The Possible Pathophysiological Role of Pancreatic Stone Protein in Sepsis and Its Potential Therapeutic Implication
Abstract
1. Introduction
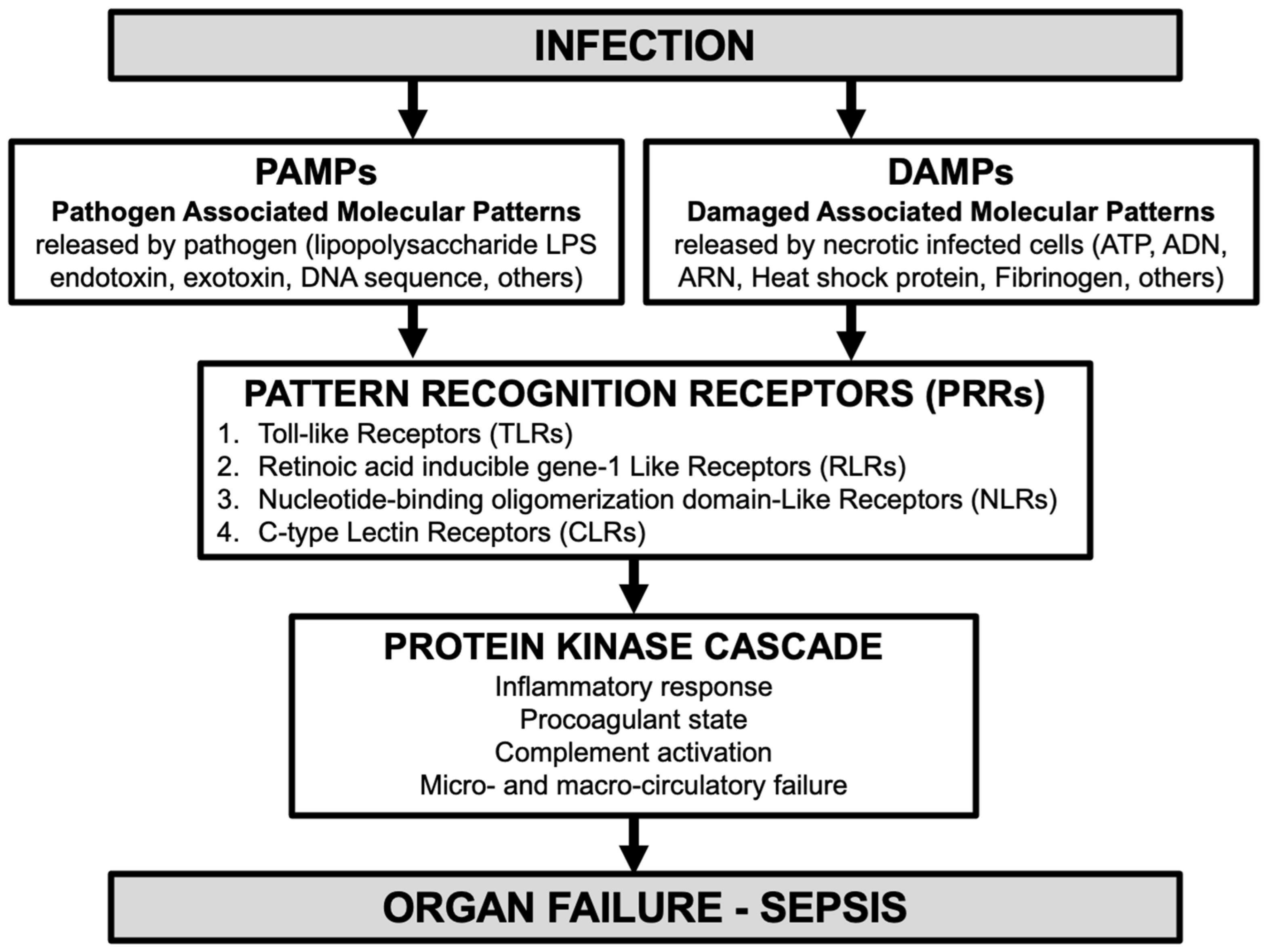
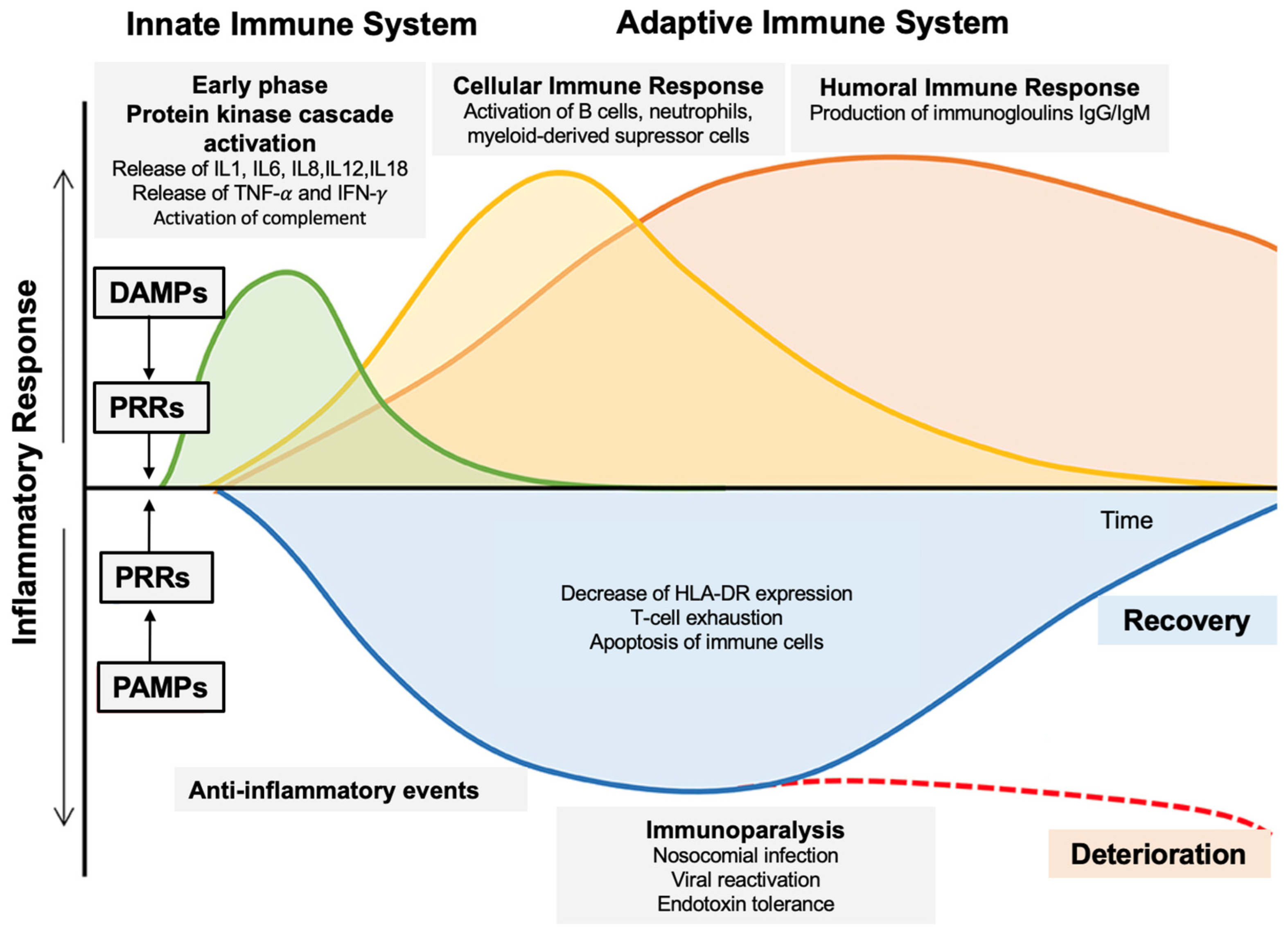
1.1. Role of Innate Immunity in Sepsis Pathophysiology [8]
1.2. Targeting Innate Immunity for Sepsis Treatment [9]
1.3. Pancreatic Stone Protein (PSP)
2. Review Objectives and Methods
3. Results
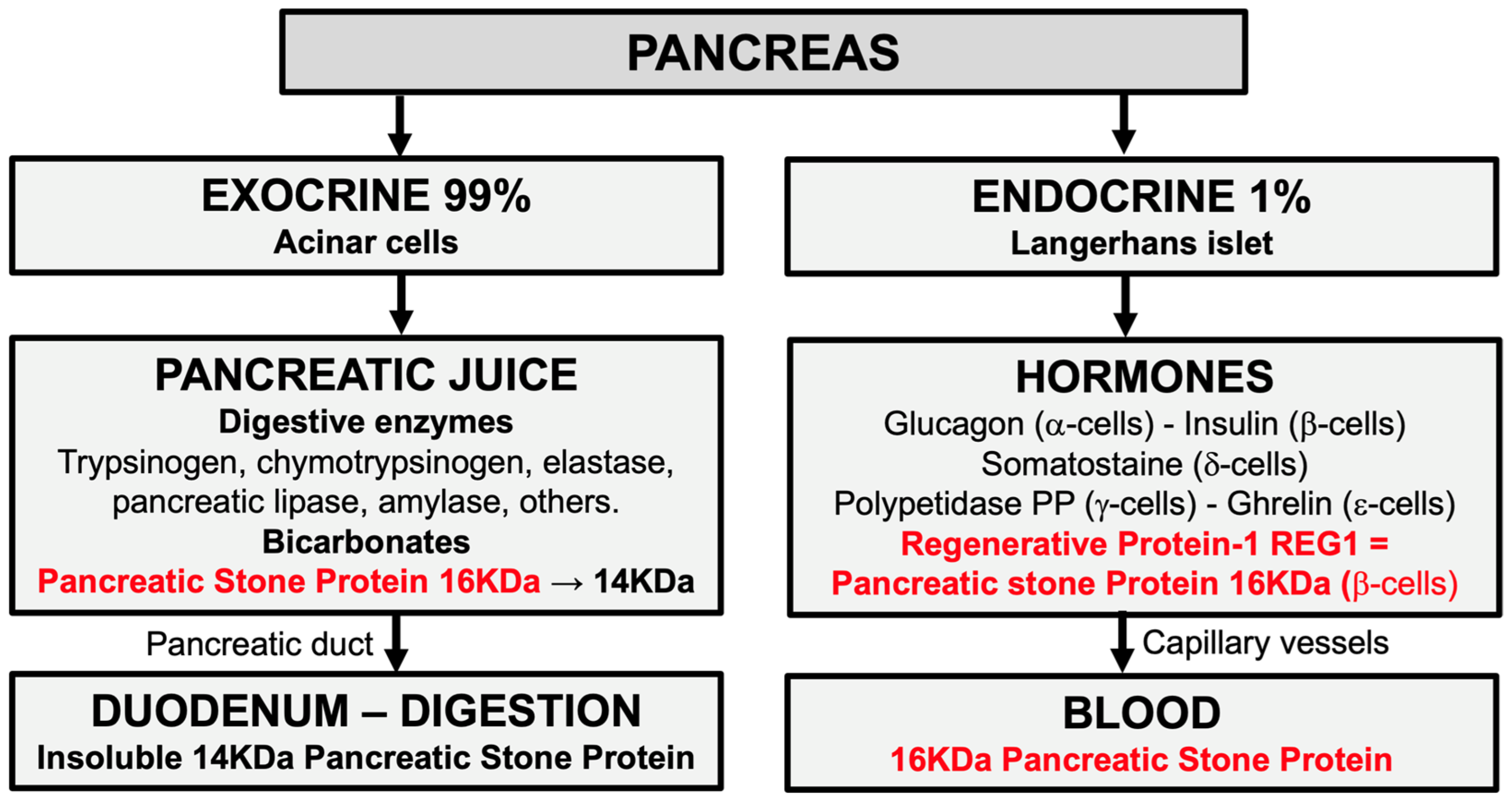
3.1. Definition and Origin of Pancreatic Stone Protein (PSP)
3.2. Physiology of Pancreatic Stone Protein (PSP)
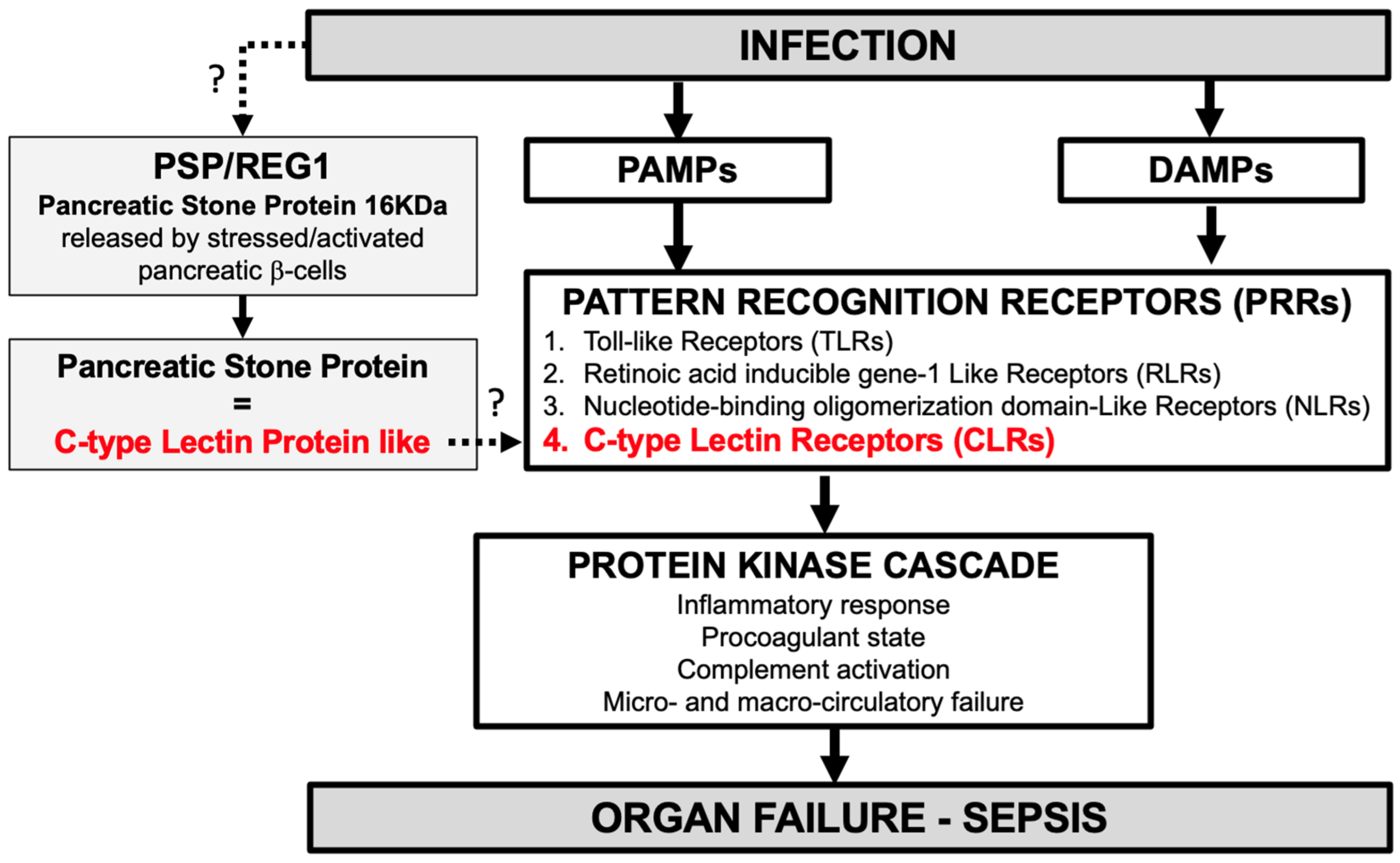
3.3. Pathophysiology of Pancreatic Stone Protein (PSP) in Infection and Sepsis [15]
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singer, M.; Deutschmann, C.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, G.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Roberts, D.; Wood, K.E.; Light, B.; Parrillo, J.E.; Sharma, S.; Suppes, R.; Feinstein, D.; Zanotti, S.; Taiberg, L.; et al. Duration of Hypotension Before Initiation of Effective Antimicrobial Therapy is the Critical Determinant of Survival in Human Septic Shock. Crit. Care Med. 2006, 34, 1589–1596. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, G.M.; French, G.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.V.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.; Giroir, B.; Randolph, A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Schlapbach, L.J.; Watson, R.S.; Sorce, L.R.; Argent, A.C.; Menon, K.; Hall, M.W.; Akech, S.; Albers, D.J.; Alpern, E.R.; Balamuth, F.; et al. International Consensus Criteria for Pediatric Sepsis and Septic Shock. JAMA 2024, 331, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Peters, M.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schlapbach, L.J.; Tasker, R.C.; Argent, A.C.; et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020, 46, 10–67. [Google Scholar] [CrossRef] [PubMed]
