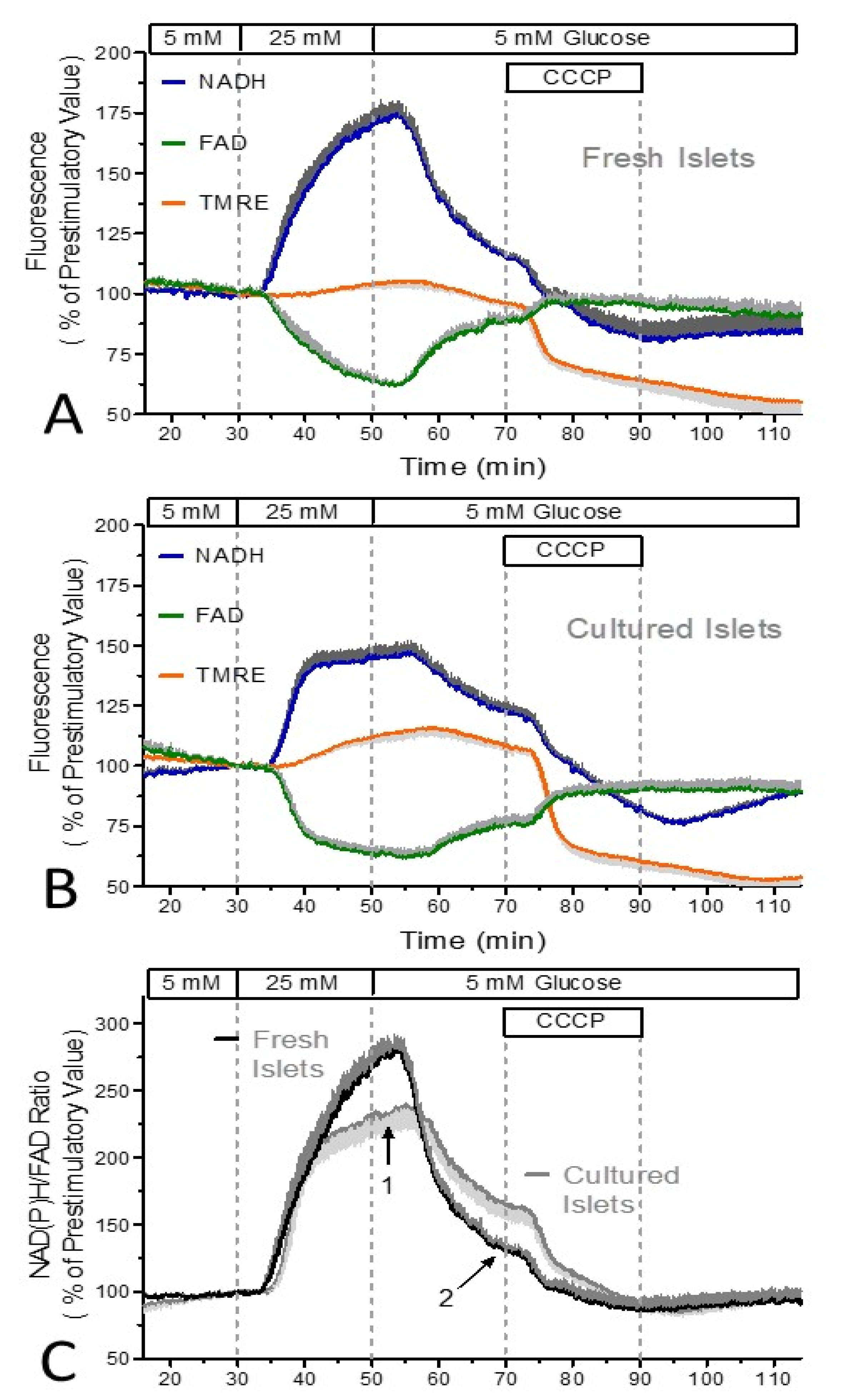
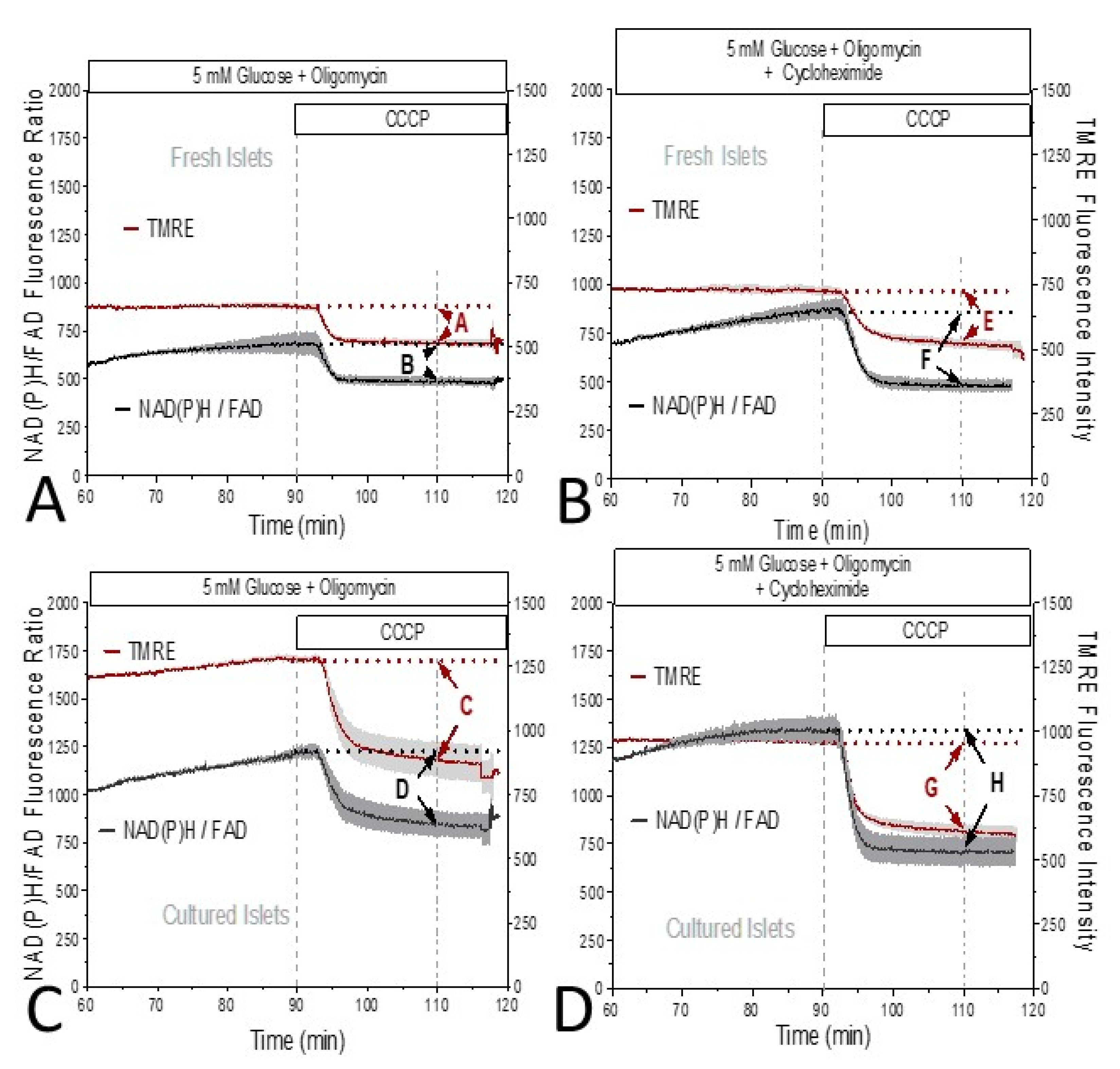
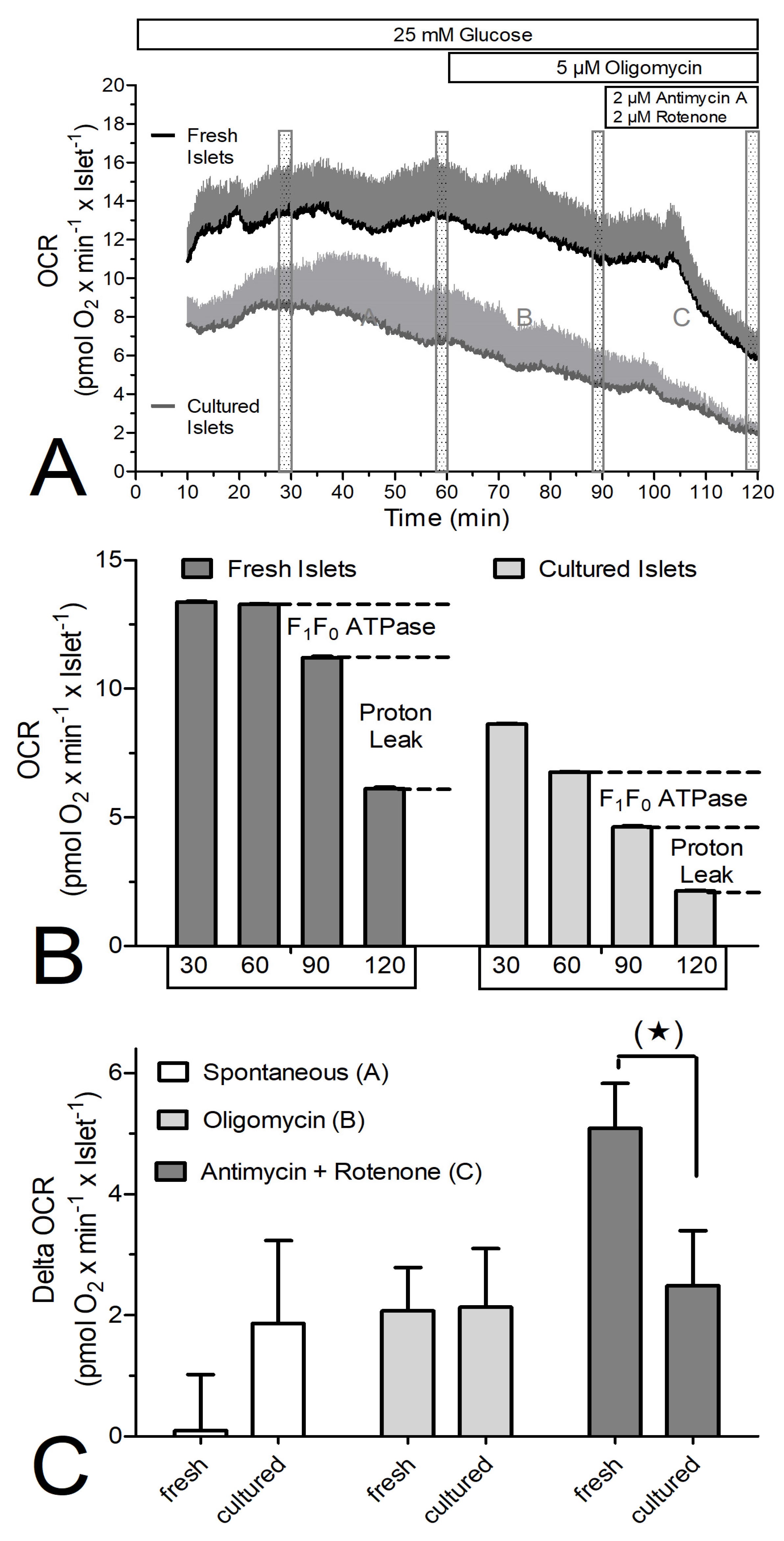
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panten, U.; Christians, J.; von Kriegstein, E.; Poser, W.; Hasselblatt, A. Studies on the mechanism of L-leucine-and alpha-ketoisocaproic acid-induced insulin release from perifused isolated pancreatic islets. Diabetologia 1974, 10, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Malaisse, W.J.; Sener, A.; Malaisse-Lagae, F.; Welsh, M.; Matthews, D.E.; Bier, D.M.; Hellerström, C. The stimulus-secretion coupling of amino acid-induced insulin release. Metabolic response of pancreatic islets of L-glutamine and L-leucine. J. Biol. Chem. 1982, 257, 8731–8737. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Sidarala, V.; Kaufman, B.A.; Soleimanpour, S.A. Mitochondrial metabolism and dynamics in pancreatic beta cell glucose sensing. Biochem. J. 2023, 480, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, S.L.; Cardone, R.L.; Foster, H.R.; Ho, T.; Potapenko, E.; Poudel, C.; VanDeusen, H.R.; Sdao, S.M.; Alves, T.C.; Zhao, X.; et al. Pyruvate Kinase Controls Signal Strength in the Insulin Secretory Pathway. Cell Metab. 2020, 32, 736–750.e5. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Ashcroft, F.M. Pancreatic β-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol. Rev. 2018, 98, 117–214. [Google Scholar] [CrossRef] [PubMed]
- Corradi, J.; Thompson, B.; Fletcher, P.A.; Bertram, R.; Sherman, A.S.; Satin, L.S. KATP channel activity and slow oscillations in pancreatic beta cells are regulated by mitochondrial ATP production. J. Physiol. 2023, 601, 5655–5667. [Google Scholar] [CrossRef] [PubMed]
- Henquin, J.C.; Meissner, H.P. Opposite effects of tolbutamide and diazoxide on 86Rb+ fluxes and membrane potential in pancreatic B cells. Biochem. Pharmacol. 1982, 31, 1407–1415. [Google Scholar] [CrossRef]
