Antioxidants as Protection against Reactive Oxygen Stress Induced by Formaldehyde (FA) Exposure: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Data Analysis
2.3. Study Quality Assessment
3. Results
3.1. Article Selection
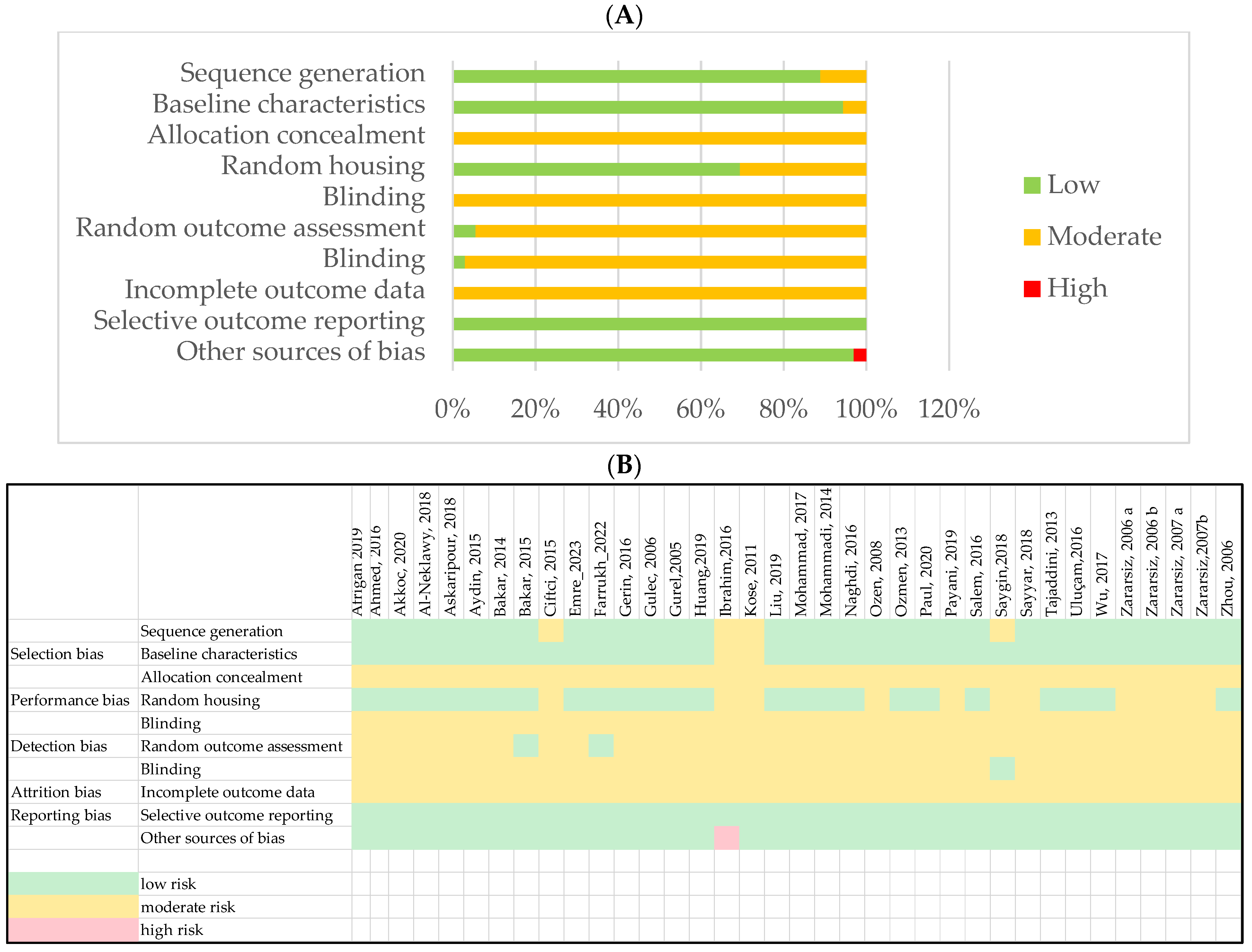
3.2. Study Selection Bias
3.3. Antioxidants Effects
3.3.1. Antioxidants Pre-Treatment: Histopathological Effects of Antioxidants before FA Exposure
3.3.2. Effects of Concomitant Administration of Antioxidants during FA Exposure
3.3.3. Effects of Administration of Antioxidants Following FA Exposure
3.3.4. Effects of Administration of Antioxidants in Humans Following FA Exposure
3.3.5. Mechanisms and Effects Induced by Antioxidants to Influence FA Exposure
4. Discussion
4.1. Promising Antioxidant Treatments
4.2. Bias and Limitations in Human Studies
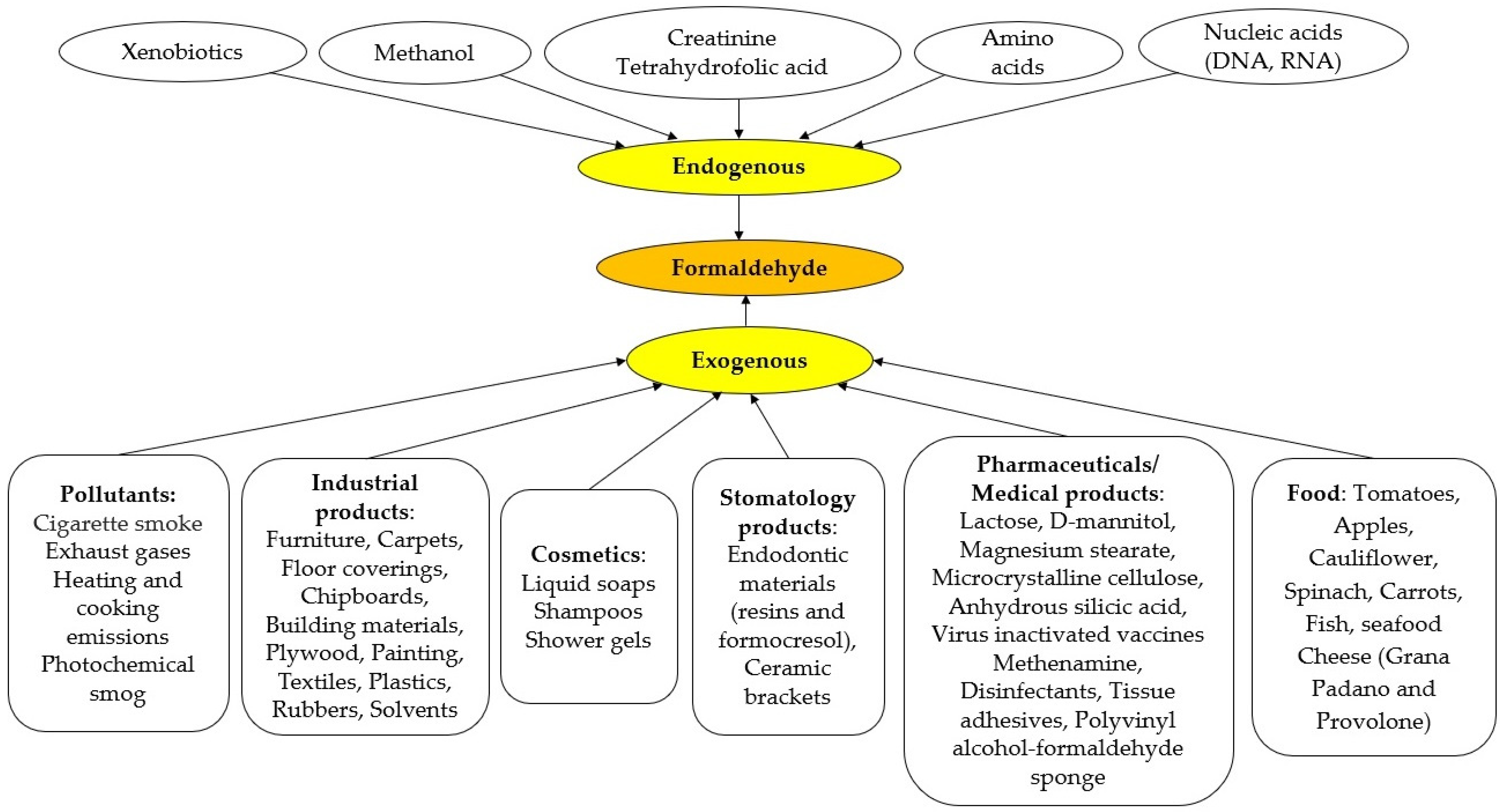
4.3. FA Exposure
4.4. Metabolism and Endogenous FA Regulation
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ibrahim, B.S.; Barioni, É.D.; Heluany, C.; Braga, T.T.; Drewes, C.C.; Costa, S.G.; Câmara, N.O.S.; Farsky, S.H.P.; Lino-dos-Santos-Franco, A. Beneficial Effects of Vitamin C Treatment on Pregnant Rats Exposed to Formaldehyde: Reversal of Immunosuppression in the Offspring. Toxicol. Appl. Pharmacol. 2016, 300, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.A.; Mahmoud, O.M.; Al Badawi, M.H.; Gab-Alla, A.A. Role of Nigella Sativa Seed Oil on Corneal Injury Induced by Formaldehyde in Adult Male Albino Rats. Folia Morphol. 2016, 75, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Jiang, Z.; Gong, B.; Dou, Y.; Song, M.; Song, X.; Tian, Y. Vitamin E Reversed Apoptosis of Cardiomyocytes Induced by Exposure to High Dose Formaldehyde During Mice Pregnancy. Int. Heart J. 2017, 58, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Akkoc, R.F.; Ogeturk, M.; Aydin, S.; Kuloglu, T.; Aydin, S. Effects of Carnosine on Apoptosis, Transient Receptor Potential Melastatin 2, and Betatrophin in Rats Exposed to Formaldehyde. Biotech. Histochem. 2021, 96, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Gulec, M.; Gurel, A.; Armutcu, F. Vitamin E Protects against Oxidative Damage Caused by Formaldehyde in the Liver and Plasma of Rats. Mol. Cell Biochem. 2006, 290, 61–67. [Google Scholar] [CrossRef] [PubMed]
