Heteronuclear Complexes with Promising Anticancer Activity against Colon Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Complexes
2.2. DNA Binding Constant Calculation
2.3. DNA Interaction
3. Results and Discussion
3.1. Synthesis and Characterisation
3.2. Solution Stability
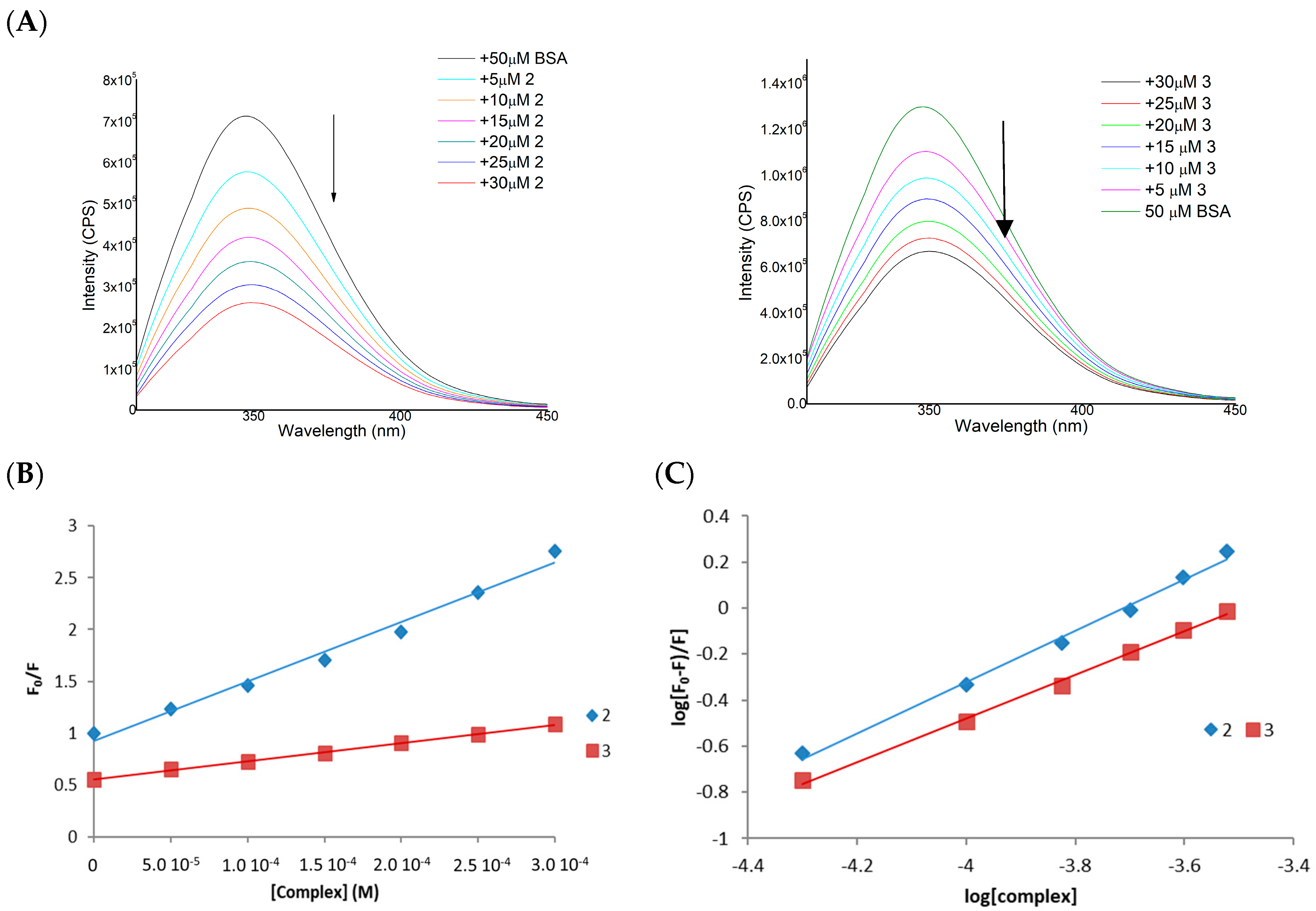
3.3. BSA Interaction
3.4. Biological Studies
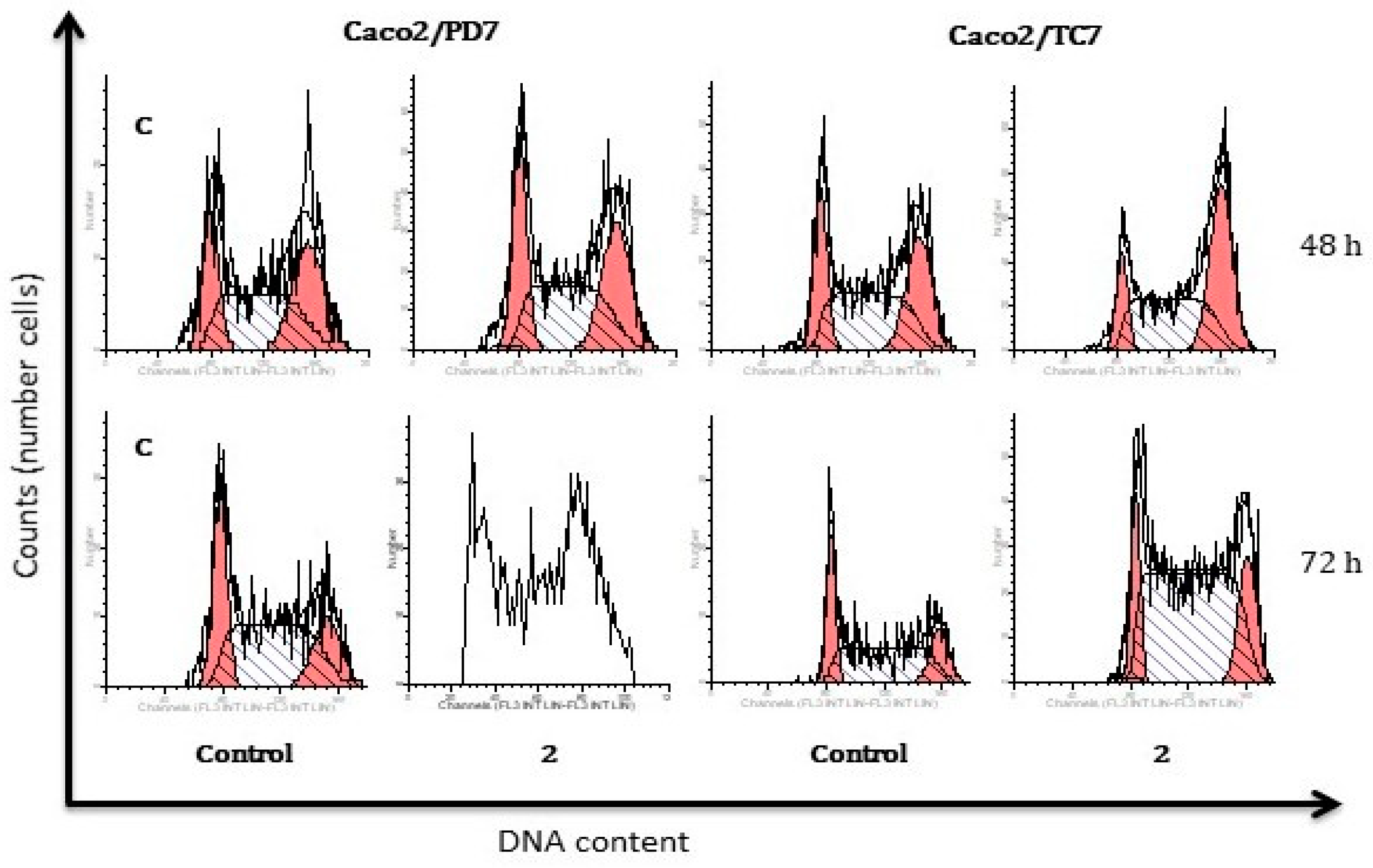
3.5. Apoptosis and Cell Cycle Studies
3.6. Effect of Heteronuclear Complexes on Intracellular Redox State
3.7. Interaction with Thioredoxin Reductase
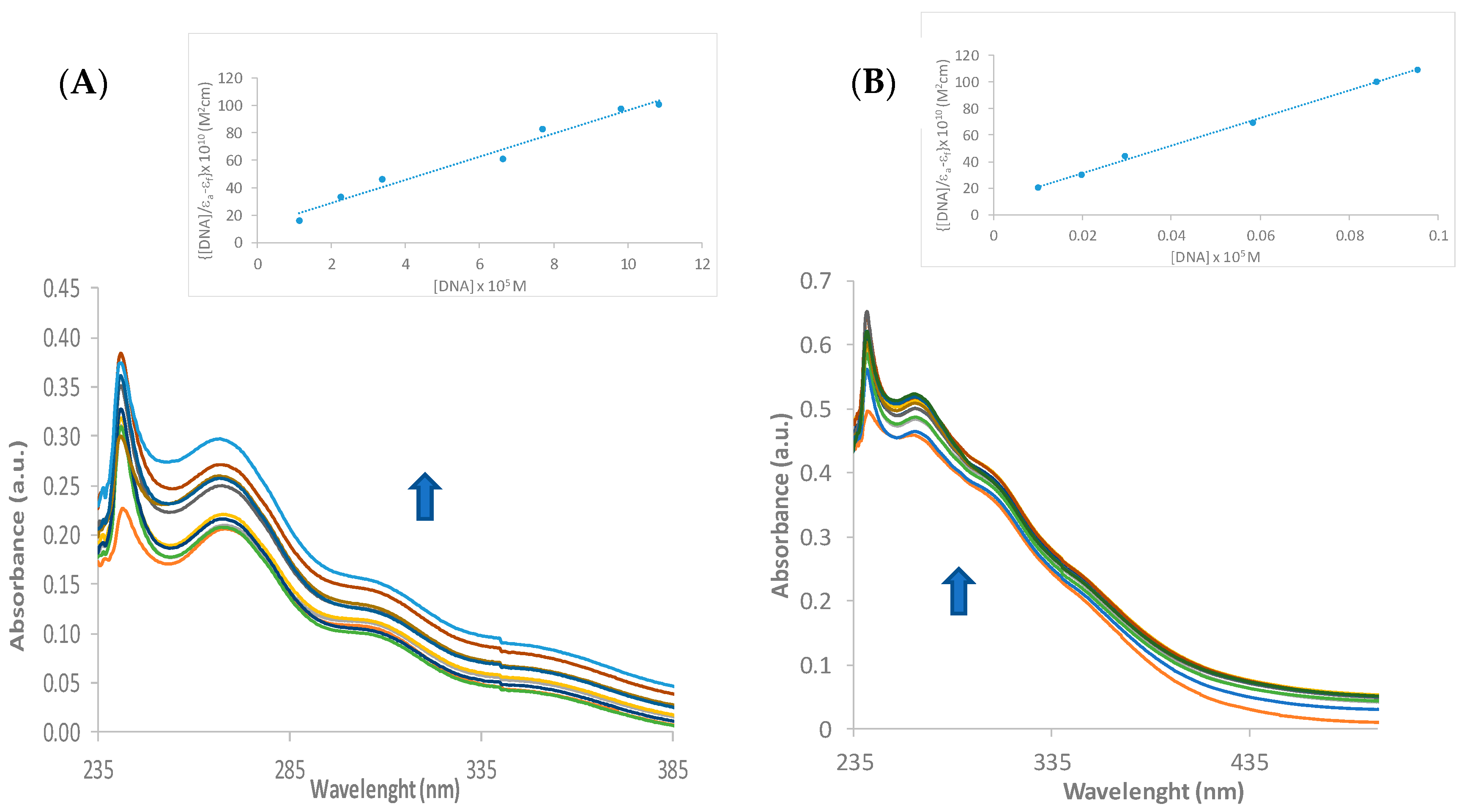
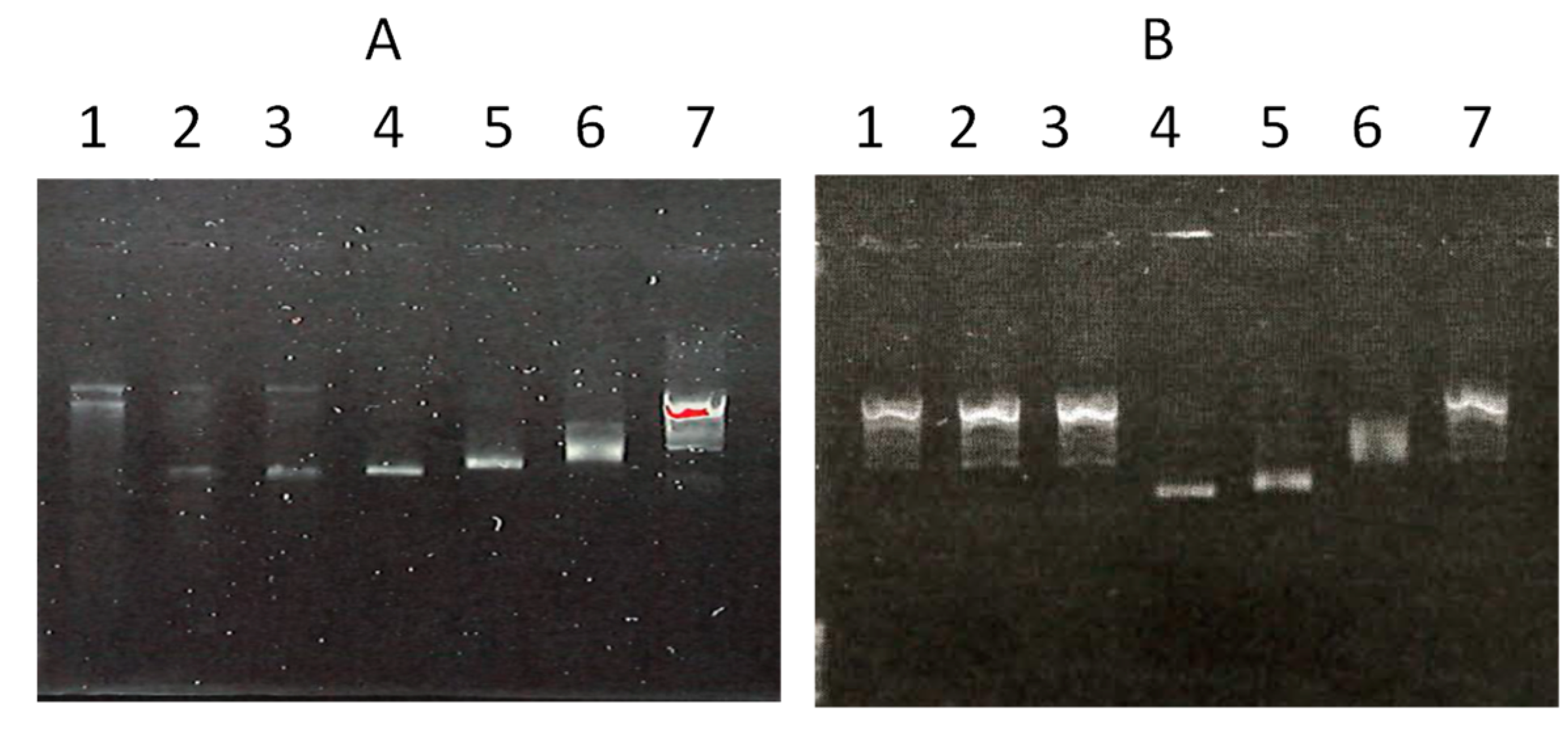
3.8. DNA Binding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ahmed, S.; Johnson, K.; Ahmed, O.; Iqbal, N. Advances in the management of colorectal cancer: From biology to treatment. Int. J. Color. Dis. 2014, 29, 1031–1042. [Google Scholar] [CrossRef]
- Adebayo, A.S.; Agbaje, K.; Adesina, S.K.; Olajubutu, O. Colorectal Cancer: Disease Process, Current Treatment Options, and Future Perspectives. Pharmaceutics 2023, 15, 2620. [Google Scholar] [CrossRef] [PubMed]
- Casini, A.; Pöthig, A. Metals in Cancer Research: Beyond Platinum Metallodrugs. ACS Cent. Sci. 2024, 10, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, S.; Aliabadi, A.; Khaksar, S. Riding the metal wave: A review of the latest developments in metal-based anticancer agents. Coord. Chem. Rev. 2024, 501, 215579. [Google Scholar] [CrossRef]
- Yeo, C.I.; Goh, C.H.P.; Tiekink, E.R.T.; Chew, J. Antibiotics: A “GOLDen” promise? Coord. Chem. Rev. 2024, 500, 215429. [Google Scholar] [CrossRef]
- Stratton, M.; Ramachandran, A.; Camacho, E.J.M.; Patil, S.; Waris, G.; Grice, K.A. Anti-fibrotic activity of gold and platinum complexes—Au(I) compounds as a new class of anti-fibrotic agents. J. Inorg. Biochem. 2020, 206, 111023. [Google Scholar] [CrossRef] [PubMed]
- Mertens, R.T.; Gukathasan, S.; Arojojoye, A.S.; Olelewe, C.; Awuah, S.G. Next Generation Gold Drugs and Probes: Chemistry and Biomedical Applications. Chem. Rev. 2023, 123, 6612–6667. [Google Scholar] [CrossRef] [PubMed]
- Essa, R.Z.; Brianna; Yeo, C.I.; Teow, S.-Y. Gold complexes and their molecular targets in colorectal cancer. J. Organomet. Chem. 2024, 1010, 123097. [Google Scholar] [CrossRef]
- Pete, S.; Roy, N.; Kar, B.; Paira, P. Construction of homo and heteronuclear Ru(II), Ir(III) and Re(I) complexes for target specific cancer therapy. Coord. Chem. Rev. 2022, 460, 214462. [Google Scholar] [CrossRef]
- Ma, L.; Li, L.; Zhu, G. Platinum-containing heterometallic complexes in cancer therapy: Advances and perspectives. Inorg. Chem. Front. 2022, 9, 2424–2453. [Google Scholar] [CrossRef]
- Giorgi, E.; Binacchi, F.; Marotta, C.; Cirri, D.; Gabbiani, C.; Pratesi, A. Highlights of New Strategies to Increase the Efficacy of Transition Metal Complexes for Cancer Treatments. Molecules 2023, 28, 273. [Google Scholar] [CrossRef] [PubMed]
- Redrado, M.; Fernández-Moreira, V.; Gimeno, M.C. Theranostics Through the Synergistic Cooperation of Heterometallic Complexes. ChemMedChem 2021, 16, 932–941. [Google Scholar] [CrossRef] [PubMed]
