The Humoral Immune Response against Human Endogenous Retroviruses in Celiac Disease: A Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Population, and Enrollment Criteria of the Study
2.2. Blood Cell Separation and Antigens
2.3. ELISA Assays
2.4. Statistical Analysis
3. Results
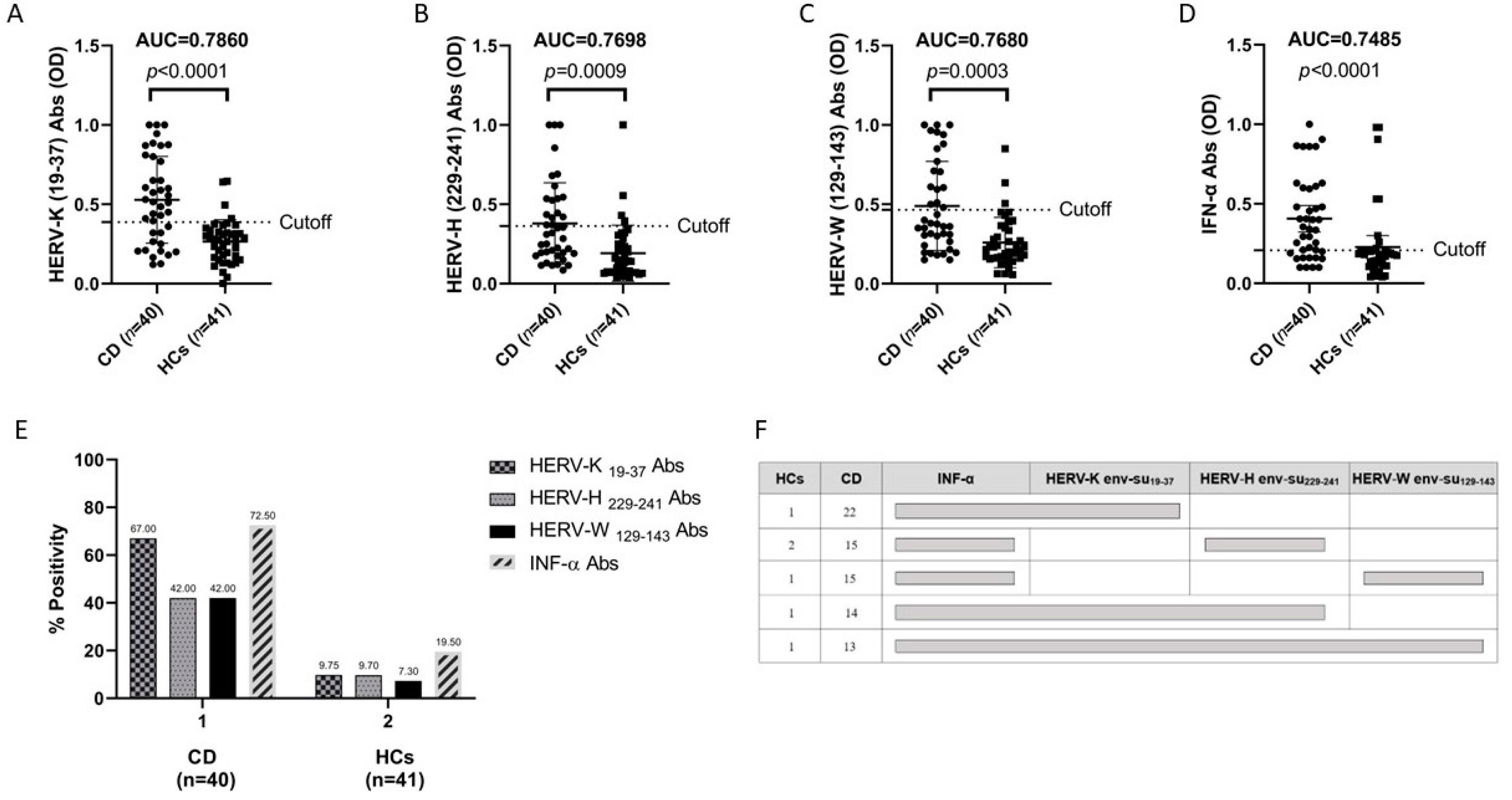
3.1. Antibody Responses to Immunogenic Epitopes of Three Families of HERVs in Patients with Celiac Disease and in HCs
3.2. Anti-HERV Profiles Relate to IFN-Alpha and Possible Synergistic Role to HERV-K, HERV-H, and HERV-W in CD in Comparison to HCs
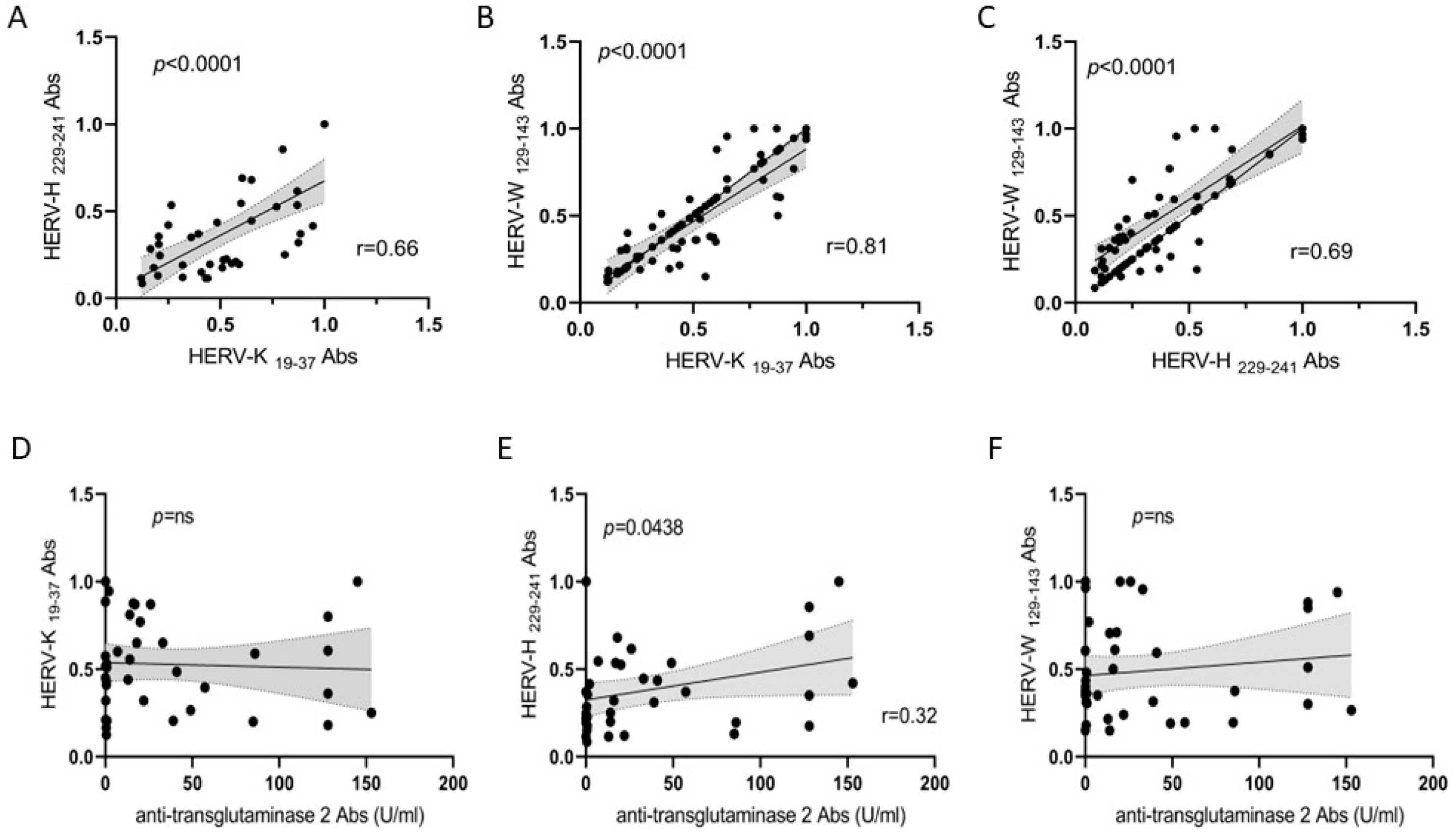
3.3. Correlation Analyses of HERV Families and Anti-TG2 Abs
3.4. Correlation Analysis between HERVs and IFN-α Abs in CD and HCs and between Anti-TG2 Abs and IFN-α in Patients with CD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, Presentation, and Diagnosis of Celiac Disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Iversen, R.; Sollid, L.M. The Immunobiology and Pathogenesis of Celiac Disease. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.F.A.; Rifkind, E.A.; Turner, I.D.; Ferguson, A. Mortality in Celiac Disease. Gastroenterology 1989, 97, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.K.T.; Prior, P.; Lane, M.R.; Pope, D.; Allan, R.N. Malignancy in Coeliac Disease-Effect of a Gluten Free Diet. Gut 1989, 30, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.N.; Gluten, M.M. Major Histocompatibility Complex, and the Small Intestine. A Molecular and Immunobiologic Approach to the Spectrum of Gluten Sensitivity (‘celiac Sprue’). Gastroenterology 1992, 102, 330–354. [Google Scholar] [CrossRef] [PubMed]
- Sollid, L.M.; Thorsby, E. HLA Susceptibility Genes in Celiac Disease: Genetic Mapping and Role in Pathogenesis. Gastroenterology 1993, 105, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Withoff, S.; Li, Y.; Jonkers, I.; Wijmenga, C. Understanding Celiac Disease by Genomics. Trends Genet. 2016, 32, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.K.; Guandalini, S.; Semrad, C.; Kupfer, S.S. A Clinician’s Guide to Celiac Disease HLA Genetics. Am. J. Gastroenterol. 2019, 114, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) Guideline for Coeliac Disease and Other Gluten-Related Disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef] [PubMed]
- Talipova, D.; Smagulova, A.; Poddighe, D. Toll-like Receptors and Celiac Disease. Int. J. Mol. Sci. 2023, 24, 265. [Google Scholar] [CrossRef]
