Differences in the Interleukin Profiles in Inattentive ADHD Prepubertal Children Are Probably Related to Conduct Disorder Comorbidity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Assessment
2.3. Treatment
2.4. Measurements
2.5. Analytical Method
2.6. Statistics
3. Results
3.1. Pro-Inflammatory Cytokines
3.1.1. Interleukin-1beta (IL-1beta)
3.1.2. Interleukin-5 (IL-5)
3.1.3. Interleukin-6 (IL-6)
3.1.4. Tumor Necrosis Factor-Alpha (TNF-α)
3.2. Anti-Inflammatory Cytokines
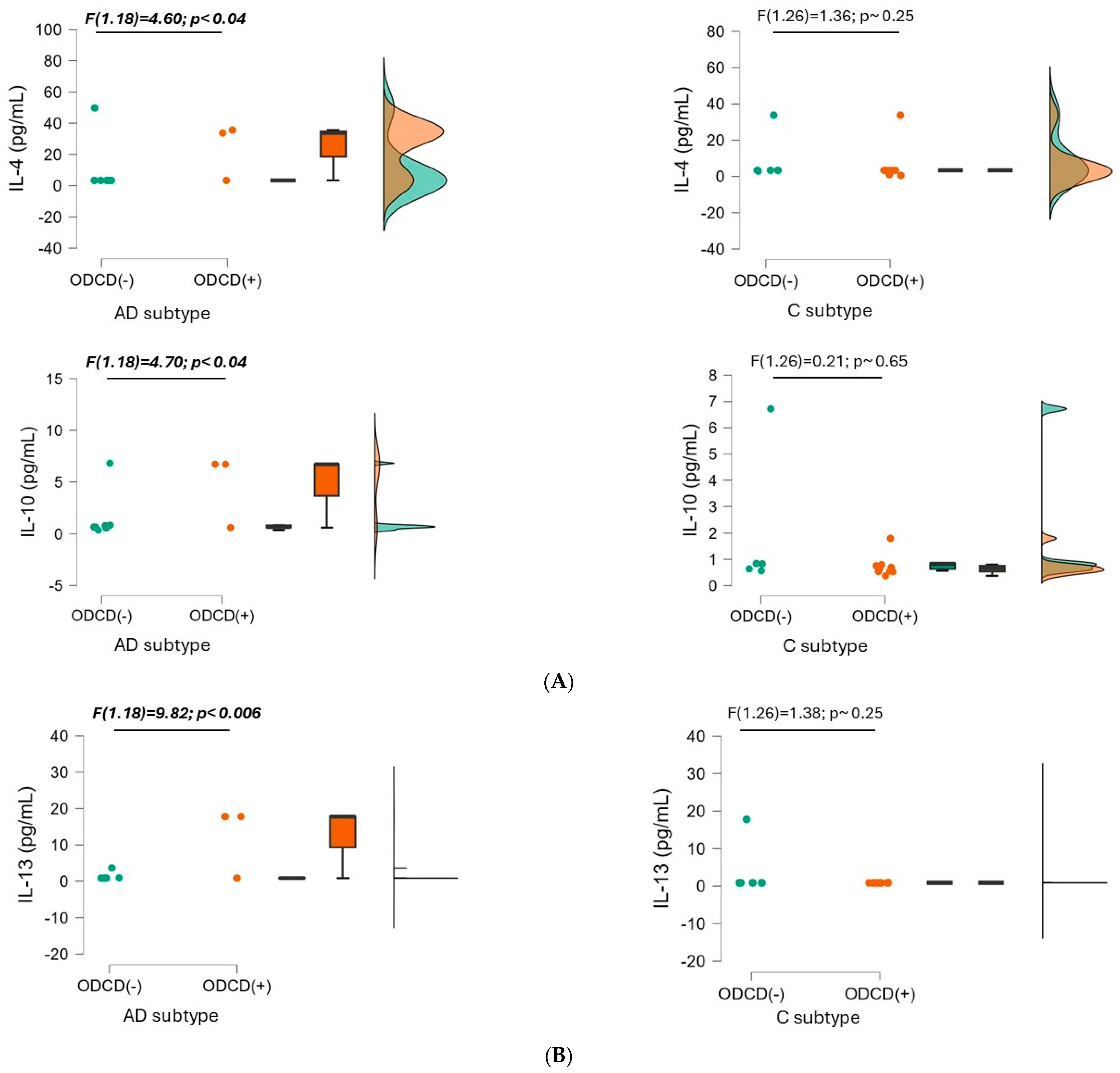
3.2.1. Interleukin-4 (IL-4)
3.2.2. Interleukin-10 (IL-10)
3.2.3. Interleukin-13 (IL-13)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faraone, S.V.; Asherson, P.; Banaschewski, T.; Biederman, J.; Buitelaar, J.K.; Ramos-Quiroga, J.A.; Rohde, L.A.; Sonuga-Barke, E.J.; Tannock, R.; Franke, B. Attention-deficit/hyperactivity disorder. Nat. Rev. Dis. Primers 2015, 1, 15020. [Google Scholar] [CrossRef]
- Wolraich, M.L.; Hagan, J.F., Jr.; Allan, C.; Chan, E.; Davison, D.; Earls, M.; Evans, S.W.; Flinn, S.K.; Froehlich, T.; Frost, J.; et al. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 2019, 144, e20192528. [Google Scholar] [CrossRef] [PubMed]
- Sayal, K.; Prasad, V.; Daley, D.; Ford, T.; Coghill, D. ADHD in children and young people: Prevalence, care pathways, and service provision. Lancet Psychiatry 2018, 5, 175–186. [Google Scholar] [CrossRef]
- de la Peña, I.C.; Pan, M.C.; Thai, C.G.; Alisso, T. Attention-Deficit/Hyperactivity Disorder Predominantly Inattentive Subtype/Presentation: Research Progress and Translational Studies. Brain Sci. 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Song, M.; Farhat, L.C.; Yon, D.K.; Lee, S.W.; Kim, M.S.; Park, S.; Oh, J.W.; Lee, S.; Cheon, K.A.; et al. Incidence, prevalence, and global burden of ADHD from 1990 to 2019 across 204 countries: Data, with critical re-analysis, from the Global Burden of Disease study. Mol. Psychiatry 2023, 28, 4823–4830. [Google Scholar] [CrossRef] [PubMed]
- APA. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; text revision; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Posner, J.; Polanczyk, G.V.; Sonuga-Barke, E. Attention-deficit hyperactivity disorder. Lancet 2020, 395, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Schiweck, C.; Arteaga-Henriquez, G.; Aichholzer, M.; Edwin Thanarajah, S.; Vargas-Cáceres, S.; Matura, S.; Grimm, O.; Haavik, J.; Kittel-Schneider, S.; Ramos-Quiroga, J.A.; et al. Comorbidity of ADHD and adult bipolar disorder: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 124, 100–123. [Google Scholar] [CrossRef]