- López-Hernández, J.E.; Nayeem, N.; Cerón-Carrasco, J.P.; Ahad, A.; Hafeez, A.; León, I.E.; Contel, M. Platinum(IV)–Gold(I) Agents with Promising Anticancer Activity: Selected Studies in 2D and 3D Triple-Negative Breast Cancer Models. Chem. Eur. J. 2023, 29, e202302045. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, M.; Bigaeva, E.; Richard, P.; Le Gendre, P.; Picquet, M.; Casini, A.; Bodio, E. New heteronuclear gold(I)-platinum(II) complexes with cytotoxic properties: Are two metals better than one? J. Inorg. Biochem. 2014, 141, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Serratrice, M.; Maiore, L.; Zucca, A.; Stoccoro, S.; Landini, I.; Mini, E.; Massai, L.; Ferraro, G.; Merlino, A.; Messori, L.; et al. Cytotoxic properties of a new organometallic platinum(ii) complex and its gold(i) heterobimetallic derivatives. Dalton Trans. 2016, 45, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Bellam, R.; Jaganyi, D.; Robinson, R.S. Heterodinuclear Ru–Pt Complexes Bridged with 2,3-Bis(pyridyl)pyrazinyl Ligands: Studies on Kinetics, Deoxyribonucleic Acid/Bovine Serum Albumin Binding and Cleavage, In Vitro Cytotoxicity, and In Vivo Toxicity on Zebrafish Embryo Activities. ACS Omega 2022, 7, 26226–26245. [Google Scholar] [CrossRef] [PubMed]
- Bellam, R.; Jaganyi, D.; Mambanda, A.; Robinson, R. Role of a 2,3-bis(pyridyl)pyrazinyl chelate bridging ligand in the reactivity of Ru(ii)–Pt(ii) dinuclear complexes on the substitution of chlorides by thiourea nucleophiles—A kinetic study. New J. Chem. 2018, 42, 12557–12569. [Google Scholar] [CrossRef]
- Ma, L.; Ma, R.; Wang, Z.; Yiu, S.-M.; Zhu, G. Heterodinuclear Pt(iv)–Ru(ii) anticancer prodrugs to combat both drug resistance and tumor metastasis. Chem. Commun. 2016, 52, 10735–10738. [Google Scholar] [CrossRef]
- Ma, L.; Lin, X.; Li, C.; Xu, Z.; Chan, C.Y.; Tse, M.K.; Shi, P.; Zhu, G. A Cancer Cell-Selective and Low-Toxic Bifunctional Heterodinuclear Pt(IV)-Ru(II) Anticancer Prodrug. Inorg. Chem. 2018, 57, 2917–2924. [Google Scholar] [CrossRef]
- Gonzalez-Pantoja, J.F.; Stern, M.; Jarzecki, A.A.; Royo, E.; Robles-Escajeda, E.; Varela-Ramirez, A.; Aguilera, R.J.; Contel, M. Titanocene-Phosphine Derivatives as Precursors to Cytotoxic Heterometallic TiAu2 and TiM (M = Pd, Pt) Compounds. Studies of Their Interactions with DNA. Inorg. Chem. 2011, 50, 11099–11110. [Google Scholar] [CrossRef] [PubMed]
- Mitra, K.; Shettar, A.; Kondaiah, P.; Chakravarty, A.R. Biotinylated Platinum(II) Ferrocenylterpyridine Complexes for Targeted Photoinduced Cytotoxicity. Inorg. Chem. 2016, 55, 5612–5622. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K. Recent developments in the chemistry of ferrocenyl secondary natural product conjugates. Coord. Chem. Rev. 2018, 366, 91–108. [Google Scholar] [CrossRef]
- Askari, B.; Rudbari, H.A.; Micale, N.; Schirmeister, T.; Maugeri, A.; Navarra, M. Anticancer study of heterobimetallic platinum(II)-ruthenium(II) and platinum(II)-rhodium(III) complexes with bridging dithiooxamide ligand. J. Organomet. Chem. 2019, 900, 120918. [Google Scholar] [CrossRef]