- Kalliomäki, M.; Satokari, R.; Lähteenoja, H.; Vähämiko, S.; Grönlund, J.; Routi, T.; Salminen, S. Expression of Microbiota, Toll-like Receptors, and Their Regulators in the Small Intestinal Mucosa in Celiac Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Vargas, J.; Green, P.H.R.; Bhagat, G. Innate Lymphoid Cells and Celiac Disease: Current Perspective. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Paolella, G.; Sposito, S.; Romanelli, A.M.; Caputo, I. Type 2 Transglutaminase in Coeliac Disease: A Key Player in Pathogenesis, Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7513. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Pender, S.L.F.; Alstead, E.; Hauer, A.C.; Lionetti, P.; MacDonald, T.T. Role of Interferon α in Promoting T Helper Cell Type 1 Responses in the Small Intestine in Coeliac Disease. Gut 2001, 48, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Di Sabatino, A.; Pickard, K.M.; Gordon, J.N.; Salvati, V.; Mazzarella, G.; Beattie, R.M.; Vossenkaemper, A.; Rovedatti, L.; Leakey, N.A.B.; Croft, N.M.; et al. Evidence for the Role of Interferon-Alfa Production by Dendritic Cells in the Th1 Response in Celiac Disease. Gastroenterology 2007, 133, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Cuoco, L.; Cianci, R.; Pandolfi, F.; Gasbarrini, G. Onset of Coeliac Disease during Treatment with Interferon for Chronic Hepatitis C. Lancet 2000, 356, 1494–1495. [Google Scholar] [CrossRef] [PubMed]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, Interferon-like Cytokines, and Their Receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Colonna, M. Type i Interferons: Diversity of Sources, Production Pathways and Effects on Immune Responses. Curr. Opin. Virol. 2011, 1, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Tovo, P.A.; Davico, C.; Marcotulli, D.; Vitiello, B.; Daprà, V.; Calvi, C.; Montanari, P.; Carpino, A.; Galliano, I.; Bergallo, M. Enhanced Expression of Human Endogenous Retroviruses, TRIM28 and SETDB1 in Autism Spectrum Disorder. Int. J. Mol. Sci. 2022, 23, 5964. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Dieterich, W.; Ehnis, T.; Bauer, M.; Donner, P.; Volta, U.; Riecken, E.O.; Schuppan, D. Identification of Tissue Transglutaminase as the Autoantigen of Celiac Disease. Nat. Med. 1997, 3, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against Type I IFNs in Patients with Life-Threatening COVID-19. Science (1979) 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Simula, E.R.; Manca, M.A.; Noli, M.; Jasemi, S.; Ruberto, S.; Uzzau, S.; Rubino, S.; Manca, P.; Sechi, L.A. Increased Presence of Antibodies against Type I Interferons and Human Endogenous Retrovirus W in Intensive Care Unit COVID-19 Patients. Microbiol. Spectr. 2022, 10, e0128022. [Google Scholar] [CrossRef] [PubMed]
- Knight, V.; Merkel, P.A.; O’Sullivan, M.D. Anticytokine Autoantibodies: Association with Infection and Immune Dysregulation. Antibodies 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Quiros-Roldan, E.; Sottini, A.; Signorini, S.G.; Serana, F.; Tiecco, G.; Imberti, L. Autoantibodies to Interferons in Infectious Diseases. Viruses 2023, 15, 1215. [Google Scholar] [CrossRef] [PubMed]