- Newcorn, J.H.; Halperin, J.M.; Jensen, P.S.; Abikoff, H.B.; Arnold, L.E.; Cantwell, D.P.; Conners, C.K.; Elliott, G.R.; Epstein, J.N.; Greenhill, L.L.; et al. Symptom profiles in children with ADHD: Effects of comorbidity and gender. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Nourredine, M.; Gering, A.; Fourneret, P.; Rolland, B.; Falissard, B.; Cucherat, M.; Geoffray, M.M.; Jurek, L. Association of Attention-Deficit/Hyperactivity Disorder in Childhood and Adolescence With the Risk of Subsequent Psychotic Disorder: A Systematic Review and Meta-analysis. JAMA Psychiatry 2021, 78, 519–529. [Google Scholar] [CrossRef]
- Riva, V.; Battaglia, M.; Nobile, M.; Cattaneo, F.; Lazazzera, C.; Mascheretti, S.; Giorda, R.; Merette, C.; Emond, C.; Maziade, M.; et al. GRIN2B predicts attention problems among disadvantaged children. Eur. Child. Adolesc. Psychiatry 2015, 24, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Larsson, H. Genetics of attention deficit hyperactivity disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef]
- Michels, N.; Clarke, G.; Olavarria-Ramírez, L.; Gómez-Martínez, S.; Diaz, L.E.; Marcos, A.; Widhalm, K.; Carvalho, L.A. Psychosocial stress and inflammation driving tryptophan breakdown in children and adolescents: A cross-sectional analysis of two cohorts. Psychoneuroendocrinology 2018, 94, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, F.M.; Pocivavsek, A. Elevated kynurenine pathway metabolism during neurodevelopment: Implications for brain and behavior. Neuropharmacology 2017, 112, 275–285. [Google Scholar] [CrossRef]
- Badawy, A.A. Tryptophan availability for kynurenine pathway metabolism across the life span: Control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology 2017, 112, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Arellano, L.; González-García, N.; Salazar-García, M.; Corona, J.C. Antioxidants as a Potential Target against Inflammation and Oxidative Stress in Attention-Deficit/Hyperactivity Disorder. Antioxidants 2020, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a risk factor for attention deficit hyperactivity disorder. Pharmacol. Biochem. Behav. 2019, 182, 22–34. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Deuschl, G. Neuroinflammation—A common thread in neurological disorders. Nat. Rev. Neurol. 2019, 15, 429–430. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, U.; Quinn, T.; Walker, D.W.; Dickinson, H. Cytokines and the neurodevelopmental basis of mental illness. Front. Neurosci. 2013, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Feghali, C.A.; Wright, T.M. Cytokines in acute and chronic inflammation. Front. Biosci. 1997, 2, d12–d26. [Google Scholar] [CrossRef]