- Hansen, J.B.; Arkhammar, P.O.; Bodvarsdottir, T.B.; Wahl, P. Inhibition of insulin secretion as a new drug target in the treatment of metabolic disorders. Curr. Med. Chem. 2004, 11, 1595–1615. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Renström, E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003, 46, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Mears, D. Regulation of insulin secretion in islets of Langerhans by Ca2+channels. J. Membr. Biol. 2004, 200, 57–66. [Google Scholar] [CrossRef]
- Henquin, J.C. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 2000, 49, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Rustenbeck, I.; Schulze, T.; Morsi, M.; Alshafei, M.; Panten, U. What Is the Metabolic Amplification of Insulin Secretion and Is It (Still) Relevant? Metabolites 2021, 11, 355. [Google Scholar] [CrossRef] [PubMed]
- Farfari, S.; Schulz, V.; Corkey, B.; Prentki, M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: Possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 2000, 49, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Panten, U.; Willenborg, M.; Schumacher, K.; Hamada, A.; Ghaly, H.; Rustenbeck, I. Acute metabolic amplification of insulin secretion in mouse islets is mediated by mitochondrial export of metabolites, but not by mitochondrial energy generation. Metabolism 2013, 62, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Morsi, M.; Schulze, T.; Früh, E.; Brüning, D.; Panten, U.; Rustenbeck, I. Fresh and cultured mouse islets differ in their response to nutrient stimulation. Endocr. Connect. 2020, 9, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Alshafei, M.; Schulze, T.; Morsi, M.; Panten, U.; Rustenbeck, I. Short-Term Inhibition of Translation by Cycloheximide Concurrently Affects Mitochondrial Function and Insulin Secretion in Islets from Female Mice. Int. J. Mol. Sci. 2023, 24, 15464. [Google Scholar] [CrossRef] [PubMed]
