Ceruloplasmin and Lipofuscin Serum Concentrations Are Associated with Presence of Hypertrophic Cardiomyopathy
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Laboratory Measurements
2.3. Statistical Analysis
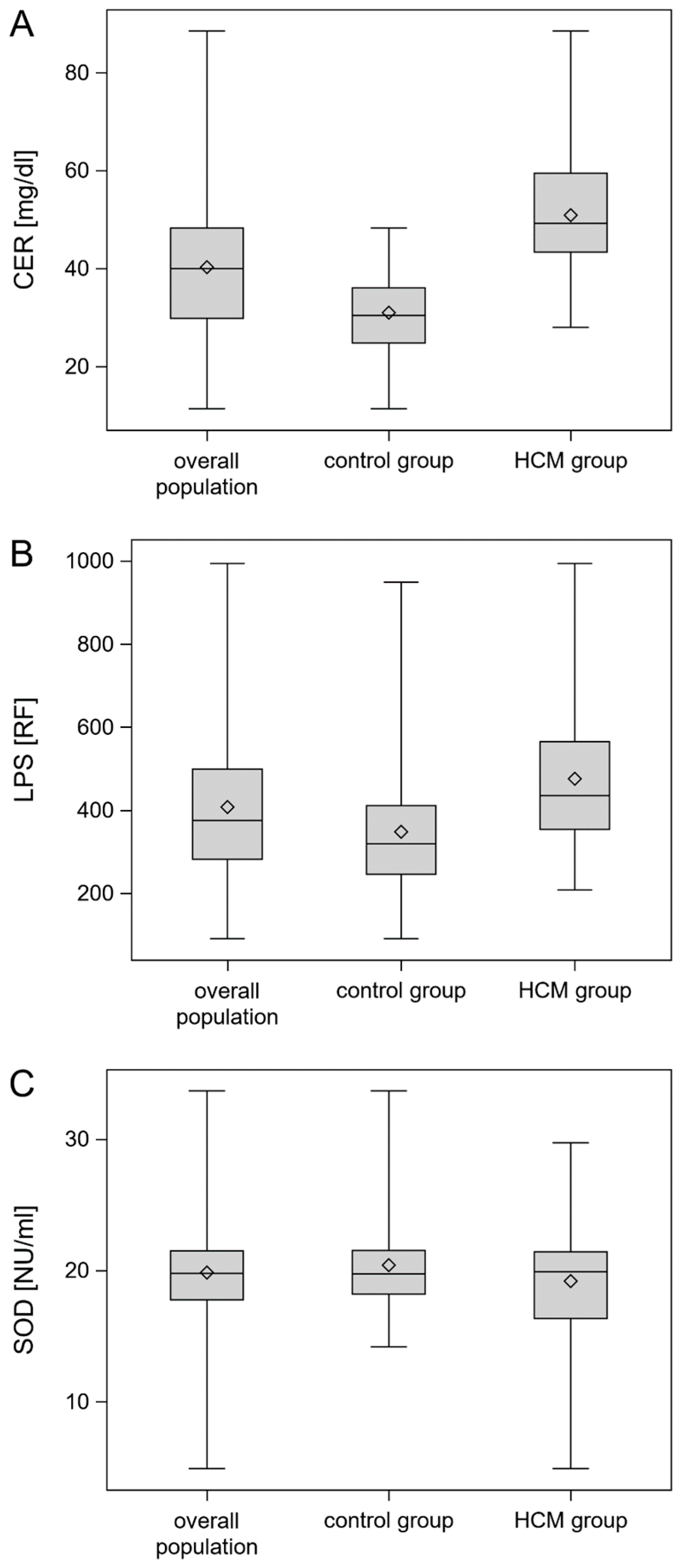
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maron, B.J.; Olivotto, I.; Spirito, P.; Casey, S.A.; Bellone, P.; Gohman, T.E.; Graham, K.J.; Burton, D.A.; Cecchi, F. Epidemiology of Hypertrophic Cardiomyopathy-Related Death: Revisited in a Large Non-Referral-Based Patient Population. Circulation 2000, 102, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Wijnker, P.J.M.; Sequeira, V.; Kuster, D.W.D.; Van Der Velden, J. Hypertrophic Cardiomyopathy: A Vicious Cycle Triggered by Sarcomere Mutations and Secondary Disease Hits. Antioxid. Redox Signal. 2019, 31, 318–358. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden Deaths in Young Competitive Athletes: Analysis of 1866 Deaths in the United States, 1980-2006. Circulation 2009, 119, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Arbelo, E.; Barriales-Villa, R.; Basso, C.; Biagini, E.; Blom, N.A.; De Boer, R.A.; et al. 2023 ESC Guidelines for the Management of Cardiomyopathies: Developed by the Task Force on the Management of Cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, L.B.; Dela, F.; Koch, J.; Hansen, C.N.; Leifsson, P.S.; Yokota, T. Impaired Cardiac Mitochondrial Oxidative Phosphorylation and Enhanced Mitochondrial Oxidative Stress in Feline Hypertrophic Cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1237–H1247. [Google Scholar] [CrossRef] [PubMed]
- Dimitrow, P.P.; Undas, A.; Wołkow, P.; Tracz, W.; Dubiel, J.S. Enhanced Oxidative Stress in Hypertrophic Cardiomyopathy. Pharmacol. Rep. 2009, 61, 491–495. [Google Scholar] [CrossRef]
- Nakamura, K.; Kusano, K.F.; Matsubara, H.; Nakamura, Y.; Miura, A.; Nishii, N.; Banba, K.; Nagase, S.; Miyaji, K.; Morita, H.; et al. Relationship between Oxidative Stress and Systolic Dysfunction in Patients with Hypertrophic Cardiomyopathy. J. Card. Fail. 2005, 11, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Szyguła-Jurkiewicz, B.; Szczurek-Wasilewicz, W.; Osadnik, T.; Frycz-Kurek, A.M.; Macioł-Skurk, K.; Małyszek-Tumidajewicz, J.; Skrzypek, M.; Romuk, E.; Gąsior, M.; Banach, M.; et al. Oxidative Stress Markers in Hypertrophic Cardiomyopathy. Medicina 2021, 58, 31. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takimoto, E.; Kass, D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2007, 49, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Oxidative Stress in Cell Death and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9030563. [Google Scholar] [CrossRef]