- Aranda, E.E.; da Luz, J.S.; Oliveira, C.C.; Divina Petersen, P.A.; Petrilli, H.M.; da Costa Ferreira, A.M. Heterobinuclear copper(II)-platinum(II) complexes with oxindolimine ligands: Interactions with DNA, and inhibition of kinase and alkaline phosphatase proteins. J. Inorg. Biochem. 2020, 203, 110863. [Google Scholar] [CrossRef] [PubMed]
- Ćoćić, D.; Jovanović-Stević, S.; Jelić, R.; Matić, S.; Popović, S.; Djurdjević, P.; Baskić, D.; Petrović, B. Homo- and hetero-dinuclear Pt(ii)/Pd(ii) complexes: Studies of hydrolysis, nucleophilic substitution reactions, DNA/BSA interactions, DFT calculations, molecular docking and cytotoxic activity. Dalton Trans. 2020, 49, 14411–14431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guan, R.; Liao, X.; Ouyang, C.; Liu, J.; Ji, L.; Chao, H. Mitochondrial DNA targeting and impairment by a dinuclear Ir–Pt complex that overcomes cisplatin resistance. Inorg. Chem. Front. 2020, 7, 1864–1871. [Google Scholar] [CrossRef]
- Vučelj, S.; Hasić, R.; Ašanin, D.; Šmit, B.; Caković, A.; Bogojeski, J.; Serafinović, M.Ć.; Marković, B.S.; Stojanović, B.; Pavlović, S.; et al. Modes of Interactions with DNA/HSA Biomolecules and Comparative Cytotoxic Studies of Newly Synthesized Mononuclear Zinc(II) and Heteronuclear Platinum(II)/Zinc(II) Complexes toward Colorectal Cancer Cells. Int. J. Mol. Sci. 2024, 25, 3027. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, I.; Bashir, M.; Arjmand, F.; Tabassum, S. Advancement of metal compounds as therapeutic and diagnostic metallodrugs: Current frontiers and future perspectives. Coord. Chem. Rev. 2021, 445, 214104. [Google Scholar] [CrossRef]
- Chandra, A.; Singh, K.; Singh, S.; Sivakumar, S.; Patra, A.K. A luminescent europium(iii)–platinum(ii) heterometallic complex as a theranostic agent: A proof-of-concept study. Dalton Trans. 2016, 45, 494–497. [Google Scholar] [CrossRef]
- Elie, B.T.; Fernández-Gallardo, J.; Curado, N.; Cornejo, M.A.; Ramos, J.W.; Contel, M. Bimetallic titanocene-gold phosphane complexes inhibit invasion, metastasis, and angiogenesis-associated signaling molecules in renal cancer. Eur. J. Med. Chem. 2019, 161, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Elie, B.T.; Pechenyy, Y.; Uddin, F.; Contel, M. A heterometallic ruthenium-gold complex displays antiproliferative, antimigratory, and antiangiogenic properties and inhibits metastasis and angiogenesis-associated proteases in renal cancer. J. Biol. Inorg. Chem. 2018, 23, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Massai, L.; Fernandez-Gallardo, J.; Guerri, A.; Arcangeli, A.; Pillozzi, S.; Contel, M.; Messori, L. Design, synthesis and characterisation of new chimeric ruthenium(II)-gold(I) complexes as improved cytotoxic agents. Dalton Trans. 2015, 44, 11067–11076. [Google Scholar] [CrossRef] [PubMed]
