The Potential Role of Butyrate in the Pathogenesis and Treatment of Autoimmune Rheumatic Diseases
Abstract
1. Introduction
2. Gut Dysbiosis and Systemic Autoimmune Disease (SAD) Development
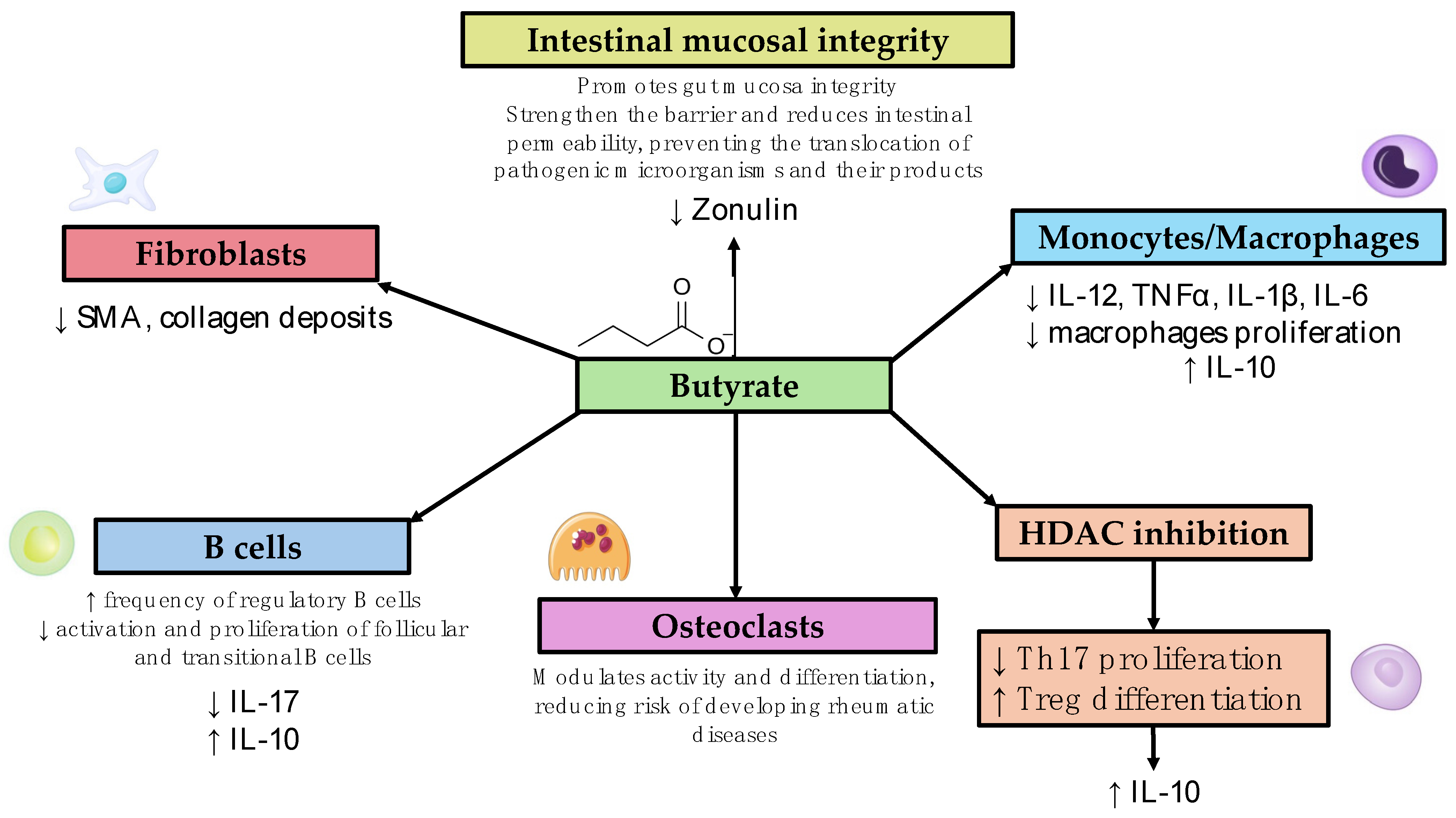
3. Diseases Listed Alphabetically (List of Studies Analyzed and the Main Findings Are Shown in Table 1, Different Mechanisms of Action Are Summarized in Figure 1)
3.1. Behçet’s Disease (BD)
3.2. Rheumatoid Arthritis (RA)
3.3. Sjogren’s Syndrome (SS)
3.4. Systemic Lupus Erythematosus (SLE)
3.5. Systemic Sclerosis (SSc)
| Authors | Year of Publication | Type of Study | Main Findings |
|---|---|---|---|
| Behçet’s Disease | |||
| Yun et al. [53] | 2018 | In vivo Cross-sectional study on 11 patients with Behçet’s disease and 10 healthy controls |
|
| Emmi et al. [54] | 2021 | In vivo Proof-of-concept randomized trial study on 17 patients with Behçet’s disease |
|
| Rheumatoid Arthritis | |||
| He J et al. [57] | 2022 | Mice model |
|
| Kim DS et al. [58] | 2018 | Mice model |
|
| Rosser EC et al. [59] | 2020 | Mice model/in vitro |
|
| Tajik N et al. [44] | 2020 | Mice model |
|
| Takahashi D et al. [56] | 2020 | Mice model |
|
| Balakrishnan B et al. [61] | 2021 | Mice model |
|
| Sjogren’s Syndrome | |||
| Kim DS et al. [64] | 2021 | Mice mode/in vitro |
|
| Moon J et al. [86] | 2020 | Cross-sectional study on 10 pSS patients, 14 subjects with dry eye symptoms, and 12 healthy controls |
|
| Cano-Ortiz et al. [87] | 2020 | Cross-sectional study on 19 pSS patients compared to 19 healthy controls |
|
| Systemic Lupus Erythematosus | |||
| He et al. [70] | 2020 | Mice model |
|
| Sanchez et al. [71] | 2020 | Mice model |
|
| Moleòn J et al. [74]; Moleòn et al. [75] | 2023; 2023 | Mice model |
|
| Widhani A et al. [76] | 2022 | Randomized, double-blind, placebo-controlled trial on 23 patients per group |
|
| Systemic Sclerosis | |||
| Park HJ et al. [84]. | 2021 | Mice model |
|
| Russo E et al. [85] | 2024 | Cross-sectional study on 26 patients |
|
4. Conclusions and Future Perspective
Funding
Conflicts of Interest
References
- Wang, Y.; Wei, J.; Zhang, W.; Doherty, M.; Zhang, Y.; Xie, H.; Li, W.; Wang, N.; Lei, G.; Zeng, C. Gut dysbiosis in rheumatic diseases: A systematic review and meta-analysis of 92 observational studies. EBioMedicine 2022, 80, 104055. [Google Scholar] [CrossRef]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Teng, F.; Klinger, C.N.; Felix, K.M.; Bradley, C.P.; Wu, E.; Tran, N.L.; Umesaki, Y.; Wu, H.-J.J. Gut Microbiota Drive Autoimmune Arthritis by Promoting Differentiation and Migration of Peyer’s Patch T Follicular Helper Cells. Immunity 2016, 44, 875–888. [Google Scholar] [CrossRef]