- Reid, A.; Miller, C.; Farrant, J.P.; Polturi, R.; Clark, D.; Ray, S.; Cooper, G.; Schmitt, M. Copper Chelation in Patients with Hypertrophic Cardiomyopathy. Open Hear. 2022, 9, e001803. [Google Scholar] [CrossRef]
- Shiva, S.; Wang, X.; Ringwood, L.A.; Xu, X.; Yuditskaya, S.; Annavajjhala, V.; Miyajima, H.; Hogg, N.; Harris, Z.L.; Gladwin, M.T. Ceruloplasmin Is a NO Oxidase and Nitrite Synthase That Determines Endocrine NO Homeostasis. Nat. Chem. Biol. 2006, 2, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, R.; Budde, H.; Zhazykbayeva, S.; Herwig, M.; Sieme, M.; Delalat, S.; Mostafi, N.; Gömöri, K.; Tangos, M.; Jarkas, M.; et al. Stress Activated Signalling Impaired Protein Quality Control Pathways in Human Hypertrophic Cardiomyopathy. Int. J. Cardiol. 2021, 344, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Lillo, R.; Graziani, F.; Franceschi, F.; Iannaccone, G.; Massetti, M.; Olivotto, I.; Crea, F.; Liuzzo, G. Inflammation across the Spectrum of Hypertrophic Cardiac Phenotypes. Heart Fail. Rev. 2023, 28, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Ellims, A.H.; Beale, A.L.; Taylor, A.J.; Murphy, A.; Dart, A.M. Systemic Inflammation Is Associated with Myocardial Fibrosis, Diastolic Dysfunction, and Cardiac Hypertrophy in Patients with Hypertrophic Cardiomyopathy. Am. J. Transl. Res. 2017, 9, 5063–5073. [Google Scholar]
- Kakimoto, Y.; Okada, C.; Kawabe, N.; Sasaki, A.; Tsukamoto, H.; Nagao, R.; Osawa, M. Myocardial Lipofuscin Accumulation in Ageing and Sudden Cardiac Death. Sci. Rep. 2019, 9, 3304. [Google Scholar] [CrossRef] [PubMed]
- Ranjbarvaziri, S.; Kooiker, K.B.; Ellenberger, M.; Fajardo, G.; Zhao, M.; Vander Roest, A.S.; Woldeyes, R.A.; Koyano, T.T.; Fong, R.; Ma, N.; et al. Altered Cardiac Energetics and Mitochondrial Dysfunction in Hypertrophic Cardiomyopathy. Circulation 2021, 144, 1714–1731. [Google Scholar] [CrossRef]
- Jóźwiak, J.J.; Studziński, K.; Tomasik, T.; Windak, A.; Mastej, M.; Catapano, A.L.; Ray, K.K.; Mikhailidis, D.P.; Toth, P.P.; Howard, G.; et al. The Prevalence of Cardiovascular Risk Factors and Cardiovascular Disease among Primary Care Patients in Poland: Results from the LIPIDOGRAM2015 Study. Atheroscler. Suppl. 2020, 42, e15–e24. [Google Scholar] [CrossRef]
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 ESC Guidelines on Diagnosis and Management of Hypertrophic Cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [CrossRef]
- Richterich, R.; Gautier, E.; Stillhart, H.; Rossi, E. The Heterogeneity of Caeruloplasmin Nd the Enzymatic Defect in Wilson’s Disease. Helv. Paediatr. Acta 1960, 15, 424–436. [Google Scholar] [PubMed]