- Lease, N.; Vasilevski, V.; Carreira, M.; de Almeida, A.; Sanau, M.; Hirva, P.; Casini, A.; Contel, M. Potential Anticancer Heterometallic Fe-Au and Fe-Pd Agents: Initial Mechanistic Insights. J. Med. Chem. 2013, 56, 5806–5818. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Moreno, E.; Gascon, S.; Rodriguez-Yoldi, M.J.; Cerrada, E.; Laguna, M. S-Propargylthiopyridine Phosphane Derivatives As Anticancer Agents: Characterization and Antitumor Activity. Organometallics 2013, 32, 3710–3720. [Google Scholar] [CrossRef]
- Johnson, A.; Marzo, I.; Gimeno, M.C. Heterobimetallic propargyl gold complexes with pi-bound copper or silver with enhanced anticancer activity. Dalton Trans. 2020, 49, 11736–11742. [Google Scholar] [CrossRef] [PubMed]
- Kaim, W.; Schwederski, B.; Klein, A. Bioinorganic Chemistry--Inorganic Elements in the Chemistry of Life: An Introduction and Guide; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef]
- Lelièvre, P.; Sancey, L.; Coll, J.-L.; Deniaud, A.; Busser, B. The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers 2020, 12, 3594. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, T.; Yuan, Y.; Li, N.; Wang, X.; Guan, J. Copper and Copper Complexes in Tumor Therapy. ChemMedChem 2024, 19, e202400060. [Google Scholar] [CrossRef]
- Pellei, M.; Del Bello, F.; Porchia, M.; Santini, C. Zinc coordination complexes as anticancer agents. Coord. Chem. Rev. 2021, 445, 214088. [Google Scholar] [CrossRef]
- Vergara, E.; Casini, A.; Sorrentino, F.; Zava, O.; Cerrada, E.; Rigobello, M.P.; Bindoli, A.; Laguna, M.; Dyson, P.J. Anticancer Therapeutics That Target Selenoenzymes: Synthesis, Characterization, in vitro Cytotoxicity, and Thioredoxin Reductase Inhibition of a Series of Gold(I) Complexes Containing Hydrophilic Phosphine Ligands. ChemMedChem 2010, 5, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Vergara, E.; Mohr, F.; de Vos, D.; Cerrada, E.; Mendia, A.; Laguna, M. Synthesis, characterization, and in vitro cytotoxicity of some gold(I) and trans platinum(II) thionate complexes containing water-soluble PTA and DAPTA ligands. X-ray crystal structures of Au(SC4H3N2)(PTA), trans-Pt(SC4H3N2)2(PTA)2, trans-Pt(SC5H4N)2(PTA)2, and trans-Pt(SC5H4N)2(DAPTA)2. Inorg. Chem. 2008, 47, 5641–5648. [Google Scholar] [CrossRef] [PubMed]
- Daigle, D.J.; Pepperman, A.B., Jr.; Vail, S.L. Synthesis of a monophosphorus analog of hexamethylenetetramine. J. Hetercycl. Chem. 1974, 11, 407–408. [Google Scholar] [CrossRef]
- Atrián-Blasco, E.; Gascón, S.; Rodríguez-Yoldi, M.J.; Laguna, M.; Cerrada, E. Synthesis of Gold(I) Derivatives Bearing Alkylated 1,3,5-Triaza-7-phosphaadamantane as Selective Anticancer Metallodrugs. Eur. J. Inorg. Chem. 2016, 2016, 2791–2803. [Google Scholar] [CrossRef]
- Pinto, M.; Robineleon, S.; Appay, M.D.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simonassmann, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human-colon carcinoma cell-line caco-2 in culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Chantret, I.; Rodolosse, A.; Barbat, A.; Dussaulx, E.; Brotlaroche, E.; Zweibaum, A.; Rousset, M. Differential Expression Of Sucrase-Isomaltase In Clones Isolated From Early And Late Passages Of The Cell-Line Caco-2—Evidence For Glucose-Dependent Negative Regulation. J. Cell Sci. 1994, 107, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Marmol, I.; Virumbrales-Munoz, M.; Quero, J.; Sanchez-De-Diego, C.; Fernandez, L.; Ochoa, I.; Cerrada, E.; Yoldi, M.J.R. Alkynyl gold(I) complex triggers necroptosis via ROS generation in colorectal carcinoma cells. J. Inorg. Biochem. 2017, 176, 123–133. [Google Scholar] [CrossRef]