- Tavernier, J.; Cornelis, S.; Devos, R.; Guisez, Y.; Plaetinck, G.; Van der Heyden, J. Structure/function analysis of human interleukin 5 and its receptor. Agents Actions Suppl. 1995, 46, 23–34. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Chomarat, P.; Banchereau, J. Interleukin-4 and interleukin-13: Their similarities and discrepancies. Int. Rev. Immunol. 1998, 17, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-González, D.; Carreón-Trujillo, S.; Alvarez-Arellano, L.; Abarca-Merlin, D.M.; Domínguez-López, P.; Salazar-García, M.; Corona, J.C. A Potential Role for Neuroinflammation in ADHD. Adv. Exp. Med. Biol. 2023, 1411, 327–356. [Google Scholar] [CrossRef] [PubMed]
- Abdel Samei, A.M.; Mahmoud, D.A.M.; Salem Boshra, B.; Abd El Moneam, M.H.E. The Interplay Between Blood Inflammatory Markers, Symptom Domains, and Severity of ADHD Disorder in Children. J. Atten. Disord. 2024, 28, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, I.; Siegl, A.; Luckhardt, S.; Wenz, S.; Friedrichsen, H.; El Jomaa, H.; Steinmann, A.; Kilencz, T.; Arteaga-Henríquez, G.; Ramos-Sayalero, C.; et al. Inflammatory biotype of ADHD is linked to chronic stress: A data-driven analysis of the inflammatory proteome. Transl. Psychiatry 2024, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, A.; Wojtacha, P.; Równiak, M.; Kolenkiewicz, M.; Huang, A.C.W. ADHD pathogenesis in the immune, endocrine and nervous systems of juvenile and maturating SHR and WKY rats. Psychopharmacology 2019, 236, 2937–2958. [Google Scholar] [CrossRef]
- Garre-Morata, L.; Haro, T.d.; González-Villén, R.; Fernández-López, M.L.; Escames, G.; Molina-Carballo, A.; Acuña-Castroviejo, D. Changes in cortisol and in oxidative/nitrosative stress indicators after ADHD treatment. Antioxidants 2024, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Allred, E.N.; Dammann, O.; Fichorova, R.N.; Hooper, S.R.; Hunter, S.J.; Joseph, R.M.; Kuban, K.; Leviton, A.; O’Shea, T.M.; Scott, M.N. Systemic Inflammation during the First Postnatal Month and the Risk of Attention Deficit Hyperactivity Disorder Characteristics among 10 year-old Children Born Extremely Preterm. J. Neuroimmune Pharmacol. 2017, 12, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Elsadek, A.E.; Al-Shokary, A.H.; Abdelghani, W.E.; Kamal, N.M.; Ibrahim, A.O.; El-Shorbagy, H.H.; Suliman, H.A.; Barseem, N.F.; Abdel Maksoud, Y.H.; Azab, S.M.; et al. Serum Levels of Interleukin-6 and Tumor Necrosis Factor Alpha in Children With Attention-Deficit Hyperactivity Disorder. J. Pediatr. Neurosci. 2020, 15, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.-C.; Mondelli, V.; Satyanarayanan, S.K.; Chiang, Y.-J.; Chen, H.-T.; Su, K.-P.; Pariante, C.M. Cortisol, Inflammatory Biomarkers and Neurotrophins in Children and Adolescents with Attention Deficit Hyperactivity Disorder (ADHD) in Taiwan. Brain Behav. Immun. 2020, 88, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M. Manual for the Children’s Depression Inventory; Multi-Health Systems: North Tonawanda, NY, USA, 1992. [Google Scholar]