- Tovo, P.A.; Opramolla, A.; Pizzol, A.; Calosso, G.; Daprà, V.; Galliano, I.; Calvi, C.; Pinon, M.; Cisarò, F.; Rigazio, C.; et al. Overexpression of Endogenous Retroviruses in Children with Celiac Disease. Eur. J. Pediatr. 2021, 180, 2429–2434. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, D.S.; Schilbert, H.M.; Dodero, V.I. Molecular and Structural Parallels between Gluten Pathogenic Peptides and Bacterial-Derived Proteins by Bioinformatics Analysis. Int. J. Mol. Sci. 2021, 22, 9278. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Scott-Sheldon, L.A.J.; Risech-Neyman, Y.; Moss, S.F.; Ludvigsson, J.F.; Green, P.H.R. Celiac Disease and Increased Risk of Pneumococcal Infection: A Systematic Review and Meta-Analysis. Am. J. Med. 2018, 131, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Stene, L.C.; Honeyman, M.C.; Hoffenberg, E.J.; Haas, J.E.; Sokol, R.J.; Emery, L.; Taki, I.; Norris, J.M.; Erlich, H.A.; Eisenbarth, G.S.; et al. Rotavirus Infection Frequency and Risk of Celiac Disease Autoimmunity in Early Childhood: A Longitudinal Study. Am. J. Gastroenterol. 2006, 101, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Oikarinen, M.; Puustinen, L.; Lehtonen, J.; Hakola, L.; Simell, S.; Toppari, J.; Ilonen, J.; Veijola, R.; Virtanen, S.M.; Knip, M.; et al. Enterovirus Infections Are Associated With the Development of Celiac Disease in a Birth Cohort Study. Front. Immunol. 2021, 11, 604529. [Google Scholar] [CrossRef] [PubMed]
- Beyerlein, A.; Donnachie, E.; Ziegler, A.G. Infections in Early Life and Development of Celiac Disease. Am. J. Epidemiol. 2017, 186, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Nobel, Y.R.; Green, P.H.R.; Blaser, M.J.; Ludvigsson, J.F. Risk of Clostridium Difficile Infection in Patients With Celiac Disease: A Population-Based Study. Am. J. Gastroenterol. 2017, 112, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.J.; Wotton, C.J.; Yeates, D.; Ahmad, T.; Jewell, D.P.; Goldacre, M.J. Pneumococcal Infection in Patients with Coeliac Disease. Eur. J. Gastroenterol. Hepatol. 2008, 20, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Canova, C.; Zabeo, V.; Pitter, G.; Romor, P.; Baldovin, T.; Zanotti, R.; Simonato, L. Association of Maternal Education, Early Infections, and Antibiotic Use with Celiac Disease: A Population-Based Birth Cohort Study in Northeastern Italy. Am. J. Epidemiol. 2014, 180, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Bouziat, R.; Hinterleitner, R.; Brown, J.J.; Stencel-Baerenwald, J.E.; Ikizler, M.; Mayassi, T.; Meisel, M.; Kim, S.M.; Discepolo, V.; Pruijssers, A.J.; et al. Reovirus Infection Triggers Inflammatory Responses to Dietary Antigens and Development of Celiac Disease. Science (1979) 2017, 356, 44–50. [Google Scholar] [CrossRef]
- Sandberg-Bennich, S.; Dahlquist, G.; Källén, B. Coeliac Disease Is Associated with Intrauterine Growth and Neonatal Infections. Acta Paediatr. Int. J. Paediatr. 2002, 91, 30–33. [Google Scholar] [CrossRef]