- Vergara, E.; Miranda, S.; Mohr, F.; Cerrada, E.; Tiekink, E.R.T.; Romero, P.; Mendia, A.; Laguna, M. Gold(I) and Palladium(II) thiolato complexes containing water-soluble phosphane ligands. Eur. J. Inorg. Chem. 2007, 2007, 2926–2933. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Yoshinari, N.; Naruse, D.; Nozaki, K.; Konno, T. Synthesis, Structures, and Luminescence Properties of Interconvertible AuI2ZnII and AuI3ZnII Complexes with Mixed Bis(diphenylphosphino)methane and d-Penicillaminate. Inorg. Chem. 2013, 52, 14368–14375. [Google Scholar] [CrossRef]
- Smoleński, P.; Benisvy, L.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Syntheses and Crystal Structures of the First Zinc Complex with 1,3,5-Triaza-7-phosphaadamantane (PTA), [ZnCl2(PTA)2], and of the Hybrid Organic–Inorganic Salts of N-Methyl-1,3,5-triaza-7-phosphaadamantane with Tetrahalozinc [PTA–Me]2 [ZnI2X2] (X = I, Cl). Eur. J. Inorg. Chem. 2009, 2009, 1181–1186. [Google Scholar] [CrossRef]
- Spinner, E. 250. The infrared spectra of some N-heteroaromatic mercaptocompounds and of their N-methyl and S-methyl derivatives. J. Chem. Soc. 1960, 1237–1242. [Google Scholar] [CrossRef]
- Sousa-Pedrares, A.; Romero, J.; Arturo García-Vázquez, J.; Luz Durán, M.; Casanova, I.; Sousa, A. Electrochemical synthesis and structural characterisation of zinc, cadmium and mercury complexes of heterocyclic bidentate ligands (N, S). Dalton Trans. 2003, 1379–1388. [Google Scholar] [CrossRef]
- Rodríguez, A.; Sousa-Pedrares, A.; García-Vázquez, J.A.; Romero, J.; Sousa, A. Synthesis and Structural Characterization of Copper(I), Silver(I) and Gold(I) Complexes with Pyrimidine-2-thionato Ligands and their Adducts with Phosphanes. Eur. J. Inorg. Chem. 2011, 2011, 3403–3413. [Google Scholar] [CrossRef]
- Radisavljević, S.; Petrović, B. Gold(III) Complexes: An Overview on Their Kinetics, Interactions With DNA/BSA, Cytotoxic Activity, and Computational Calculations. Front. Chem. 2020, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Eftink, M. Fluorescence Techniques for Studying Protein Structure. In Methods of Biochemical Analysis: Protein Structure Determination; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 35, pp. 127–205. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: Baltimore, MD, USA, 2006; pp. 277–330. [Google Scholar]
- Galassi, R.; Luciani, L.; Gambini, V.; Vincenzetti, S.; Lupidi, G.; Amici, A.; Marchini, C.; Wang, J.B.; Pucciarelli, S. Multi-Targeted Anticancer Activity of Imidazolate Phosphane Gold(I) Compounds by Inhibition of DHFR and TrxR in Breast Cancer Cells. Front. Chem. 2021, 8, 602845. [Google Scholar] [CrossRef] [PubMed]