- IARC. Formaldehyde, 2-Butoxyethanol and 1-Tert-Butoxypropan-2-Ol. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer, Ed.; IARC: Lyon, France, 2006; ISBN 978-92-832-1288-1. [Google Scholar]
- Aydin, S.; Ogeturk, M.; Kuloglu, T.; Kavakli, A.; Aydin, S. Effect of Carnosine Supplementation on Apoptosis and Irisin, Total Oxidant and Antioxidants Levels in the Serum, Liver and Lung Tissues in Rats Exposed to Formaldehyde Inhalation. Peptides 2015, 64, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, G.; Aksoy, A.; Cenesiz, S.; Sogut, M.U.; Yarim, G.F.; Nisbet, C.; Guvenc, D.; Ertekin, A. Therapeutic Role of Curcumin in Oxidative DNA Damage Caused by Formaldehyde. Microsc. Res. Tech. 2015, 78, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Yuan, L.; Wei, C.; Zhao, Y.; Qian, Y.; Ma, P.; Ding, S.; Yang, X.; Wang, X. Effects of Combined Exposure to Formaldehyde and Benzene on Immune Cells in the Blood and Spleen in Balb/c Mice. Environ. Toxicol. Pharmacol. 2016, 45, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, R.J.; Schofield, C.J. Deciphering Functions of Intracellular Formaldehyde: Linking Cancer and Aldehyde Metabolism. Biochemistry 2018, 57, 904–906. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; He, X.; Yang, P.; Zong, T.; Sun, P.; Sun, R.; Yu, T.; Jiang, Z. The Cellular Function and Molecular Mechanism of Formaldehyde in Cardiovascular Disease and Heart Development. J. Cell. Mol. Med. 2021, 25, 5358–5371. [Google Scholar] [CrossRef]
- Umansky, C.; Morellato, A.E.; Pontel, L.B. Illuminating Cellular Formaldehyde. Nat. Commun. 2021, 12, 580. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.M.; Gentry, R.; Fitch, S.; Lu, K.; Clewell, H.J. An Updated Mode of Action and Human Relevance Framework Evaluation for Formaldehyde-Related Nasal Tumors. Crit. Rev. Toxicol. 2020, 50, 919–952. [Google Scholar] [CrossRef] [PubMed]