- Manghera, M.; Ferguson-Parry, J.; Lin, R.; Douville, R.N. NF-ΚB and IRF1 Induce Endogenous Retrovirus K Expression via Interferon-Stimulated Response Elements in Its 5′ Long Terminal Repeat. J. Virol. 2016, 90, 9338–9349. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montojo, M.; Fathi, S.; Rastegar, C.; Simula, E.R.; Doucet-O’Hare, T.; Cheng, Y.H.H.; Abrams, R.P.M.; Pasternack, N.; Malik, N.; Bachani, M.; et al. TDP-43 Proteinopathy in ALS Is Triggered by Loss of ASRGL1 and Associated with HML-2 Expression. Nat. Commun. 2024, 15, 4163. [Google Scholar] [CrossRef]
- Li, W.; Lee, M.H.; Henderson, L.; Tyagi, R.; Bachani, M.; Steiner, J.; Campanac, E.; Hoffman, D.A.; Von Geldern, G.; Johnson, K.; et al. Human Endogenous Retrovirus-K Contributes to Motor Neuron Disease. Sci. Transl. Med. 2015, 7, 307ra153. [Google Scholar] [CrossRef] [PubMed]
- Simula, E.R.; Arru, G.; Zarbo, I.R.; Solla, P.; Sechi, L.A. Tdp-43 and Herv-k Envelope-specific Immunogenic Epitopes Are Recognized in Als Patients. Viruses 2021, 13, 2301. [Google Scholar] [CrossRef] [PubMed]
- Dubowsky, M.; Theunissen, F.; Carr, J.M.; Rogers, M.L. The Molecular Link Between TDP-43, Endogenous Retroviruses and Inflammatory Neurodegeneration in Amyotrophic Lateral Sclerosis: A Potential Target for Triumeq, an Antiretroviral Therapy. Mol. Neurobiol. 2023, 60, 6330–6345. [Google Scholar] [CrossRef]
- Arru, G.; Galleri, G.; Deiana, G.A.; Zarbo, I.R.; Sechi, E.; Bo, M.; Cadoni, M.P.L.; Corda, D.G.; Frau, C.; Simula, E.R.; et al. Herv-k Modulates the Immune Response in Als Patients. Microorganisms 2021, 9, 1784. [Google Scholar] [CrossRef]
- Küry, P.; Nath, A.; Créange, A.; Dolei, A.; Marche, P.; Gold, J.; Giovannoni, G.; Hartung, H.P.; Perron, H. Human Endogenous Retroviruses in Neurological Diseases. Trends Mol. Med. 2018, 24, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Harz, C.; Sanchez, L.; Pereira, G.C.; Maldener, E.; Heras, S.R.; Ostrow, L.W.; Ravits, J.; Batra, R.; Meese, E.; et al. Transcriptional Profiling of HERV-K(HML-2) in Amyotrophic Lateral Sclerosis and Potential Implications for Expression of HML-2 Proteins. Mol. Neurodegener. 2018, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Garson, J.A.; Usher, L.; Al-Chalabi, A.; Huggett, J.; Day, E.F.; McCormick, A.L. Quantitative Analysis of Human Endogenous Retrovirus-K Transcripts in Postmortem Premotor Cortex Fails to Confirm Elevated Expression of HERV-K RNA in Amyotrophic Lateral Sclerosis. Acta Neuropathol. Commun. 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Montojo, M.; Li, W.; Nath, A. Technical Considerations in Detection of HERV-K in Amyotrophic Lateral Sclerosis: Selection of Controls and the Perils of QPCR. Acta Neuropathol. Commun. 2019, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Levet, S.; Charvet, B.; Bertin, A.; Deschaumes, A.; Perron, H.; Hober, D. Human Endogenous Retroviruses and Type 1 Diabetes. Curr. Diab Rep. 2019, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Noli, M.; Meloni, G.; Manca, P.; Cossu, D.; Palermo, M.; Sechi, L.A. Herv-w and Mycobacterium Avium Subspecies Paratuberculosis Are at Play in Pediatric Patients at Onset of Type 1 Diabetes. Pathogens 2021, 10, 1135. [Google Scholar] [CrossRef]