- Binacchi, F.; Guarra, F.; Cirri, D.; Marzo, T.; Pratesi, A.; Messori, L.; Gabbiani, C.; Biver, T. On the Different Mode of Action of Au(I)/Ag(I)-NHC Bis-Anthracenyl Complexes Towards Selected Target Biomolecules. Molecules 2020, 25, 5446. [Google Scholar] [CrossRef]
- Atrian-Blasco, E.; Gascon, S.; Rodriguez-Yoldi, M.J.; Laguna, M.; Cerrada, E. Novel Gold(I) Thiolate Derivatives Synergistic with 5-Fluorouracil as Potential Selective Anticancer Agents in Colon Cancer. Inorg. Chem. 2017, 56, 8562–8579. [Google Scholar] [CrossRef] [PubMed]
- Kharpan, B.; Shyam, A.; Nandi, R.; Paul, S.; Paul, P.C.; Mondal, P.; Kumar, D.; Choudhury, S.; Ray, S. Antiparasitic activity, DNA/BSA binding interaction, molecular docking and DFT studies of mesogenic L-leucine based Schiff base and its derivatize Cu(II) and Zn(II) complexes. J. Mol. Struct. 2024, 1304, 137633. [Google Scholar] [CrossRef]
- Ansari, M.F.; Arjmand, F. Synthesis and characterization of copper(II) norcraugsodine complexes: Their in vitro binding studies with therapeutic targets (ct-DNA/tRNA/BSA), cleavage, and cytotoxicity profile. J. Mol. Struct. 2024, 1304, 137692. [Google Scholar] [CrossRef]
- Garcia-Moreno, E.; Gascon, S.; Atrian-Blasco, E.; Rodriguez-Yoldi, M.J.; Cerrada, E.; Laguna, M. Gold(I) complexes with alkylated PTA (1,3,5-triaza-7-phosphaadamantane) phosphanes as anticancer metallodrugs. Eur. J. Med. Chem. 2014, 79, 164–172. [Google Scholar] [CrossRef]
- Abas, E.; Pena-Martinez, R.; Aguirre-Ramírez, D.; Rodriguez-Dieguez, A.; Laguna, M.; Grasa, L. New selective thiolate gold(i) complexes inhibit the proliferation of different human cancer cells and induce apoptosis in primary cultures of mouse colon tumors. Dalton Trans. 2020, 49, 1915–1927. [Google Scholar] [CrossRef]
- Moal, V.L.L.; Servin, A.L. Pathogenesis of Human Enterovirulent Bacteria: Lessons from Cultured, Fully Differentiated Human Colon Cancer Cell Lines. Microbiol. Mol. Biol. Rev. 2013, 77, 380–439. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Hasan, A.A.; Kalinina, E.; Tatarskiy, V.; Shtil, A. The Thioredoxin System of Mammalian Cells and Its Modulators. Biomedicines 2022, 10, 1757. [Google Scholar] [CrossRef] [PubMed]
- Arnér, E.S.J. Effects of Mammalian Thioredoxin Reductase Inhibitors. In Reactive Oxygen Species: Network Pharmacology and Therapeutic Applications; Schmidt, H.H.H.W., Ghezzi, P., Cuadrado, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 289–309. [Google Scholar]
- Lu, Y.; Zhao, X.; Li, K.; Luo, G.; Nie, Y.; Shi, Y.; Zhou, Y.; Ren, G.; Feng, B.; Liu, Z.; et al. Thioredoxin-like protein 2 is overexpressed in colon cancer and promotes cancer cell metastasis by interaction with ran. Antioxid. Redox Signal. 2013, 19, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.-J.; Geng, W.-S.; Wang, Z.-Q.; Chen, L.; Zeng, X.-S. The role of thioredoxin system in cancer: Strategy for cancer therapy. Cancer Chemother. Pharmacol. 2019, 84, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Muangthong, T.; Chusangnin, P.; Hassametto, A.; Tanomrat, R.; Suwannalert, P. Thioredoxin Reductase-1 as a Potential Biomarker in Fibroblast-Associated HCT116 Cancer Cell Progression and Dissemination in a Zebrafish Model. Cancers 2022, 15, 56. [Google Scholar] [CrossRef]