- Culver, A.; Leone, M. Physiopathologie du sepsis et du choc septique. In Manuel D’infectiologie Appliquée en Anesthésie Réanimation et Médecine Péri-Opératoire; Presses universitaires François-Rabelais: Tours, France, 2021; pp. 43–50. [Google Scholar]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis—Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.; Laterre, P.; Francois, B.; LaRosa, S.P.; Angus, D.C.; Mira, J.P.; Wittebole, X.; Dugernier, T.; Perrotin, D.; Tidswell, M.; et al. Effet of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: The ACCESS randomized trial. JAMA 2013, 309, 1154–1162. [Google Scholar] [CrossRef]
- Farooq, M.; Batool, M.; Suk Kim, M.; Choi, S. Toll-like Receptors as a Therapeutic Target in the Era of Immunotherapies. Front. Cell Dev. Biol. 2021, 9, 756315. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Michaela, G.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 9, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Geddes, K.; Magalhaes, J.; Girardin, S. Unleashing the therapeutic potential of NOD-like receptors. Nat. Rev. Drug Discov. 2009, 8, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Mnich, M.; van Dalen, R.; van Sorge, N. C-Type Lectin Receptors in Host Defense Against Bacterial Pathogens. Front. Cell Infect. Microbiol. 2020, 10, 309. [Google Scholar] [CrossRef] [PubMed]
- Ventura, F.; Eggimann, P.; Daix, T.; François, B.; Pugin, J. Pancreatic stone protein measurement to screen and diagnose sepsis in the context of the Surviving Sepsis Campaign recommendations. Med. Res. Arch. 2024, 11, 1–19. [Google Scholar] [CrossRef]
- Prazak, J.; Irincheeva, I.; Llewelyn, M.; Eggimann, P.; Que, Y.-A. Accuracy of Pancreatic Stone Protein for the diagnosis of infection in hospitalized adults. A systematic reviwe and individual patient level meta-analysis. Crit. Care 2021, 25, 182. [Google Scholar] [CrossRef] [PubMed]