- Tovo, P.A.; Rabbone, I.; Tinti, D.; Galliano, I.; Trada, M.; Daprà, V.; Cerutti, F.; Bergallo, M. Enhanced Expression of Human Endogenous Retroviruses in New-Onset Type 1 Diabetes: Potential Pathogenetic and Therapeutic Implications. Autoimmunity 2020, 53, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Noli, M.; Meloni, G.; Ruberto, S.; Jasemi, S.; Simula, E.R.; Cossu, D.; Bo, M.; Palermo, M.; Sechi, L.A. HERV-K Envelope Protein Induces Long-Lasting Production of Autoantibodies in T1DM Patients at Onset in Comparison to ZNT8 Autoantibodies. Pathogens 2022, 11, 1188. [Google Scholar] [CrossRef] [PubMed]
- Levet, S.; Joanou, J.; Queruel, N.; Pierquin, J.; Perron, H.J.F. HERV-W-Env Involvement in Human T1D Pathogenesis—New Insights from Two Mouse Models. Diabetes 2018, 67, 160-LB. [Google Scholar] [CrossRef]
- Tugnet, N.; Rylance, P.; Roden, D.; Trela, M.; Nelson, P. Human Endogenous Retroviruses (HERVs) and Autoimmune Rheumatic Disease: Is There a Link? Open Rheumatol. J. 2013, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Stearrett, N.; Dawson, T.; Rahnavard, A.; Bachali, P.; Bendall, M.L.; Zeng, C.; Caricchio, R.; Pérez-Losada, M.; Grammer, A.C.; Lipsky, P.E.; et al. Expression of Human Endogenous Retroviruses in Systemic Lupus Erythematosus: Multiomic Integration With Gene Expression. Front. Immunol. 2021, 12, 661437. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Mei, X.; Zhao, D.; Sun, Y.; Song, J.; Pan, W.; Shi, W. DNA Methylation Modulates HERV-E Expression in CD4+ T Cells from Systemic Lupus Erythematosus Patients. J. Dermatol. Sci. 2015, 77, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.; Rylance, P.; Roden, D.; Trela, M.; Tugnet, N. Viruses as Potential Pathogenic Agents in Systemic Lupus Erythematosus. Lupus 2014, 23, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Januchowski, R.; Prokop, J.; Jagodzinski, P.P. Role of Epigenetic DNA Alterations in the Pathogenesis of Systemic Lupus Erythematosus. J. Appl. Genet. 2004, 45, 237–248. [Google Scholar] [PubMed]
- Balada, E.; Ordi-Ros, J.; Vilardell-Tarrés, M. Molecular Mechanisms Mediated by Human Endogenous Retroviruses (HERVs) in Autoimmunity. Rev. Med. Virol. 2009, 19, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Hervé, C.A.; Lugli, E.B.; Brand, A.; Griffiths, D.J.; Venables, P.J.W. Autoantibodies to Human Endogenous Retrovirus-K Are Frequently Detected in Health and Disease and React with Multiple Epitopes. Clin. Exp. Immunol. 