- Shen, X.; Xia, Y.; Lu, H.; Zheng, P.; Wang, J.; Chen, Y.; Xu, C.; Qiu, C.; Zhang, Y.; Xiao, Z.; et al. Synergistic targeting of TrxR1 and ATM/AKT pathway in human colon cancer cells. Biomed. Pharmacother. 2024, 174, 116507. [Google Scholar] [CrossRef]
- Zhang, J.M.; Li, X.M.; Han, X.; Liu, R.J.; Fang, J.G. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Marmol, I.; Montanel-Perez, S.; Royo, J.C.; Gimeno, M.C.; Villacampa, M.D.; Rodriguez-Yoldi, M.J.; Cerrada, E. Gold(I) and Silver(I) Complexes with 2-Anilinopyridine-Based Heterocycles as Multitarget Drugs against Colon Cancer. Inorg. Chem. 2020, 59, 17732–17745. [Google Scholar] [CrossRef] [PubMed]
- Marmol, I.; Sanchez-De-Diego, C.; Dieste, A.P.; Cerrada, E.; Yoldi, M.J.R. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Quero, J.; Royo, J.C.; Fodor, B.; Gimeno, M.C.; Osada, J.; Rodríguez-Yoldi, M.J.; Cerrada, E. Sulfonamide-Derived Dithiocarbamate Gold(I) Complexes Induce the Apoptosis of Colon Cancer Cells by the Activation of Caspase 3 and Redox Imbalance. Biomedicines 2022, 10, 1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Selvaraju, K.; Saei, A.A.; D’Arcy, P.; Zubarev, R.A.; Arner, E.S.J.; Linder, S. Repurposing of auranofin: Thioredoxin reductase remains a primary target of the drug. Biochimie 2019, 162, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Pierre, V.C.; Barton, J.K. Metallo-intercalators and metallo-insertors. Chem. Commun. 2007, 44, 4565–4579. [Google Scholar] [CrossRef] [PubMed]
- Pages, B.J.; Ang, D.L.; Wright, E.P.; Aldrich-Wright, J.R. Metal complex interactions with DNA. Dalton Trans. 2015, 44, 3505–3526. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Wani, W.A.; Saleem, K.; Hsieh, M.-F. Anticancer metallodrugs of glutamic acid sulphonamides: In silico, DNA binding, hemolysis and anticancer studies. RSC Adv. 2014, 4, 29629–29641. [Google Scholar] [CrossRef]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug-DNA interactions and their study by UV-Visible, fluorescence spectroscopies and cyclic voltametry. J. Photochem. Photobiol. B 2013, 124, 1–19. [Google Scholar] [CrossRef]
- Radisavljevic, S.; Cocic, D.; Jovanovic, S.; Smit, B.; Petkovic, M.; Milivojevic, N.; Planojevic, N.; Markovic, S.; Petrovic, B. Synthesis, characterization, DFT study, DNA/BSA-binding affinity, and cytotoxicity of some dinuclear and trinuclear gold(III) complexes. J. Biol. Inorg. Chem. 2019, 24, 1057–1076. [Google Scholar] [CrossRef]
- Alsaeedi, M.S.; Babgi, B.A.; Hussien, M.A.; Abdellattif, M.H.; Humphrey, M.G. DNA-Binding and Anticancer Activity of Binuclear Gold(I) Alkynyl Complexes with a Phenanthrenyl Bridging Ligand. Molecules 2020, 25, 1033. [Google Scholar] [CrossRef] [PubMed]