- Keel, M.; Haerter, L.; Reding, T.; Sun, L.K.; Hersberger, M.; Seifert, B.; Bimmler, D.; Graf, R. Pancreatic stone protein is highly increased during posttraumatic sepsis and activates neutrophil granulocytes. Crit. Care Med. 2009, 37, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Llewlyn, M.; Berger, M.; Gregory, M.; Ramaiah, R.; Taylor, A.L.; Curdt, I.; Lajaunias, F.; Graf, R.; Blincko, S.J.; Drage, S.; et al. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Crit. Care 2013, 17, R60. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.J.; Niggemann, P.; Buehler, P.K.; Lehner, F.; Schweizer, R.; Rittirsch, D.; Fuchs, N.; Waldner, M.; Steiger, P.; Giovanoli, P.; et al. Pancreatic Stone Protein Predicts Sepsis in Severely Burned Patients Irrespective of Trauma Severity A Monocentric Observational Study. Ann. Surgey 2020, 274, e1179–e1186. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.J.; Csordas, A.; Falk, V.; Slankamenac, K.; Rudiger, A.; Schönrath, F.; Cetina Biefer, H.R.; Starck, C.T.; Graf, R. Pancreatic Stone Protein Predicts Postoperative Infection in Cardiac Surgey Patients Irrespective of Cardiopulmonary Bypass or Surgical Technique. PLoS ONE 2015, 10, e0120276. [Google Scholar] [CrossRef]
- Pugin, J.; Daix, T.; Pagani, J.; Morri, D.; Giacomucci, A.; Dequin, P.F.; Guitton, C.; Que, Y.A.; Zani, G.; Brealey, D.; et al. Serial measurement of pancreatic stone protein for the early detection of sepsis in intensive care unit patients: A prospective multicentric study. Crit. Care 2021, 25, 151. [Google Scholar] [CrossRef]
- Filippidis, P.; Hovius, L.; Tissot, F.; Orasch, C.; Flückiger, U.; Siegemud, M.; Pagani, J.L.; Eggimann, P.; Marchetti, O.; Lamoth, F.; et al. Serial monitoring of pancreatic stone protein for the detection of sepsis in intensive care unit patients with complicated abdominal surgery: A prospective, longitudinal cohort study. J. Crit. Care 2024, 82, 154722. [Google Scholar] [CrossRef]