2002, 128, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Freimanis, G.; Hooley, P.; Ejtehadi, H.D.; Ali, H.A.; Veitch, A.; Rylance, P.B.; Alawi, A.; Axford, J.; Nevill, A.; Murray, P.G.; et al. A Role for Human Endogenous Retrovirus-K (HML-2) in Rheumatoid Arthritis: Investigating Mechanisms of Pathogenesis. Clin. Exp. Immunol. 2010, 160, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, R.P.; Wildschutte, J.H.; Russo, C.; Coffin, J.M. Identification, Characterization, and Comparative Genomic Distribution of the HERV-K (HML-2) Group of Human Endogenous Retroviruses. Retrovirology 2011, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, A.A. Human Endogenous Retrovirus HERV-K(HML-2) Rec Expression and Transcriptional Activities in Normal and Rheumatoid Arthritis Synovia. Chemtracts 2007, 19, 16–23. [Google Scholar]
- Ko, E.-J.; Cha, H.-J. The Roles of Human Endogenous Retroviruses (HERVs) in Inflammation. Kosin Med. J. 2021, 36, 69–78. [Google Scholar] [CrossRef]
- Mameli, G.; Erre, G.L.; Caggiu, E.; Mura, S.; Cossu, D.; Bo, M.; Cadoni, M.L.; Piras, A.; Mundula, N.; Colombo, E.; et al. Identification of a HERV-K Env Surface Peptide Highly Recognized in Rheumatoid Arthritis (RA) Patients: A Cross-Sectional Case–Control Study. Clin. Exp. Immunol. 2017, 189, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, C.; Ricceri, L.; Matteucci, C.; De Felice, A.; Tartaglione, A.M.; Argaw-Denboba, A.; Pica, F.; Grelli, S.; Calamandrei, G.; Sinibaldi Vallebona, P.; et al. High Expression of Endogenous Retroviruses from Intrauterine Life to Adulthood in Two Mouse Models of Autism Spectrum Disorders. Sci. Rep. 2018, 8, 629. [Google Scholar] [CrossRef] [PubMed]
- Carta, A.; Manca, M.A.; Scoppola, C.; Simula, E.R.; Noli, M.; Ruberto, S.; Conti, M.; Zarbo, I.R.; Antonucci, R.; Sechi, L.A.; et al. Antihuman Endogenous Retrovirus Immune Response and Adaptive Dysfunction in Autism. Biomedicines 2022, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, E.; Cipriani, C.; Matteucci, C.; Benvenuto, A.; Coniglio, A.; Argaw-Denboba, A.; Toschi, N.; Bucci, I.; Miele, M.T.; Grelli, S.; et al. Children with Autism Spectrum Disorder and Their Mothers Share Abnormal Expression of Selected Endogenous Retroviruses Families and Cytokines. Front. Immunol. 2019, 10, 2244. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, C.; Giudice, M.; Petrone, V.; Fanelli, M.; Minutolo, A.; Miele, M.T.; Toschi, N.; Maracchioni, C.; Siracusano, M.; Benvenuto, A.; et al. Modulation of Human Endogenous Retroviruses and Cytokines Expression in Peripheral Blood Mononuclear Cells from Autistic Children and Their Parents. Retrovirology 2022, 19, 26. [Google Scholar] [CrossRef]