- Oyanagui, Y. Reevaluation of Assay Methods and Establishment of Kit for Superoxide Dismutase Activity. Anal. Biochem. 1984, 142, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, M.; Miura, T.; Mizutani, K.; Aibara, K. Fluorescent Substances in Mouse and Human Sera as a Parameter of in Vivo Lipid Peroxidation. Biochim. Biophys. Acta 1985, 834, 196–204. [Google Scholar] [PubMed]
- Volchegorskiĭ, I.A.; Shaposhnik, I.I.; Alekseev, E.N.; Kharchenkova, N.V. Levels of lipid peroxidation products and ceruloplasmin in blood as characteristics of tolerance to physical load in hypertrophic cardiomyopathy. Klin. Lab. Diagn. 2002, 2, 11–13. [Google Scholar]
- Volchegorskiĭ, I.A.; Shaposhnik, I.I.; Alekseev, E.N.; Kharchenkova, N.V. Indices of the lipid peroxidation--antioxidant defense system as markers of chronic cardiac failure in cardiomyopathies. Klin. Med. 2003, 81, 26–28. [Google Scholar]
- Potluri, R.; Roberts, N.; Miller, C.; Brooks, N.; Ray, S.; Cooper, G.; Schmitt, M. Impaired Copper Homeostasis in Patients with Hypertrophic Cardiomyopathy. Eur. Heart J. 2012, 33, 741–742. [Google Scholar]
- Maung, M.T.; Carlson, A.; Olea-Flores, M.; Elkhadragy, L.; Schachtschneider, K.M.; Navarro-Tito, N.; Padilla-Benavides, T. The Molecular and Cellular Basis of Copper Dysregulation and Its Relationship with Human Pathologies. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21810. [Google Scholar] [CrossRef] [PubMed]
- Franssen, C.; Chen, S.; Unger, A.; Korkmaz, H.I.; De Keulenaer, G.W.; Tschöpe, C.; Leite-Moreira, A.F.; Musters, R.; Niessen, H.W.M.; Linke, W.A.; et al. Myocardial Microvascular Inflammatory Endothelial Activation in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2016, 4, 312–324. [Google Scholar] [CrossRef]
- Radi, R. Protein Tyrosine Nitration: Biochemical Mechanisms and Structural Basis of Functional Effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Hammadah, M.; Fan, Y.; Wu, Y.; Hazen, S.L.; Tang, W.H.W. Prognostic Value of Elevated Serum Ceruloplasmin Levels in Patients with Heart Failure. J. Card. Fail. 2014, 20, 946–952. [Google Scholar] [CrossRef]
- Nozynski, J.; Zakliczynski, M.; Konecka-Mrowka, D.; Zakliczynska, H.; Pijet, M.; Zembala-Nozynska, E.; Lange, D.; Zembala, M. Advanced Glycation End Products and Lipofuscin Deposits Share the Same Location in Cardiocytes of the Failing Heart. Exp. Gerontol. 2013, 48, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Wang, H.J.; Tan, Y.Z.; Wang, Y.L.; Yu, S.N.; Li, Z.H. Reducing lipofuscin accumulation and cardiomyocytic senescence of aging heart by enhancing autophagy. Exp. Cell Res. 2021, 403, 112585. [Google Scholar] [CrossRef] [PubMed]