- Rasouli-Saravani, A.; Jahankhani, K.; Moradi, S.; Gorgani, M.; Shafaghat, Z.; Mirsanei, Z.; Mehmandar, A.; Mirzaei, R. Role of microbiota short-chain fatty acids in the pathogenesis of autoimmune diseases. Biomed. Pharmacother. 2023, 162, 114620. [Google Scholar] [CrossRef]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7, 17–44. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Tian, Y.; Huang, C.; Li, D.; Zhong, Q.; Ma, X. Interaction between Microbes and Host Intestinal Health: Modulation by Dietary Nutrients and Gut-Brain-Endocrine-Immune Axis. Curr. Protein Pept. Sci. 2015, 16, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Høverstad, T.; Midtvedt, T.; Bøhmer, T. Short-Chain Fatty Acids in Intestinal Content of Germfree Mice Monocontaminated with Escherichia Coli or Clostridium Difficile. Scand. J. Gastroenterol. 1985, 20, 373–380. [Google Scholar] [CrossRef]
- Säemann, M.D.; Böhmig, G.A.; Österreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stöckl, J.; Hörl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar] [CrossRef]
- Willemsen, L.E.M.; Koetsier, M.A.; Van Deventer, S.J.H.; Van Tol, E.A.F. Short chain fatty acids stimulate epithelial mucin 2 expression through differential effects on prostaglandin E(1) and E(2) production by intestinal myofibroblasts. Gut 2003, 52, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; Van Der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef]
- Canani, R.B.; Costanzo, M.; Di Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, B.G.; Kim, J.H.; Chun, J.; Im, J.P.; Kim, J.S. Sodium butyrate inhibits the NF-kappa B signaling pathway and histone deacetylation, and attenuates experimental colitis in an IL-10 independent manner. Int. Immunopharmacol. 2017, 51, 47–56. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- López, P.; De Paz, B.; Rodríguez-Carrio, J.; Hevia, A.; Sánchez, B.; Margolles, A.; Suárez, A. Th17 responses and natural IgM antibodies are related to gut microbiota composition in systemic lupus erythematosus patients. Sci. Rep. 2016, 6, 24072. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrio, J.; López, P.; Sánchez, B.; González, S.; Gueimonde, M.; Margolles, A.; Reyes-Gavilán, C.G.d.L.; Suárez, A. Intestinal Dysbiosis Is Associated with Altered Short-Chain Fatty Acids and Serum-Free Fatty Acids in Systemic Lupus Erythematosus. Front. Immunol. 2017, 8, 23. [Google Scholar] [CrossRef]
- Opazo, M.C.; Ortega-Rocha, E.M.; Coronado-Arrázola, I.; Bonifaz, L.C.; Boudin, H.; Neunlist, M.; Bueno, S.M.; Kalergis, A.M.; Riedel, C.A. Intestinal Microbiota Influences Non-intestinal Related Autoimmune Diseases. Front. Microbiol. 2018, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Bellocchi, C.; Fernández-Ochoa, Á.; Montanelli, G.; Vigone, B.; Santaniello, A.; Milani, C.; Quirantes-Piné, R.; Borrás-Linares, I.; Ventura, M.; Segura-Carrettero, A.; et al. Microbial and metabolic multi-omic correlations in systemic sclerosis patients. Ann. N. Y. Acad. Sci. 2018, 1421, 97–109. [Google Scholar] [CrossRef]