- de Jong, S.; Newhouse, S.J.; Patel, H.; Lee, S.; Dempster, D.; Curtis, C.; Paya-Cano, J.; Murphy, D.; Wilson, C.E.; Horder, J.; et al. Immune signatures and disorder-specific patterns in a cross-disorder gene expression analysis. Br. J. Psychiatry 2016, 209, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Zayats, T.; Athanasiu, L.; Sonderby, I.; Djurovic, S.; Westlye, L.T.; Tamnes, C.K.; Fladby, T.; Aase, H.; Zeiner, P.; Reichborn-Kjennerud, T.; et al. Genome-wide analysis of attention deficit hyperactivity disorder in Norway. PLoS ONE 2015, 10, e0122501. [Google Scholar] [CrossRef] [PubMed]
- Hegvik, T.A.; Instanes, J.T.; Haavik, J.; Klungsøyr, K.; Engeland, A. Associations between attention-deficit/hyperactivity disorder and autoimmune diseases are modified by sex: A population-based cross-sectional study. Eur. Child. Adolesc. Psychiatry 2018, 27, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Instanes, J.T.; Halmøy, A.; Engeland, A.; Haavik, J.; Furu, K.; Klungsøyr, K. Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers With Inflammatory and Immune System Diseases. Biol. Psychiatry 2017, 81, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.H.; Elgohary, T.M.; Nosair, N.A. Serum Interleukin-6 Level in Children With Attention-Deficit Hyperactivity Disorder (ADHD). J. Child. Neurol. 2019, 34, 61–67. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M.; Joseph, R.M.; Kuban, K.C.; Allred, E.N.; Ware, J.; Coster, T.; Fichorova, R.N.; Dammann, O.; Leviton, A. Elevated blood levels of inflammation-related proteins are associated with an attention problem at age 24 mo in extremely preterm infants. Pediatr. Res. 2014, 75, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Corominas-Roso, M.; Armario, A.; Palomar, G.; Corrales, M.; Carrasco, J.; Richarte, V.; Ferrer, R.; Casas, M.; Ramos-Quiroga, J.A. IL-6 and TNF-alpha in unmedicated adults with ADHD: Relationship to cortisol awakening response. Psychoneuroendocrinology 2017, 79, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Perez, R.; Crespo, J.F.; Rodríguez, J.; Salcedo, G. Profilin is a relevant melon allergen susceptible to pepsin digestion in patients with oral allergy syndrome. J. Allergy Clin. Immunol. 2003, 111, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Neale, S.A.; Copeland, C.S.; Salt, T.E. Effect of VGLUT inhibitors on glutamatergic synaptic transmission in the rodent hippocampus and prefrontal cortex. Neurochem. Int. 2014, 73, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.; Su, K.P.; Mondelli, V.; Pariante, C.M. Cortisol and inflammatory biomarker levels in youths with attention deficit hyperactivity disorder (ADHD): Evidence from a systematic review with meta-analysis. Transl. Psychiatry 2021, 11, 430. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Wójta-Kempa, M.; Samochowiec, J.; Schiweck, C.; Aichholzer, M.; Reif, A.; Samochowiec, A.; Stańczykiewicz, B. Peripheral blood inflammatory markers in patients with attention deficit/hyperactivity disorder (ADHD): A systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 118, 110581. [Google Scholar] [CrossRef] [PubMed]