- Panten, U.; Christians, J.; von Kriegstein, E.; Poser, W.; Hasselblatt, A. Effect of carbohydrates upon fluorescence of reduced pyridine nucleotides from perifused isolated pancreatic islets. Diabetologia 1973, 9, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Panten, U.; Ishida, H. Fluorescence of oxidized flavoproteins from perifused isolated pancreatic islets. Diabetologia 1975, 11, 569–573. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scaduto, R.C., Jr.; Grotyohann, L.W. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys. J. 1999, 76, 469–477. [Google Scholar] [CrossRef]
- Crowley, L.C.; Christensen, M.E.; Waterhouse, N.J. Measuring Mitochondrial Transmembrane Potential by TMRE Staining. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Chi, H.; Bhosale, G.; Duchen, M.R. Assessing the Redox Status of Mitochondria Through the NADH/FAD2+ Ratio in Intact Cells. In Methods in Molecular Biology; Humana: New York, NY, USA, 2022; Volume 2497, pp. 313–318. [Google Scholar] [CrossRef]
- Doliba, N.M.; Wehrli, S.L.; Vatamaniuk, M.Z.; Qin, W.; Buettger, C.W.; Collins, H.W.; Matschinsky, F.M. Metabolic and ionic coupling factors in amino acid-stimulated insulin release in pancreatic beta-HC9 cells. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1507–E1519. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.; Morsi, M.; Reckers, K.; Brüning, D.; Seemann, N.; Panten, U.; Rustenbeck, I. Metabolic amplification of insulin secretion is differentially desensitized by depolarization in the absence of exogenous fuels. Metabolism 2017, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brüning, D.; Morsi, M.; Früh, E.; Scherneck, S.; Rustenbeck, I. Metabolic Regulation of Hormone Secretion in Beta-Cells and Alpha-Cells of Female Mice: Fundamental Differences. Endocrinology 2022, 163, bqac125. [Google Scholar] [CrossRef] [PubMed]
- Bowler, M.W.; Montgomery, M.G.; Leslie, A.G.; Walker, J.E. How azide inhibits ATP hydrolysis by the F-ATPases. Proc. Natl. Acad. Sci. USA 2006, 103, 8646–8649. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A. Azide as a probe of co-operative interactions in the mitochondrial F1-ATPase. Biochim. Biophys. Acta 1989, 974, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.C.; Mlady, G.W.; Kwon, Y.H.; Rose, G.M. Chronic in vivo sodium azide infusion induces selective and stable inhibition of cytochrome c oxidase. J. Neurochem. 1996, 66, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- Valdebenito, G.E.; Chacko, A.R.; Duchen, M.R. The mitochondrial ATP synthase as an ATP consumer-a surprising therapeutic target. EMBO J. 2023, 42, e114141. [Google Scholar] [CrossRef]
- Devenish, R.J.; Prescott, M.; Boyle, G.M.; Nagley, P. The oligomycin axis of mitochondrial ATP synthase: OSCP and the proton channel. J. Bioenerg. Biomembr. 2000, 32, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Poetsch, T.; Ju, J.; Eyler, D.E.; Dang, Y.; Bhat, S.; Merrick, W.C.; Green, R.; Shen, B.; Liu, J.O. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat. Chem. Biol. 2010, 6, 209–217. [Google Scholar] [CrossRef]
- Garcia-Barrado, M.J.; Ravier, M.A.; Rolland, J.F.; Gilon, P.; Nenquin, M.; Henquin, J.C. Inhibition of protein synthesis sequentially impairs distinct steps of stimulus-secretion coupling in pancreatic beta cells. Endocrinology 2001, 142, 299–307. [Google Scholar] [CrossRef]
- Curry, D.L.; Bennett, L.L.; Grodsky, G.M. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology 1968, 83, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Sando, H.; Borg, J.; Steiner, D.F. Studies on the secretion of newly synthesized proinsulin and insulin from isolated rat islets of Langerhans. J. Clin. Investig. 1972, 51, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Heinz, S.; Freyberger, A.; Lawrenz, B.; Schladt, L.; Schmuck, G.; Ellinger-Ziegelbauer, H. Mechanistic Investigations of the Mitochondrial Complex I Inhibitor Rotenone in the Context of Pharmacological and Safety Evaluation. Sci. Rep. 2017, 7, 45465. [Google Scholar] [CrossRef] [PubMed]
- Rieske, J.S.; Lipton, S.H.; Baum, H.; Silman, H.I. Factors affecting the binding of antimycin A to complex 3 of the mitochondrial respiratory chain. J. Biol. Chem. 1967, 242, 4888–4896. [Google Scholar] [CrossRef] [PubMed]