- Luan, M.; Shang, Z.; Teng, Y.; Chen, X.; Zhang, M.; Lv, H.; Zhang, R. The shared and specific mechanism of four autoimmune diseases. Oncotarget 2017, 8, 108355–108374. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Luckey, D.; Taneja, V. The gut microbiome in autoimmunity: Sex matters. Clin. Immunol. 2015, 159, 154. [Google Scholar] [CrossRef] [PubMed]
- Meehan, C.J.; Beiko, R.G. A Phylogenomic View of Ecological Specialization in the Lachnospiraceae, a Family of Digestive Tract-Associated Bacteria. Genome Biol. Evol. 2014, 6, 703. [Google Scholar] [CrossRef] [PubMed]
- Bellocchi, C.; Fernández-Ochoa, Á.; Montanelli, G.; Vigone, B.; Santaniello, A.; Quirantes-Piné, R.; Borrás-Linares, I.; Gerosa, M.; Artusi, C.; Gualtierotti, R.; et al. Identification of a Shared Microbiomic and Metabolomic Profile in Systemic Autoimmune Diseases. J. Clin. Med. 2019, 8, 1291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jameson, E.; Crosatti, M.; Schäfer, H.; Rajakumar, K.; Bugg, T.D.H.; Chen, Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc. Natl. Acad. Sci. USA 2014, 111, 4268–4273. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic Butyrate-Producing Communities in Humans: An Overview Using Omics Data. mSystems 2017, 2, e00130-17. [Google Scholar] [CrossRef] [PubMed]
- Amir, I.; Bouvet, P.; Legeay, C.; Gophna, U.; Weinberger, A. Eisenbergiella tayi gen. nov., sp. nov., isolated from human blood. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 3, 907–914. [Google Scholar] [CrossRef]
- Sandri, M.; Dal Monego, S.; Conte, G.; Sgorlon, S.; Stefanon, B. Raw meat based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet. Res. 2016, 13, 65. [Google Scholar] [CrossRef]
- Ganesan, A.; Chaussonnerie, S.; Tarrade, A.; Dauga, C.; Bouchez, T.; Pelletier, E.; Le Paslier, D.; Sghir, A. Cloacibacillus evryensis gen. nov., sp. nov., a novel asaccharolytic, mesophilic, amino-acid-degrading bacterium within the phylum “Synergistetes”, isolated from an anaerobic sludge digester. Int. J. Syst. Evol. Microbiol. 2008, 58, 2003–2012. [Google Scholar] [CrossRef]
- Treede, I.; Braun, A.; Sparla, R.; Kühnel, M.; Giese, T.; Turner, J.R.; Anes, E.; Kulaksiz, H.; Füllekrug, J.; Stremmel, W.; et al. Anti-inflammatory effects of phosphatidylcholine. J. Biol. Chem. 2007, 282, 27155. [Google Scholar] [CrossRef]
- Schreiber, R.; Zechner, R. Lipolysis meets inflammation: Arachidonic acid mobilization from fat. J. Lipid Res. 2014, 55, 2447. [Google Scholar] [CrossRef]
- Robbins, A.; Hentzien, M.; Toquet, S.; Didier, K.; Servettaz, A.; Pham, B.-N.; Giusti, D. Diagnostic Utility of Separate Anti-Ro60 and Anti-Ro52/TRIM21 Antibody Detection in Autoimmune Diseases. Front. Immunol. 2019, 10, 444. [Google Scholar] [CrossRef]
- Temmoku, J.; Sato, S.; Fujita, Y.; Asano, T.; Suzuki, E.; Kanno, T.; Furuya, M.Y.; Matsuoka, N.; Kobayashi, H.; Watanabe, H.; et al. Clinical significance of myositis-specific autoantibody profiles in Japanese patients with polymyositis/dermatomyositis. Medicine 2019, 98, e15578. [Google Scholar] [CrossRef]