- Caye, A.; Spadini, A.V.; Karam, R.G.; Grevet, E.H.; Rovaris, D.L.; Bau, C.H.; Rohde, L.A.; Kieling, C. Predictors of persistence of ADHD into adulthood: A systematic review of the literature and meta-analysis. Eur. Child. Adolesc. Psychiatry 2016, 25, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Ferencova, N.; Visnovcova, Z.; Ondrejka, I.; Hrtanek, I.; Bujnakova, I.; Kovacova, V.; Macejova, A.; Tonhajzerova, I. Peripheral Inflammatory Markers in Autism Spectrum Disorder and Attention Deficit/Hyperactivity Disorder at Adolescent Age. Int. J. Mol. Sci. 2023, 24, 11710. [Google Scholar] [CrossRef] [PubMed]
- Molina-Carballo, A.; Naranjo-Gómez, A.; Uberos, J.; Justicia-Martínez, F.; Ruiz-Ramos, M.J.; Cubero-Millán, I.; Contreras-Chova, F.; Augustin-Morales, M.D.; Khaldy-Belkadi, H.; Muñoz-Hoyos, A. Methylphenidate effects on blood serotonin and melatonin levels may help to synchronise biological rhythms in children with ADHD. J. Psychiatr. Res. 2013, 47, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, L.; Molina-Carballo, A.; Cubero-Millán, I.; Checa-Ros, A.; Machado-Casas, I.; Blanca-Jover, E.; Jerez-Calero, A.; Madrid-Fernández, Y.; Uberos, J.; Muñoz-Hoyos, A. Indole Tryptophan Metabolism and Cytokine S100B in Children with Attention-Deficit/Hyperactivity Disorder: Daily Fluctuations, Responses to Methylphenidate, and Interrelationship with Depressive Symptomatology. J. Child. Adolesc. Psychopharmacol. 2020, 30, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Cubero-Millán, I.; Molina-Carballo, A.; Machado-Casas, I.; Fernández-López, L.; Martínez-Serrano, S.; Tortosa-Pinto, P.; Ruiz-López, A.; Luna-del-Castillo, J.D.; Uberos, J.; Muñoz-Hoyos, A. Methylphenidate ameliorates depressive comorbidity in ADHD children without any modification on differences in serum melatonin concentration between ADHD subtypes. Int. J. Mol. Sci. 2014, 15, 17115–17129. [Google Scholar] [CrossRef] [PubMed]
- Oades, R.D.; Dauvermann, M.R.; Schimmelmann, B.G.; Schwarz, M.J.; Myint, A.M. Attention-deficit hyperactivity disorder (ADHD) and glial integrity: S100B, cytokines and kynurenine metabolism–Effects of medication. Behav. Brain Funct. BBF 2010, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, T.I.; Landaas, E.T.; Hegvik, T.A.; Ulvik, A.; Halmoy, A.; Ueland, P.M.; Haavik, J. Serum concentrations of kynurenines in adult patients with attention-deficit hyperactivity disorder (ADHD): A case-control study. Behav. Brain Funct. 2015, 11, 36. [Google Scholar] [CrossRef] [PubMed]