- Gukasjan, R.; Raptis, D.; Schulz, H.-U.; Halangk, W.; Graf, R. Pancreatic stone protein predicts outcome in patients with peritonitis in the ICU. Crit. Care Med. 2013, 41, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.A.; Guessous, I.; Dupuis-Lozeron, E.; Rodrigues Alves de Oliveira, C.; Ferreira Oliveira, C.; Graf, R.; Seematter, G.; Revelly, J.P.; Pagani, J.L.; Liaudet, L.; et al. Prognostication of mortality in critically ill patients with severe infections. Chest 2015, 148, 674–682. [Google Scholar] [CrossRef]
- de Guardinia-Romualdo, L.G.; Berger, M.; Jimenéz-Santos, E.; Rebollo-Acebes, S.; Jimenez-sanchez, R.; Esteban-Torrella, P.; Hernando-Holgado, A.; Ortin-Freire, A.; Albaladejo-Oton, M.D. Pancreatic stone protein and soluble CD25 for infection and sepsis in an Emergency Department. Eur. J. Clin. Investig. 2017, 47, 297–304. [Google Scholar] [CrossRef]
- Guadiana-Romualdo, L.; Albaladejo-Oton, M.; Berger, M.; Jimenez-santos, E.; Jimenez-Sanchez, R.; Esteban-Torrella, P.; Rebello-Acebes, S.; Hernando-Holgado, A.; Ortin-Freire, A.; Trujillo-Santos, J. Prognostic performance of pancreatic stone protein in critically ill patients with sepsis. Biomark. Med. 2019, 13, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Zuercher, P.; Moser, A.; de Guadiana-Romualdo, L.; Llewelyn, M.J.; Graf, R.; Reding, T.; Eggimann, P.; Que, Y.A.; Prazak, J. Discriminative performance of pancreatic stone protein in predicting ICU mortality and infection severity in adult patients with infection: A systematic review and individual patient level meta-analysis. Infection 2023, 51, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Boeck, L.; Graf, R.; Eggimann, P.; Pargger, H.; Raptis, D.A.; Smyrnios, N.; Thakkar, N.; Siegemund, M.; Rakic, J.; Tamm, M.; et al. Pancreatic stone