- Chu, X.J.; Cao, N.W.; Zhou, H.Y.; Meng, X.; Guo, B.; Zhang, H.-Y.; Li, B.-Z. The oral and gut microbiome in rheumatoid arthritis patients: A systematic review. Rheumatology 2021, 60, 1054–1066. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Zhang, P.; Song, C.; Pan, F.; Li, G.; Peng, L.; Yang, Y.; Wei, Z.; Huang, F. Gut microbiota changes in patients with spondyloarthritis: A systematic review. Semin. Arthritis Rheum. 2022, 52, 151925. [Google Scholar] [CrossRef]
- Yadav, S.K.; Boppana, S.; Ito, N.; Mindur, J.E.; Mathay, M.T.; Patel, A.; Dhib-Jalbut, S.; Ito, K. Gut dysbiosis breaks immunological tolerance toward the central nervous system during young adulthood. Proc. Natl. Acad. Sci. USA 2017, 114, E9318–E9327. [Google Scholar] [CrossRef]
- Li, B.; Selmi, C.; Tang, R.; Gershwin, M.E.; Ma, X. The microbiome and autoimmunity: A paradigm from the gut-liver axis. Cell Mol. Immunol. 2018, 15, 595–609. [Google Scholar] [CrossRef]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 535, 75–84. [Google Scholar] [CrossRef]
- Horvat, S.; Rupnik, M. Interactions between Clostridioides difficile and fecal microbiota in in vitro batch model: Growth, sporulation, and microbiota changes. Front. Microbiol. 2018, 9, 1633. [Google Scholar] [CrossRef]
- Frost, F.; Kacprowski, T.; Rühlemann, M.; Pietzner, M.; Bang, C.; Franke, A.; Nauck, M.; Völker, U.; Völzke, H.; Dörr, M.; et al. Long-term instability of the intestinal microbiome is associated with metabolic liver disease, low microbiota diversity, diabetes mellitus and impaired exocrine pancreatic function. Gut 2021, 70, 522–530. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Joyce Wu, H.J.; Mauro, D.; Schett, G.; Ciccia, F. The gut-joint axis in rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 224–237. [Google Scholar] [CrossRef]
- Arrieta, M.C.; Bistritz, L.; Meddings, J.B. Alterations in intestinal permeability. Gut 2006, 55, 1512. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Frech, M.; Schulz, O.; Schälter, F.; Lucas, S.; Azizov, V.; Dürholz, K.; Steffen, F.; Omata, Y.; Rings, A.; et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020, 11, 1995. [Google Scholar] [CrossRef]
- Wu, H.J.; Ivanov, I.I.; Darce, J.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Benoist, C.; Mathis, D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010, 32, 815. [Google Scholar] [CrossRef]
- Marietta, E.V.; Murray, J.A.; Luckey, D.H.; Jeraldo, P.R.; Lamba, A.; Patel, R.; Luthra, H.S.; Mangalam, A.; Taneja, V. Suppression of Inflammatory Arthritis by Human Gut-Derived Prevotella histicola in Humanized Mice. Arthritis Rheumatol. 2016, 68, 2878–2888. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Silva, C.; Greenfield, J.; Liu, W.Q.; Metz, L.M.; Yong, V.W. Biomarkers of intestinal barrier function in multiple sclerosis are associated with disease activity. Mult. Scler. 2020, 26, 1340–1350. [Google Scholar] [CrossRef]