- Gentry, P.R.; Rodricks, J.V.; Turnbull, D.; Bachand, A.; Van Landingham, C.; Shipp, A.M.; Albertini, R.J.; Irons, R. Formaldehyde Exposure and Leukemia: Critical Review and Reevaluation of the Results from a Study that Is the Focus for Evidence of Biological Plausibility. Crit. Rev. Toxicol. 2013, 43, 661–670. [Google Scholar] [CrossRef]
- Arts, J.H.E.; Rennen, M.A.J.; De Heer, C. Inhaled Formaldehyde: Evaluation of Sensory Irritation in Relation to Carcinogenicity. Regul. Toxicol. Pharmacol. 2006, 44, 144–160. [Google Scholar] [CrossRef]
- Kwak, K.; Paek, D.; Park, J. Occupational Exposure to Formaldehyde and Risk of Lung Cancer: A Systematic Review and Meta-analysis. Am. J. Ind. Med. 2020, 63, 312–327. [Google Scholar] [CrossRef]
- Catalani, S.; Donato, F.; Madeo, E.; Apostoli, P.; De Palma, G.; Pira, E.; Mundt, K.A.; Boffetta, P. Occupational Exposure to Formaldehyde and Risk of Non-Hodgkin Lymphoma: A Meta-Analysis. BMC Cancer 2019, 19, 1245. [Google Scholar] [CrossRef]
- Allegra, A.; Spatari, G.; Mattioli, S.; Curti, S.; Innao, V.; Ettari, R.; Allegra, A.G.; Giorgianni, C.; Gangemi, S.; Musolino, C. Formaldehyde Exposure and Acute Myeloid Leukemia: A Review of the Literature. Medicina 2019, 55, 638. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-C.; Kim, I.; Song, J.; Park, J. Does Formaldehyde Have a Causal Association with Nasopharyngeal Cancer and Leukaemia? Ann. Occup. Environ. Med. 2018, 30, 5. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, M.; Stewart, P.A.; Lubin, J.H.; Beane Freeman, L.E.; Hornung, R.W.; Herrick, R.F.; Hoover, R.N.; Fraumeni, J.F.; Blair, A.; Hayes, R.B. Mortality from Lymphohematopoietic Malignancies and Brain Cancer Among Embalmers Exposed to Formaldehyde. JNCI J. Natl. Cancer Inst. 2009, 101, 1696–1708. [Google Scholar] [CrossRef]
- Tulpule, K.; Hohnholt, M.C.; Dringen, R. Formaldehyde Metabolism and Formaldehyde-induced Stimulation of Lactate Production and Glutathione Export in Cultured Neurons. J. Neurochem. 2013, 125, 260–272. [Google Scholar] [CrossRef]
- İnci, M.; Zararsız, İ.; Davarcı, M.; Görür, S. Toxic Effects of Formaldehyde on the Urinary System. Turk. J. Urol. 2013, 39, 48–52. [Google Scholar] [CrossRef]