- Schulz, N.; Kluth, O.; Jastroch, M.; Schürmann, A. Minor role of mitochondrial respiration for fatty-acid induced insulin secretion. Int. J. Mol. Sci. 2013, 14, 18989–18998. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, J.D.; Sereda, S.B.; Stiles, L.; Elorza, A.; Allister, E.M.; Neilson, A.; Ferrick, D.A.; Wheeler, M.B.; Shirihai, O.S. A novel high-throughput assay for islet respiration reveals uncoupling of rodent and human islets. PLoS ONE 2012, 7, e33023. [Google Scholar] [CrossRef] [PubMed]
- Robson-Doucette, C.A.; Sultan, S.; Allister, E.M.; Wikstrom, J.D.; Koshkin, V.; Bhattacharjee, A.; Prentice, K.J.; Sereda, S.B.; Shirihai, O.S.; Wheeler, M.B. Beta-cell uncoupling protein 2 regulates reactive oxygen species production, which influences both insulin and glucagon secretion. Diabetes 2011, 60, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.M.; Castegna, A.; Amorese, D.; Marmo, R.; Calcagnile, V.M.; Palmieri, L.; Ricquier, D.; et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M. The ADP and ATP transport in mitochondria and its carrier. Biochim. Biophys. Acta 2008, 778, 1978–2021. [Google Scholar] [CrossRef]
- Taddeo, E.P.; Alsabeeh, N.; Baghdasarian, S.; Wikstrom, J.D.; Ritou, E.; Sereda, S.; Erion, K.; Li, J.; Stiles, L.; Abdulla, M.; et al. Mitochondrial Proton Leak Regulated by Cyclophilin D Elevates Insulin Secretion in Islets at Nonstimulatory Glucose Levels. Diabetes 2020, 69, 131–145. [Google Scholar] [CrossRef]
- Jung, S.R.; Reed, B.J.; Sweet, I.R. A highly energetic process couples calcium influx through L-type calcium channels to insulin secretion in pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E717–E727. [Google Scholar] [CrossRef]