- Ciccia, F.; Guggino, G.; Rizzo, A.; Alessandro, R.; Luchetti, M.M.; Milling, S.; Saieva, L.; Cypers, H.; Stampone, T.; Di Benedetto, P.; et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2017, 76, 1123–1132. [Google Scholar] [CrossRef]
- Kim, J.W.; Kwok, S.K.; Choe, J.Y.; Park, S.H. Recent Advances in Our Understanding of the Link between the Intestinal Microbiota and Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2019, 20, 4871. [Google Scholar] [CrossRef]
- Matei, D.E.; Menon, M.; Alber, D.G.; Smith, A.M.; Nedjat-Shokouhi, B.; Fasano, A.; Magill, L.; Duhlin, A.; Bitoun, S.; Gleizes, A.; et al. Intestinal barrier dysfunction plays an integral role in arthritis pathology and can be targeted to ameliorate disease. Med 2021, 2, 864–883.e9. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, T.H.; Ekici, M.; Çolpak, A.İ.; Brown, K.L.; Karadağ, Ö.; Balci-Peynircioglu, B. Behçet’s syndrome: Recent advances to aid diagnosis. Clin. Exp. Med. 2023, 23, 4079–4090. [Google Scholar] [CrossRef] [PubMed]
- Joubert, M.; André, M.; Barnich, N.; Billard, E. Microbiome and Behçet’s disease: A systematic review. Clin. Exp. Rheumatol. 2023, 41, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Kim, K.; Lee, E.S.; Park, S. The Suppressive Effect of Butyrate and Bromopyruvate on Inflammatory Cytokine Production and Short Chain Fatty Acid Receptor Expression by Blood Mononuclear Cells in Patients with Behçet’s Disease. Ann. Dermatol. 2018, 30, 566. [Google Scholar] [CrossRef] [PubMed]
- Emmi, G.; Bettiol, A.; Niccolai, E.; Ramazzotti, M.; Amedei, A.; Pagliai, G.; Taddei, N.; Sofi, F.; Fiorillo, C.; Prisco, D.; et al. Butyrate-Rich Diets Improve Redox Status and Fibrin Lysis in Behçet’s Syndrome. Circ. Res. 2021, 128, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Golpour, F.; Abbasi-Alaei, M.; Babaei, F.; Mirzababaei, M.; Parvardeh, S.; Mohammadi, G.; Nassiri-Asl, M. Short chain fatty acids, a possible treatment option for autoimmune diseases. Biomed. Pharmacother. 2023, 163, 114763. [Google Scholar] [CrossRef]
- Takahashi, D.; Hoshina, N.; Kabumoto, Y.; Maeda, Y.; Suzuki, A.; Tanabe, H.; Isobe, J.; Yamada, T.; Muroi, K.; Yanagisawa, Y.; et al. Microbiota-derived butyrate limits the autoimmune response by promoting the differentiation of follicular regulatory T cells. EBioMedicine 2020, 58, 102913. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chu, Y.; Li, J.; Meng, Q.; Liu, Y.; Jin, J.; Wang, Y.; Wang, J.; Huang, B.; Shi, L.; et al. Intestinal butyrate-metabolizing species contribute to autoantibody production and bone erosion in rheumatoid arthritis. Sci. Adv. 2022, 8, 1511. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kwon, J.E.; Lee, S.H.; Kim, E.K.; Ryu, J.-G.; Jung, K.-A.; Choi, J.-W.; Park, M.-J.; Moon, Y.-M.; Park, S.-H.; et al. Attenuation of Rheumatoid Inflammation by Sodium Butyrate Through Reciprocal Targeting of HDAC2 in Osteoclasts and HDAC8 in T Cells. Front. Immunol. 2018, 9, 1525. [Google Scholar] [CrossRef] [PubMed]