- Athanassiadis, B.; George, G.A.; Abbott, P.V.; Wash, L.J. A Review of the Effects of Formaldehyde Release from Endodontic Materials. Int. Endod. J. 2015, 48, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; He, R. Metabolism of Formaldehyde In Vivo. In Formaldehyde and Cognition; Springer: Dordrecht, The Netherlands, 2017; pp. 21–46. ISBN 978-94-024-1175-1. [Google Scholar]
- Smith, A.E. Formaldehyde. Occup. Med. 1992, 42, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Emre, E.; Ogeturk, M.; Aydın, S.; Kuloglu, T.; Aksu, F.; Kavakli, A. Carvacrol Protects Rat Liver Exposed to Formaldehyde by Regulating Oxidative Stress, and Asprosin and Subfatin Hormones. Biotech. Histochem. 2023, 98, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Payani, S.; Mamatha, C.; Chandraprakash, C.; Bhaskar, M. Protective Role of (Bronco-T) against Formaldehyde Induced Antioxidant, Oxidative and Histopathological Changes in Lung of Male Wistar Rats. Toxicol. Rep. 2019, 6, 718–726. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Morsy, F.A.; El-Nabarawy, S.K.; Ahmed, M.A.; Ali, N.A. Lycopene: An Effective Neuroprotective Option against Neurodeterioration Induced by Formaldehyde Inhalation. Comp. Clin. Pathol. 2016, 25, 1171–1184. [Google Scholar] [CrossRef]
- Huang, J.; Lu, Y.; Zhang, B.; Yang, S.; Zhang, Q.; Cui, H.; Lu, X.; Zhao, Y.; Yang, X.; Li, R. Antagonistic Effect of Epigallocatechin-3-Gallate on Neurotoxicity Induced by Formaldehyde. Toxicology 2019, 412, 29–36. [Google Scholar] [CrossRef]
- Zararsiz, I.; Kus, I.; Akpolat, N.; Songur, A.; Ogeturk, M.; Sarsilmaz, M. Protective Effects of Omega-3 Essential Fatty Acids against Formaldehyde-Induced Neuronal Damage in Prefrontal Cortex of Rats. Cell Biochem. Funct. 2006, 24, 237–244. [Google Scholar] [CrossRef]
- Zararsiz, I.; Sarsilmaz, M.; Tas, U.; Kus, I.; Meydan, S.; Ozan, E. Protective Effect of Melatonin against Formaldehyde-Induced Kidney Damage in Rats. Toxicol. Ind. Health 2007, 23, 573–579. [Google Scholar] [CrossRef]
- Ozen, O.A.; Kus, M.A.; Kus, I.; Alkoc, O.A.; Songur, A. Protective Effects of Melatonin against Formaldehyde-Induced Oxidative Damage and Apoptosis in Rat Testes: An Immunohistochemical and Biochemical Study. Syst. Biol. Reprod. Med. 2008, 54, 169–176. [Google Scholar] [CrossRef]