protein: A marker of organ failure and outcome in ventilator-associated pneumonia. Chest 2011, 140, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.A.; Delodder, F.; Guessous, I.; Graf, R.; Bain, M.; Calandra, T.; Liaudet, L.; Eggimann, P. Pancreatic stone protein as an early biomarker predicting mortality in a prospective cohort of patients with sepsis requiring ICU management. Crit. Care 2012, 16, R114. [Google Scholar] [CrossRef]
- Hu, P.; Lu, Y.H.; Deng, W.; Li, Q.; Zhao, N.; Shao, Q.; Wu, L.; Wang, X.Z.; Qian, K.J.; Liu, F. The critical role of pancreatic stone protein/regenerating protein in sepsis-related multiorgan failure. Front. Med. 2023, 10, 1172529. [Google Scholar] [CrossRef]
- Graf, R. Pancreatic stone protein—Sepsis and the riddles of the exocrine pancreas. Pancreatology 2020, 20, 301–304. [Google Scholar] [CrossRef]
- De Caro, A.; Lohse, J.; Sarles, H. Characterization of a protein isolated from pancreatic calculi of men suffering from chronic calcifying pancreatitis. Biochem. Biophys. Res. Commun. 1979, 87, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Multinger, L.; De Caro, A.; Lombardo, D.; Campese, D.; Sarles, H. Pancreatic stone protein, a phosphoprotein which inhibits calcium carbonate precipitation from human pancreatic juice. Biochem. Biophys. Res. Commun. 1983, 110, 69–74. [Google Scholar]
- De Reggi, M.; Gharib, B.; Patard, L.; Stoven, V. Lithostathine, the presumed pancreatic stone inhibitor, does not interact specifically with calcium carbonate crystals. J. Biol. Chem. 1998, 273, 4967–4971. [Google Scholar] [CrossRef] [PubMed]
- Terazono, K.; Yamamoto, H.; Takasawa, S.; Shiga, K.; Yonemura, Y.; Tochino, Y.; Okamoto, H. A novel gene activated in regenerating islets. J. Biol. Chem. 1988, 263, 2111–2114. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Yonekura, T.; Terazono, K.; Yamamoto, H.; Okamoto, H. Complete nucleotide sequence of human reg gene and its expression in normal and tumoral tissues. The reg protein, pancreatic stone protein, and pancreatic thread protein are one and the same product of the gene. J. Biol. Chem. 1990, 265, 7432–7439. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.X.; Hayakawa, T.; Ko, S.; Ishiguro, H.; Kitagawa, M. Pancreatic stone protein/regenerating protein family in pancreatic and gastrointestinal diseases. Intern. Med. 2011, 50, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Reding, T.; Palmiere, C.; Pazhepurackel, C.; Schiesser, M.; Bimmler, D.; Schlegel, A.; Süss, U.; Steiner, S.; Mancina, L.; Seleznik, G.; et al. The pancreas responds to remote damage and systemic stress by secretion of the pancreatic secretory proteins PSP/regI and PAP/regIII. Oncotarget 2017, 8, 30162–30174. [Google Scholar] [CrossRef] [PubMed]
- Schiesser, M.; Bimmler, D.; Frick, T.; Graf, R. Conformational changes of pancreatitis-associated protein (pap) activated by trypsin lead to insoluble protein aggregates. Pancreas 2001, 22, 186–192. [Google Scholar] [CrossRef]
- Hayakawa, T.; Kondo, T.; Shibata, T.; Kitagawa, M.; Sakai, Y.; Sobajima, H.; Tanikawa, M.; Nakae, Y.; Hayakawa, S.; Katsuzaki, T.; et al. Serum pancreatic stone protein in pancreatic diseases. Int. J. Pancreatol. 1993, 13, 97–103. [Google Scholar] [CrossRef]
- Satomura, Y.; Sawabu, N.; Mouri, I.; Yamakawa, O.; Watanabe, H.; Motoo, Y.; Okai, T.; Ito, T.; Kaneda, K.; Okamoto, H. Measurement of serum psp/reg-protein concentration in various diseases with a newly developed enzyme-linked immunosorbent assay. J. Gastroenterol. 1995, 30, 643–650. [Google Scholar] [CrossRef]
- Cummings, R.; McEver, R. C-type Lectins. In Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2009. [Google Scholar]