- Dimiza, F.; Perdih, F.; Tangoulis, V.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Interaction of copper(II) with the non-steroidal anti-inflammatory drugs naproxen and diclofenac: Synthesis, structure, DNA- and albumin-binding. J. Inorg. Biochem. 2011, 105, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, C.K.; Sung, C.M.; Zimmerman, J.P.; Hill, D.T.; Mong, S.; Crooke, S.T. Interactions of gold coordination-complexes with DNA. Biochem. Pharmacol. 1986, 35, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, T.; Valletta, E.; Ferino, G.; Isaia, F.; Pani, A.; Vascellari, S.; Castellano, C.; Demartin, F.; Cabiddu, M.G.; Cadoni, E. Novel coumarins and related copper complexes with biological activity: DNA binding, molecular docking and in vitro antiproliferative activity. J. Inorg. Biochem. 2017, 177, 101–109. [Google Scholar] [CrossRef]




| Complex | H6 | H5 | H4, H3 | NCH2N (AB System) | NCH2P |
|---|---|---|---|---|---|
| [Au(Spy)(PTA)] | 8.14 (dt) | 6.86 (ddd) | 7.35 (m) | 4.52 and 4.34 | 4.35 (s) |
| [Cu{Au(Spy)(PTA)}2]PF6 (1) | 8.42 (br s) | 7.09 (brs) | 7.54 (br s) | 4.43 and 4.34 | 4.25 (s) |
| [Zn{Au(PTA)(Spy)}2](NO3)2 (2) | 8.13 (d) | 6.87 (ddd) | 7.36 (td), 7.29 (d) | 4.53 and 4.35 | 4.35 (s) |
| [Zn{Au(PTA)(Spy)}2]Cl2 (3) | 8.13 (d) | 6.87 (ddd) | 7.36 (td), 7.30 (d) | 4.53 and 4.35 | 4.35 (s) |
| Complex | Spy | ||||
|---|---|---|---|---|---|
| υ(C=C), υ(C=N) | Ring Deformation | PTA | Au-S | Au-P | |
| [Au(Spy)(PTA)] | 1648, 1570, 1548 | 1121, 783, 625 | 1440, 1409 | 289 | 271 |
| 1 | 1635, 1570(sh), 1550 1583 | 1127, 756, 649 | 1452, 1412 | 283 | 270 |
| 2 | 1640, 1570(sh), 1548 1590 | 1137, 758, 648 | 1451, 1410 | 288 | 271 |
| 3 | 1640, 1571(sh), 1548 1589 | 1138, 759, 647 | 1449, 1410 | 292 | 276 |
| Complex | KSV (M−1) | Kb (M−1) | n |
|---|---|---|---|
| [Cu{Au(Spy)(PTA)}2]PF6 (1) | 5.73 × 103 | 1.37 × 104 | 1.11 |
| [Zn{Au(PTA)(Spy)}2](NO3)2 (2) | 1.76 × 103 | 1.71 × 103 | 0.95 |
| Complex | IC50 (μM) [a] | ||
|---|---|---|---|
| Caco-2/PD7 | Caco-2/TC7 | logP | |
| [Au(Spy)(PTA)] | 2.64 ± 0.25 | 2.03 ± 0.02 | |
| [Cu{Au(Spy)(PTA)}2]PF6 (1) | 0.16 ± 0.02 | 0.13 ± 0.07 | 0.84 |
| [Zn{Au(PTA)(Spy)}2](NO3)2 (2) | 2.18 ± 0.24 | 1.94 ± 0.81 | 0.47 |
| Auranofin | 1.8 ± 0.1 | 2.1 ± 0.4 | −2.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atrián-Blasco, E.; Sáez, J.; Rodriguez-Yoldi, M.J.; Cerrada, E. Heteronuclear Complexes with Promising Anticancer Activity against Colon Cancer. Biomedicines 2024, 12, 1763. https://doi.org/10.3390/biomedicines12081763
Atrián-Blasco E, Sáez J, Rodriguez-Yoldi MJ, Cerrada E. Heteronuclear Complexes with Promising Anticancer Activity against Colon Cancer. Biomedicines. 2024; 12(8):1763. https://doi.org/10.3390/biomedicines12081763
Chicago/Turabian StyleAtrián-Blasco, Elena, Javier Sáez, Maria Jesús Rodriguez-Yoldi, and Elena Cerrada. 2024. "Heteronuclear Complexes with Promising Anticancer Activity against Colon Cancer" Biomedicines 12, no. 8: 1763. https://doi.org/10.3390/biomedicines12081763
APA StyleAtrián-Blasco, E., Sáez, J., Rodriguez-Yoldi, M. J., & Cerrada, E. (2024). Heteronuclear Complexes with Promising Anticancer Activity against Colon Cancer. Biomedicines, 12(8), 1763. https://doi.org/10.3390/biomedicines12081763