- Rosser, E.C.; Piper, C.J.M.; Matei, D.E.; Blair, P.A.; Rendeiro, A.F.; Orford, M.; Alber, D.G.; Krausgruber, T.; Catalan, D.; Klein, N.; et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020, 31, 837–851.e10. [Google Scholar] [CrossRef]
- Cao, S.; Budina, E.; Raczy, M.M.; Solanki, A.; Nguyen, M.; Beckman, T.N.; Reda, J.W.; Hultgren, K.; Ang, P.S.; Slezak, A.J.; et al. A serine-conjugated butyrate prodrug with high oral bioavailability suppresses autoimmune arthritis and neuroinflammation in mice. Nat. Biomed. Eng. 2024, 8, 611–627. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Luckey, D.; Bodhke, R.; Chen, J.; Marietta, E.; Jeraldo, P.; Murray, J.; Taneja, V. Prevotella histicola Protects From Arthritis by Expansion of Allobaculum and Augmenting Butyrate Production in Humanized Mice. Front. Immunol. 2021, 12, 609644. [Google Scholar] [CrossRef]
- Wang, F.; Zhufeng, Y.; Chen, Z.; Xu, J.; Cheng, Y. The composition and function profile of the gut microbiota of patients with primary Sjögren’s syndrome. Clin. Rheumatol. 2023, 42, 1315–1326. [Google Scholar] [CrossRef]
- van der Meulen, T.A.; Harmsen, H.J.M.; Vila, A.V.; Kurilshikov, A.; Liefers, S.C.; Zhernakova, A.; Fu, J.; Wijmenga, C.; Weersma, R.K.; de Leeuw, K.; et al. Shared gut, but distinct oral microbiota composition in primary Sjögren’s syndrome and systemic lupus erythematosus. J. Autoimmun. 2019, 97, 77–87. [Google Scholar] [CrossRef]
- Kim, D.S.; Woo, J.S.; Min, H.K.; Choi, J.-W.; Moon, J.H.; Park, M.-J.; Kwok, S.-K.; Park, S.-H.; Cho, M.-L. Short-chain fatty acid butyrate induces IL-10-producing B cells by regulating circadian-clock-related genes to ameliorate Sjögren’s syndrome. J. Autoimmun. 2021, 119, 102611. [Google Scholar] [CrossRef]
- Deng, C.; Xiao, Q.; Fei, Y. A Glimpse Into the Microbiome of Sjögren’s Syndrome. Front. Immunol. 2022, 13, 918619. [Google Scholar] [CrossRef]
- Akhil, A.; Bansal, R.; Anupam, K.; Tandon, A.; Bhatnagar, A. Systemic lupus erythematosus: Latest insight into etiopathogenesis. Rheumatol. Int. 2023, 43, 1381. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Silverman, G.J. The Microbiome and Systemic Lupus Erythematosus. N. Engl. J. Med. 2018, 378, 2236–2237. [Google Scholar] [CrossRef]
- Mohd, R.; Chin, S.F.; Shaharir, S.S.; Cham, Q.S. Involvement of Gut Microbiota in SLE and Lupus Nephritis. Biomedicines 2023, 11, 653. [Google Scholar] [CrossRef]
- Hevia, A.; Milani, C.; López, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; González, S.; Suárez, A.; Gueimonde, M.; et al. Intestinal Dysbiosis Associated with Systemic Lupus Erythematosus. mBio 2014, 5, 1548–1562. [Google Scholar] [CrossRef]
- He, H.; Xu, H.; Xu, J.; Zhao, H.; Lin, Q.; Zhou, Y.; Nie, Y. Sodium Butyrate Ameliorates Gut Microbiota Dysbiosis in Lupus-Like Mice. Front. Nutr. 2020, 7, 604283. [Google Scholar] [CrossRef]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B cell-intrinsic epigenetic modulation of antibody responses by dietary fiber-derived short-chain fatty acids. Nat. Commun. 2020, 11, 60. [Google Scholar] [CrossRef]