- Iovanna, J.; Frigerio, J.M.; Dusetti, N.; Ramare, F.; Raibaud, P.; Dagorn, J.C. Lithostathine, an inhibitor of CaCO3 crystal growth in pancreatic juice, induces bacterial aggregation. Pancreas 1993, 8, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Tatemichi, N.; Takahashi, C.; Hayakawa, S.; Shibata, T.; Kitagawa, M.; Sobajima, H.; Nakae, Y. Enzyme immunoassay and characterization of pancreatic stone proteins in human urine. J. Clin. Lab. Anal. 1993, 7, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Costantini, E.; Carlin, M.; Porta, M.; Brizzi, M.F. Type 2 diabetes mellitus and sepsis: State of the art, certainties and missing evidence. Acta Diabetol. 2021, 58, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Nugent, K. Hyperglycemia, Insulin, and Insulin Resistance in Sepsis. Am. J. Med. Sci. 2021, 361, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.; Abreau, D.; Mahadevan, J.; Asada, R.; Kries, K.; Graf, R.; Marshall, B.A.; Hershey, T.; Urano, F. Pancreatic stone protein/regenerating protein is a potential biomarker for endoplasmic reticulum stress in beta cells. Sci. Rep. 2019, 9, 5199. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.J.; Buehler, P.K.; Niggemann, P.; Rittirsch, D.; Schweizer, R.; Waldner, M.; Giovanoli, P.; Cinelli, P.; Reding, T.; Graf, R.; et al. Expression of Pancreatic Stone Protein is Unaffected by Trauma and Subsequent Surgery in Burn Patients. World J. Surg. 2020, 44, 3000–3009. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.J.; Rittirsch, D.; Buehler, P.K.; Schweizer, R.; Giovanoli, P.; Cinelli, P.; Plock, J.A.; Reding, T.; Graf, R. Response of routine inflammatory biomarkers and novel Pancreatic Stone Protein to inhalation injury and its interference with sepsis detection in severely burned patients. Burns 2021, 47, 338–348. [Google Scholar] [CrossRef]
| M. Keel et al., 2009 [17] Human clinical study | PSP activates polymorphonuclear neutrophils and microcirculatory failure. |