- Balestrieri, E.; Arpino, C.; Matteucci, C.; Sorrentino, R.; Pica, F.; Alessandrelli, R.; Coniglio, A.; Curatolo, P.; Rezza, G.; Macciardi, F.; et al. HERVs Expression in Autism Spectrum Disorders. PLoS ONE 2012, 7, e48831. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cao, M.; Iduma, P.; Karachaliou, N.; Santarpia, M.; Blanco, J.; Rosell, R. Human Endogenous Retroviruses and Cancer. Cancer Biol. Med. 2016, 13, 483–488. [Google Scholar] [PubMed]
- Gao, Y.; Yu, X.F.; Chen, T. Human Endogenous Retroviruses in Cancer: Expression, Regulation and Function (Review). Oncol. Lett. 2021, 21, 121. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, M.; Sbravati, F.; Allegri, D.; Labriola, F.; Lombardo, V.; Spisni, E.; Zarbo, C.; Alvisi, P. Is the Clinical Pattern of Pediatric Celiac Disease Changing? A Thirty-Years Real-Life Experience of an Italian Center. Ital. J. Pediatr. 2021, 47, 235. [Google Scholar] [CrossRef] [PubMed]
- Arru, G.; Sechi, E.; Mariotto, S.; Farinazzo, A.; Mancinelli, C.; Alberti, D.; Ferrari, S.; Gajofatto, A.; Capra, R.; Monaco, S.; et al. Antibody Response against HERV-W Env Surface Peptides Differentiates Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder. Mult. Scler. J. Exp. Transl. Clin. 2017, 3, 2055217317742425. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.N.; Roden, D.; Nevill, A.; Freimanis, G.L.; Trela, M.; Ejtehadi, H.D.; Bowman, S.; Axford, J.; Veitch, A.M.; Tugnet, N.; et al. Rheumatoid Arthritis Is Associated with IgG Antibodies to Human Endogenous Retrovirus Gag Matrix: A Potential Pathogenic Mechanism of Disease? J. Rheumatol. 2014, 41, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Reynier, F.; Verjat, T.; Turrel, F.; Imbert, P.E.; Marotte, H.; Mougin, B.; Miossec, P. Increase in Human Endogenous Retrovirus HERV-K (HML-2) Viral Load in Active Rheumatoid Arthritis. Scand. J. Immunol. 2009, 70, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Dopkins, N.; O’Mara, M.M.; Singh, B.; Marston, J.L.; Bendall, M.L.; Nixon, D.F. How Human Endogenous Retroviruses Interact with the Microbiota in Health and Disease. Trends Microbiol. 2022, 30, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Jabri, B.; Sollid, L.M. T Cells in Celiac Disease. J. Immunol. 2017, 198, 3005–3014. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Newell, E.W.; Glanville, J.; Fernandez-Becker, N.; Khosla, C.; Chien, Y.H.; Davis, M.M. Dietary Gluten Triggers Concomitant Activation of CD4+ and CD8+ Aβ T Cells and Γλ T Cells in Celiac Disease. Proc. Natl. Acad. Sci. USA 2013, 110, 13073–13078. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, E.M.; Lundin, K.E.A.; Krajči, P.; Scott, H.; Sollid, L.M.; Brandtzaeg, P. Gluten Specific, HLA-DQ Restricted T Cells from Coeliac Mucosa Produce Cytokines with Th1 or Th0 Profile Dominated by Interferon. Gut 1995, 37, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Turelli, P.; Castro-Diaz, N.; Marzetta, F.; Kapopoulou, A.; Raclot, C.; Duc, J.; Tieng, V.; Quenneville, S.; Trono, D. Interplay of TRIM28 and DNA Methylation in Controlling Human Endogenous Retroelements. Genome Res. 2014, 24, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Rowe, H.M.; Jakobsson, J.; Mesnard, D.; Rougemont, J.; Reynard, S.; Aktas, T.; Maillard, P.V.; Layard-Liesching, H.; Verp, S.; Marquis, J.; et al. KAP1 Controls Endogenous Retroviruses in Embryonic Stem Cells. Nature 2010, 463, 237–240. [Google Scholar] [CrossRef] [PubMed]