- Guo, X.; Yang, X.; Li, Q.; Shen, X.; Zhong, H.; Yang, Y. The Microbiota in Systemic Lupus Erythematosus: An Update on the Potential Function of Probiotics. Front. Pharmacol. 2021, 12, 759095. [Google Scholar] [CrossRef]
- de la Visitación, N.; Robles-Vera, I.; Toral, M.; Duarte, J. Protective Effects of Probiotic Consumption in Cardiovascular Disease in Systemic Lupus Erythematosus. Nutrients 2019, 11, 2676. [Google Scholar] [CrossRef] [PubMed]
- Moleón, J.; González-Correa, C.; Miñano, S.; Robles-Vera, I.; de la Visitación, N.; Barranco, A.M.; Gómez-Guzmán, M.; Sánchez, M.; Riesco, P.; Guerra-Hernández, E.; et al. Protective effect of microbiota-derived short chain fatty acids on vascular dysfunction in mice with systemic lupus erythematosus induced by toll like receptor 7 activation. Pharmacol. Res. 2023, 198, 106997. [Google Scholar] [CrossRef]
- Moleón, J.; González-Correa, C.; Robles-Vera, I.; Miñano, S.; de la Visitación, N.; Barranco, A.M.; Martín-Morales, N.; O’valle, F.; Mayo-Martínez, L.; García, A.; et al. Targeting the gut microbiota with dietary fibers: A novel approach to prevent the development cardiovascular complications linked to systemic lupus erythematosus in a preclinical study. Gut Microbes 2023, 15, 2247053. [Google Scholar] [CrossRef]
- Widhani, A.; Djauzi, S.; Suyatna, F.D.; Dewi, B.E. Changes in Gut Microbiota and Systemic Inflammation after Synbiotic Supplementation in Patients with Systemic Lupus Erythematosus: A Randomized, Double-Blind, Placebo-Controlled Trial. Cells 2022, 11, 3419. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic sclerosis. Lancet 2023, 401, 304. [Google Scholar] [CrossRef]
- McMahan, Z.H.; Kulkarni, S.; Chen, J.; Chen, J.Z.; Xavier, R.J.; Pasricha, P.J.; Khanna, D. Systemic sclerosis gastrointestinal dysmotility: Risk factors, pathophysiology, diagnosis and management. Nat. Rev. Rheumatol. 2023, 19, 166–181. [Google Scholar] [CrossRef]
- Volkmann, E.R.; McMahan, Z. Gastrointestinal involvement in systemic sclerosis: Pathogenesis, assessment, and treatment. Curr. Opin. Rheumatol. 2022, 34, 328. [Google Scholar] [CrossRef]
- Polkowska-Pruszyńska, B.; Gerkowicz, A.; Rawicz-Pruszyński, K.; Krasowska, D. Gut microbiome in systemic sclerosis: A potential therapeutic target. Adv. Dermatol. Allergol./Postȩpy Dermatol. Alergol. 2022, 39, 101. [Google Scholar] [CrossRef]
- Kim, S.; Park, H.J.; Lee, S.I. The Microbiome in Systemic Sclerosis: Pathophysiology and Therapeutic Potential. Int. J. Mol. Sci. 2022, 23, 16154. [Google Scholar] [CrossRef]
- Patrone, V.; Puglisi, E.; Cardinali, M.; Schnitzler, T.S.; Svegliati, S.; Festa, A.; Gabrielli, A.; Morelli, L. Gut microbiota profile in systemic sclerosis patients with and without clinical evidence of gastrointestinal involvement. Sci. Rep. 2017, 7, 14874. [Google Scholar] [CrossRef]