| Baseline ADHD | Post-Treatment ADHD | Statistics | ||
|---|---|---|---|---|
| (n = 31) | (n = 31) | t | p | |
| Age (years) | 7.65 ± 1.33 | 8.1 ± 1.5 | 1.24 | 0.21 |
| Height (cm) | 130.31 ± 9.49 | 133.50 ± 8.03 | 1.43 | 0.16 |
| Weight (kg) | 33.03 ± 8.08 | 35.9 ± 7.77 | 1.16 | 0.15 |
| Body Mass Index (kg/m2) | 19.28 ± 4.42 | 19.90 ± 3.34 | 0.62 | 0.54 |
| Attention Deficit (ADHD-AD) | 17.48 ± 4.42 | 5.38 ± 4.25 | 10.99 | 0.00001 *** |
| Combined ADHD (ADHD-C) | 13.19 ± 8.92 | 3.59 ± 4.29 | 5.4 | 0.00001 *** |
| OD Conduct Disorder (ODCD) | 10.55 ± 7.24 | 4.25 ± 6.16 | 3.69 | 0.0005 *** |
| CDI total score (CDI) | 10.2 ± 5.63 | 7.69 ± 3.05 | 2.18 | 0.03 * |
| Anxiety total score (SCAS) | 30.42 ± 15.49 | 16.93 ± 13.15 | 3.7 | 0.0005 *** |
| KBIT total score | 101.23 ± 11.4 | |||
| Interleukins | Inattentive ADHD (n = 13) | Combined ADHD (n = 18) | |||
|---|---|---|---|---|---|
| Baseline | Post-MPH | Baseline | Post-MPH | ||
| Pro-inflammatory | IL-1beta | 2.26 ± 3.91 | 2.83 ± 4.17 | 1.55 ± 3.41 | 1.72 ± 3.35 |
| IL-5 | 14.96 ±18.21 | 17.83 ± 26.32 | 17.36 ± 32.21 | 9.21 ± 14.65 | |
| IL-6 | 35.75 ± 67.13 | 37.38 ± 89.61 | 15.41 ± 24.85 | 10.81 ± 19.96 | |
| TNF-alpha | 4.39 ± 4.75 | 7.00 ± 10.19 | 4.03 ± 2.55 | 7.49 ± 8.24 | |
| Anti-inflammatory | IL-4 | 14.25 ± 18.04 | 17.95 ± 29.13 | 7.29 ± 11.25 | 7.23± 10.48 |
| IL-10 | 2.49 ± 2.95 | 2.27 ± 2.87 | 1.16 ± 1.63 | 1.77 ± 2.15 | |
| IL-13 | 4.54 ± 7.04 | 2.89 ± 5.29 | 2.08 ± 4.52 | 3.49 ± 6.09 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Villén, R.; Fernández-López, M.L.; Checa-Ros, A.; Tortosa-Pinto, P.; Aguado-Rivas, R.; Garre-Morata, L.; Acuña-Castroviejo, D.; Molina-Carballo, A. Differences in the Interleukin Profiles in Inattentive ADHD Prepubertal Children Are Probably Related to Conduct Disorder Comorbidity. Biomedicines 2024, 12, 1818. https://doi.org/10.3390/biomedicines12081818
González-Villén R, Fernández-López ML, Checa-Ros A, Tortosa-Pinto P, Aguado-Rivas R, Garre-Morata L, Acuña-Castroviejo D, Molina-Carballo A. Differences in the Interleukin Profiles in Inattentive ADHD Prepubertal Children Are Probably Related to Conduct Disorder Comorbidity. Biomedicines. 2024; 12(8):1818. https://doi.org/10.3390/biomedicines12081818
Chicago/Turabian StyleGonzález-Villén, Raquel, María Luisa Fernández-López, Ana Checa-Ros, Pilar Tortosa-Pinto, Raquel Aguado-Rivas, Laura Garre-Morata, Darío Acuña-Castroviejo, and Antonio Molina-Carballo. 2024. "Differences in the Interleukin Profiles in Inattentive ADHD Prepubertal Children Are Probably Related to Conduct Disorder Comorbidity" Biomedicines 12, no. 8: 1818. https://doi.org/10.3390/biomedicines12081818