- Radu, R.I.; Bold, A.; Pop, O.T.; Mălăescu, D.G.; Gheorghişor, I.; Mogoantă, L. Histological and Immunohistochemical Changes of the Myocardium in Dilated Cardiomyopathy. Rom. J. Morphol. Embryol. = Rev. Roum. Morphol. Embryol. 2012, 53, 269–275. [Google Scholar]
- Korovila, I.; Hugo, M.; Castro, J.P.; Weber, D.; Höhn, A.; Grune, T.; Jung, T. Proteostasis, Oxidative Stress and Aging. Redox Biol. 2017, 13, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Brunk, U.T.; Jones, C.B.; Sohal, R.S. A Novel Hypothesis of Lipofuscinogenesis and Cellular Aging Based on Interactions between Oxidative Stress and Autophagocytosis. Mutat. Res. 1992, 275, 395–403. [Google Scholar] [CrossRef]
- Unno, K.; Isobe, S.; Izawa, H.; Cheng, X.W.; Kobayashi, M.; Hirashiki, A.; Yamada, T.; Harada, K.; Ohshima, S.; Noda, A.; et al. Relation of Functional and Morphological Changes in Mitochondria to Myocardial Contractile and Relaxation Reserves in Asymptomatic to Mildly Symptomatic Patients with Hypertrophic Cardiomyopathy. Eur. Heart J. 2009, 30, 1853–1862. [Google Scholar] [CrossRef]

| General Population N = 182 # | Control Group N = 97 | Hypertrophic Cardiomyopathy Group N = 85 | p * | |
|---|---|---|---|---|
| Baseline data | ||||
| Age, years | 53.0 (40–63) | 52.0 (40–63) | 52.5 (40–63.5) | 0.9271 |
| Female, n (%) | 107 (58.8) | 57 (58.8) | 50 (58.8) | 0.9934 |
| SBP, mmHg | 130 (120–136) | 130.0 (120.0–140.0) | 125.0 (120.0–130.00) | 0.0397 * |
| DBP, mmHg | 80 (70–83) | 80.0 (75.0–88.0) | 80.0 (70.0–80.0) | 0.0002 * |
| Coronary heart disease, n (%) | 33 (18.1) | 13 (13.4) | 20 (23.5) | 0.0769 |
| Hypertension, n (%) | 94 (51.6) | 52 (53.6) | 42 (49.4) | 0.5719 |
| Type 2 diabetes, n (%) | 3 (1.6) | 0 (0) | 3 (3.5) | 0.1374 |
| Hypercholesterolemia, n (%) | 102 (56) | 51 (52.6) | 34 (65.4) | 0.1322 |
| BMI, kg/m2 | 28.3 (4.3) | 28.3 (4.1) | 28.4 (4.5) | 0.8286 |
| HBA1c, % | 5.6 (5.2–6.4) | 5.5 (5.2–6.0) | 5.6 (5.1–6.1) | 0.9779 |
| Parameter | Control Group N = 97 # | Hypertrophic Cardiomyopathy Group N = 52 | p * |
|---|---|---|---|
| SOD | 19.7 (18.2–21.5) | 19.89 (16.3–21.4) | 0.1962 |
| CER | 30.4 (24.8–36.0) | 49.2 (43.3–59.5) | <0.0001 * |
| LPS | 319.0 (246.0–411.0) | 435.05 (353.8–565.0) | <0.0001 * |
| Parameters | Hypertrophic Cardiomyopathy Group N = 85 # | Parameters | Hypertrophic Cardiomyopathy Group N = 85 |
|---|---|---|---|
| HCM with LVOT obstruction, n (%) | 37 (43.5) | TAPSE, mm | 22.0 (21.0–26.0) |
| SAM, n (%) | 28 (32.9) | RVSP, mmHg | 15.0 (1.0–25.0) |
| Alcohol septum ablation, n (%) | 8 (9.4) | WBC, ×109/L | 6.3 (5.3–7.3) |
| Septal myectomy, n (%) | 4 (4.7) | Hemoglobin, mmol/L | 8.9 (8.2–9.4) |
| Coronary heart disease, n (%) | 20 (23.5) | Creatinine, µmol/L | 87.0 (75.0–106.0) |