- Rountree, A.M.; Reed, B.J.; Cummings, B.P.; Jung, S.R.; Stanhope, K.L.; Graham, J.L.; Griffen, S.C.; Hull, R.L.; Havel, P.J.; Sweet, I.R. Loss of coupling between calcium influx, energy consumption and insulin secretion associated with development of hyperglycaemia in the UCD-T2DM rat model of type 2 diabetes. Diabetologia 2013, 56, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. Oxidative stress: The vulnerable beta-cell. Biochem. Soc. Trans. 2008, 36, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Roma, L.P.; Jonas, J.C. Nutrient Metabolism, Subcellular Redox State, and Oxidative Stress in Pancreatic Islets and β-Cells. J. Mol. Biol. 2020, 32, 1461–1493. [Google Scholar] [CrossRef] [PubMed]
- Fridlyand, L.E.; Philipson, L.H. Glucose sensing in the pancreatic beta cell: A computational systems analysis. Theor. Biol. Med. Model. 2010, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef]
- Noguchi, H.; Naziruddin, B.; Jackson, A.; Shimoda, M.; Ikemoto, T.; Fujita, Y.; Chujo, D.; Takita, M.; Peng, H.; Sugimoto, K.; et al. Fresh islets are more effective for islet transplantation than cultured islets. Cell Transplant. 2012, 21, 517–523. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshafei, M.; Morsi, M.; Reschke, J.; Rustenbeck, I. The Proton Leak of the Inner Mitochondrial Membrane Is Enlarged in Freshly Isolated Pancreatic Islets. Biomedicines 2024, 12, 1747. https://doi.org/10.3390/biomedicines12081747
Alshafei M, Morsi M, Reschke J, Rustenbeck I. The Proton Leak of the Inner Mitochondrial Membrane Is Enlarged in Freshly Isolated Pancreatic Islets. Biomedicines. 2024; 12(8):1747. https://doi.org/10.3390/biomedicines12081747
Chicago/Turabian StyleAlshafei, Mohammed, Mai Morsi, Julia Reschke, and Ingo Rustenbeck. 2024. "The Proton Leak of the Inner Mitochondrial Membrane Is Enlarged in Freshly Isolated Pancreatic Islets" Biomedicines 12, no. 8: 1747. https://doi.org/10.3390/biomedicines12081747
APA StyleAlshafei, M., Morsi, M., Reschke, J., & Rustenbeck, I. (2024). The Proton Leak of the Inner Mitochondrial Membrane Is Enlarged in Freshly Isolated Pancreatic Islets. Biomedicines, 12(8), 1747. https://doi.org/10.3390/biomedicines12081747