| CX. Jin et al., 2011 [37] Literature review | PSP is structurally similar to C-type lectin-like proteins. |
| L. Boeck et al., 2011 [28] Clinical study | PSP was correlated with the SOFA score from ventilator-associated pneumonia (VAP) onset up to day 7. |
| P. Hu et al., 2023 [30] Animal study | After PSP injection in mice, level of inflammatory factors, PMN tissue infiltration, key parameters reflecting organ failure, disease severity score, and mortality were directly correlated with the doses of PSP injected. |
| P. Hu et al., 2023 [30] Clinical study | PSP was correlated with mechanical ventilation, vasopressors administration at admission and during the stay, SOFA score, and renal replacement therapy (RRT). |
| P. Zuercher et al., 2023 [27] Meta-analysis | PSP discriminates mild infection, severe infection, sepsis, and septic shock. The 28-day mortality rate increased continuously for each ascending quartile of PSP level at admission. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura, F.; Tissières, P. The Possible Pathophysiological Role of Pancreatic Stone Protein in Sepsis and Its Potential Therapeutic Implication. Biomedicines 2024, 12, 1790. https://doi.org/10.3390/biomedicines12081790
Ventura F, Tissières P. The Possible Pathophysiological Role of Pancreatic Stone Protein in Sepsis and Its Potential Therapeutic Implication. Biomedicines. 2024; 12(8):1790. https://doi.org/10.3390/biomedicines12081790
Chicago/Turabian StyleVentura, François, and Pierre Tissières. 2024. "The Possible Pathophysiological Role of Pancreatic Stone Protein in Sepsis and Its Potential Therapeutic Implication" Biomedicines 12, no. 8: 1790. https://doi.org/10.3390/biomedicines12081790
APA StyleVentura, F., & Tissières, P. (2024). The Possible Pathophysiological Role of Pancreatic Stone Protein in Sepsis and Its Potential Therapeutic Implication. Biomedicines, 12(8), 1790. https://doi.org/10.3390/biomedicines12081790