| AF, n (%) | 20 (23.5) | Platelets, ×109/L | 203.0 (184.0–245.0) |
| nsVT present, n (%) | 43 (50.6) | Total bilirubin, µmol/L | 10.30 (7.4–12.8) |
| HR mean,/min | 70.00 (63.00–74.00) | Albumin, g/L | 47.0 (45.0–50.0) |
| ICD, n (%) | 31 (36.5) | Uric acid, µmol/L | 375.00 (299.0–438.0) |
| Beta-blokers, n(%) | 76 (89.4) | Potassium, µmol/L | 4.50 (4.2–4.7) |
| Cordarone, n (%) | 10 (11.8) | Sodium, mmol/L | 141.0 (139.0–142.0) |
| ACEI/ARB, n (%) | 39 (45.9) | Fibrinogen, mg/dL | 338.0 (291.0–387.0) |
| MRA, n (%) | 19 (22.4) | AST, U/L | 23.0 (19.0–31.0) |
| Verapamil, n (%) | 23 (27.1) | ALT, U/L | 23.0 (16.0–35.0) |
| Statin, n (%) | 45 (52.9) | ALP, U/L | 73.0 (58.0–89.0) |
| 6MWT, n (%) | 510.0 (440.0–593.8) | GGTP, U/L | 33.00 (21.0–60.0) |
| IVS max, mm | 20.0 (17.0–22.0) | Total cholesterol, mmol/L | 4.6 (3.8–5.3) |
| LVEDD, mm | 48.0 (43.0–52.0) | LDL, mmol/L | 2.5 (2.0–3.2) |
| LA, mm | 43.0 (37.0–48.0) | hs-CRP, mg/L | 1.32 (0.8–2.7) |
| LVEF, % | 55.5 (54.5–63.0) | Ferritin, μg/L | 84.0 (37.0–165.0) |
| RVDD, mm | 35.0 (33.0–39.0) | NT-proBNP, pg/mL | 333.0 (134.5–863.5) |
| AUC [±95 CI] | Cutoff | Sensitivity [±95 CI] | Specificity [±95 CI] | Accuracy | |
|---|---|---|---|---|---|
| SOD | 0.556 [0.469–0.643] | <16.74 | 0.34 [0.24–0.45] | 0.94 [0.87–0.98] | 0.66 [0.59–0.73] |
| CER | 0.924 [0.887–0.960] | ≥40.83 | 0.84 [0.74–0.91] | 0.88 [0.79–0.93] | 0.86 [0.80–0.91] |
| LPS | 0.740 [0.669–0.812] | ≥389.6 | 0.66 [0.55–0.76] | 0.72 [0.62–0.81] | 0.69 [0.62–0.76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smyła-Gruca, W.; Szczurek-Wasilewicz, W.; Skrzypek, M.; Romuk, E.; Karmański, A.; Jurkiewicz, M.; Gąsior, M.; Osadnik, T.; Banach, M.; Jóźwiak, J.J.; et al. Ceruloplasmin and Lipofuscin Serum Concentrations Are Associated with Presence of Hypertrophic Cardiomyopathy. Biomedicines 2024, 12, 1767. https://doi.org/10.3390/biomedicines12081767
Smyła-Gruca W, Szczurek-Wasilewicz W, Skrzypek M, Romuk E, Karmański A, Jurkiewicz M, Gąsior M, Osadnik T, Banach M, Jóźwiak JJ, et al. Ceruloplasmin and Lipofuscin Serum Concentrations Are Associated with Presence of Hypertrophic Cardiomyopathy. Biomedicines. 2024; 12(8):1767. https://doi.org/10.3390/biomedicines12081767
Chicago/Turabian StyleSmyła-Gruca, Wiktoria, Wioletta Szczurek-Wasilewicz, Michał Skrzypek, Ewa Romuk, Andrzej Karmański, Michał Jurkiewicz, Mariusz Gąsior, Tadeusz Osadnik, Maciej Banach, Jacek J. Jóźwiak, and et al. 2024. "Ceruloplasmin and Lipofuscin Serum Concentrations Are Associated with Presence of Hypertrophic Cardiomyopathy" Biomedicines 12, no. 8: 1767. https://doi.org/10.3390/biomedicines12081767