- Kabel, A.M.; Omar, M.S.; Elmaaboud, M.A.A. Amelioration of bleomycin-induced lung fibrosis in rats by valproic acid and butyrate: Role of nuclear factor kappa-B, proinflammatory cytokines and oxidative stress. Int. Immunopharmacol. 2016, 39, 335–342. [Google Scholar] [CrossRef]
- Park, H.J.; Jeong, O.Y.; Chun, S.H.; Cheon, Y.H.; Kim, M.; Kim, S.; Lee, S.-I. Butyrate Improves Skin/Lung Fibrosis and Intestinal Dysbiosis in Bleomycin-Induced Mouse Models. Int. J. Mol. Sci. 2021, 22, 2765. [Google Scholar] [CrossRef]
- Russo, E.; Lepri, G.; Baldi, S.; Fioretto, B.S.; Romano, E.; El Aoufy, K.; Ramazzotti, M.; Rosa, I.; Ghezzi, G.; DI Gloria, L.; et al. Pos0044 Deciphering the Gut Microbiota of Very Early Systemic Sclerosis (Vedoss) Patients: A Taxonomic and Functional Characterization. Sci. Abstr. 2024, 83, 289. [Google Scholar] [CrossRef]
- Moon, J.; Choi, S.H.; Yoon, C.H.; Kim, M.K. Gut dysbiosis is prevailing in Sjögren’s syndrome and is related to dry eye severity. PLoS ONE 2020, 15, e0229029. [Google Scholar] [CrossRef]
- Cano-Ortiz, A.; Laborda-Illanes, A.; Plaza-Andrades, I.; del Pozo, A.M.; Cuadrado, A.V.; de Mora, M.R.C.; Leiva-Gea, I.; Sanchez-Alcoholado, L.; Queipo-Ortuño, M.I. Connection between the Gut Microbiome, Systemic Inflammation, Gut Permeability and FOXP3 Expression in Patients with Primary Sjögren’s Syndrome. Int. J. Mol. Sci. 2020, 21, 8733. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coccia, C.; Bonomi, F.; Lo Cricchio, A.; Russo, E.; Peretti, S.; Bandini, G.; Lepri, G.; Bartoli, F.; Moggi-Pignone, A.; Guiducci, S.; et al. The Potential Role of Butyrate in the Pathogenesis and Treatment of Autoimmune Rheumatic Diseases. Biomedicines 2024, 12, 1760. https://doi.org/10.3390/biomedicines12081760
Coccia C, Bonomi F, Lo Cricchio A, Russo E, Peretti S, Bandini G, Lepri G, Bartoli F, Moggi-Pignone A, Guiducci S, et al. The Potential Role of Butyrate in the Pathogenesis and Treatment of Autoimmune Rheumatic Diseases. Biomedicines. 2024; 12(8):1760. https://doi.org/10.3390/biomedicines12081760
Chicago/Turabian StyleCoccia, Carmela, Francesco Bonomi, Anna Lo Cricchio, Edda Russo, Silvia Peretti, Giulia Bandini, Gemma Lepri, Francesca Bartoli, Alberto Moggi-Pignone, Serena Guiducci, and et al. 2024. "The Potential Role of Butyrate in the Pathogenesis and Treatment of Autoimmune Rheumatic Diseases" Biomedicines 12, no. 8: 1760. https://doi.org/10.3390/biomedicines12081760
APA StyleCoccia, C., Bonomi, F., Lo Cricchio, A., Russo, E., Peretti, S., Bandini, G., Lepri, G., Bartoli, F., Moggi-Pignone, A., Guiducci, S., Del Galdo, F., Furst, D. E., Matucci Cerinic, M., & Bellando-Randone, S. (2024). The Potential Role of Butyrate in the Pathogenesis and Treatment of Autoimmune Rheumatic Diseases. Biomedicines, 12(8), 1760. https://doi.org/10.3390/biomedicines12081760