| CD n = 40 | HCs n = 41 | |
|---|---|---|
| Age, years | 33 ± 21.59 | 31.4 ± 21.16 |
| Female sex, n (%) | 25 (62.5) | 26 (65) |
| Anti-transglutaminase 2 (TG2) antibodies (U/mL), n (%) | 35 (87.5) | - |
| Anti-endomysium Abs, n (%) | 13 (32.5) | - |
| Anti-deamidated gliadin Abs IgA (U/mL), n (%) | 19 (47.5) | - |
| Anti-deamidated gliadin Abs IgG (U/mL), n (%) | 17 (42.5) | - |
| Age | N | Subject | INF-α | p | HERV-K | p | HERV-H | p | HERV-W | p |
|---|---|---|---|---|---|---|---|---|---|---|
| 0–11 | 10 | CD | 8 | 0.0300 | 7 | 0.0274 | 6 | 0.0743 * | 7 | 0.0062 |
| (80%) | (70%) | (60%) | (70%) | |||||||
| 12 | HCs | 3 | 2 | 2 | 1 | |||||
| (25%) | (16.66%) | (16.66%) | (8.33%) | |||||||
| 12–18 | 5 | CD | 4 | 0.2063 | 3 | 0.5238 | 1 | 1.0000 | 2 | 1.0000 |
| (80%) | (60%) | (20%) | (40%) | |||||||
| 5 | HCs | 1 | 1 | 1 | 1 | |||||
| (20%) | (20%) | (20%) | (20%) | |||||||
| 19–40 | 8 | CD | 6 | 0.0406 | 7 | 0.0014 | 3 | 0.5692 | 3 | 0.5692 |
| (75%) | (87.5%) | (37.5%) | (37.5%) | |||||||
| 7 | HCs | 1 | 0 | 1 | 1 | |||||
| (14.28%) | (0%) | (14.28%) | (14.28%) | |||||||
| 41–51 | 7 | CD | 5 | 0.1319 | 4 | 0.1189 | 4 | 0.1189 | 4 | 0.1189 |
| (71.42%) | (57.14%) | (57.14%) | (57.14%) | |||||||
| 8 | HCs | 2 | 1 | 0 | 0 | |||||
| (25%) | (12.5%) | (0%) | (0%) | |||||||
| 52–81 | 10 | CD | 6 | 0.0573 * | 6 | 0.0573 * | 3 | 0.2105 | 1 | 1.0000 |
| (60%) | (60%) | (30%) | (10%) | |||||||
| 9 | HCs | 1 | 0 | 0 | 0 | |||||
| (11.11%) | (0%) | (0%) | (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bo, M.; Manetti, R.; Biggio, M.L.; Sechi, L.A. The Humoral Immune Response against Human Endogenous Retroviruses in Celiac Disease: A Case–Control Study. Biomedicines 2024, 12, 1811. https://doi.org/10.3390/biomedicines12081811
Bo M, Manetti R, Biggio ML, Sechi LA. The Humoral Immune Response against Human Endogenous Retroviruses in Celiac Disease: A Case–Control Study. Biomedicines. 2024; 12(8):1811. https://doi.org/10.3390/biomedicines12081811
Chicago/Turabian StyleBo, Marco, Roberto Manetti, Maria Luigia Biggio, and Leonardo A. Sechi. 2024. "The Humoral Immune Response against Human Endogenous Retroviruses in Celiac Disease: A Case–Control Study" Biomedicines 12, no. 8: 1811. https://doi.org/10.3390/biomedicines12081811
APA StyleBo, M., Manetti, R., Biggio, M. L., & Sechi, L. A. (2024). The Humoral Immune Response against Human Endogenous Retroviruses in Celiac Disease: A Case–Control Study. Biomedicines, 12(8), 1811. https://doi.org/10.3390/biomedicines12081811