- Lü, J.-M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Sayyar, Z.; Yazdinezhad, A.; Hassan, M.; Jafari Anarkooli, I. Protective Effect of Matricaria Chamomilla Ethanolic Extract on Hippocampal Neuron Damage in Rats Exposed to Formaldehyde. Oxid. Med. Cell Longev. 2018, 2018, 6414317. [Google Scholar] [CrossRef] [PubMed]
- Afrigan, L.; Jafari Anarkooli, I.; Sohrabi, D.; Abdanipour, A.; Yazdinezhad, A.; Sayyar, Z.; Ghorbanlou, M.; Arianmanesh, M. The Effect of Hydroethanolic Extract of Matricaria Chamomilla on the Reproductive System of Male Rats Exposed to Formaldehyde. Andrologia 2019, 51, e13362. [Google Scholar] [CrossRef]
- PRISMA 2020 Flow Diagram. Available online: https://www.prisma-statement.org/prisma-2020-flow-diagram (accessed on 9 July 2024).
- Paul, M.; Sohag, M.S.U.; Khan, A.; Barman, R.K.; Wahed, M.I.I.; Khan, M.R.I. Pumpkin (Cucurbita Maxima) Seeds Protect against Formaldehyde-Induced Major Organ Damages. Heliyon 2020, 6, e04587. [Google Scholar] [CrossRef] [PubMed]
- Zararsiz, I.; Kus, I.; Ogeturk, M.; Akpolat, N.; Kose, E.; Meydan, S.; Sarsilmaz, M. Melatonin Prevents Formaldehyde-Induced Neurotoxicity in Prefrontal Cortex of Rats: An Immunohistochemical and Biochemical Study. Cell Biochem. Funct. 2007, 25, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Bakar, E.; Ulucam, E.; Cerkezkayabekir, A. Protective Effects of Proanthocyanidin and Vitamin E against Toxic Effects of Formaldehyde in Kidney Tissue. Biotech. Histochem. 2015, 90, 69–78. [Google Scholar] [CrossRef]
- Gerin, F.; Erman, H.; Erboga, M.; Sener, U.; Yilmaz, A.; Seyhan, H.; Gurel, A. The Effects of Ferulic Acid Against Oxidative Stress and Inflammation in Formaldehyde-Induced Hepatotoxicity. Inflammation 2016, 39, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Bakar, E.; Ulucam, E.; Cerkezkayabekir, A. Investigation of the Protective Effects of Proanthocyanidin and Vitamin E against the Toxic Effect Caused by Formaldehyde on the Liver Tissue. Environ. Toxicol. 2015, 30, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Gurel, A.; Coskun, O.; Armutcu, F.; Kanter, M.; Ozen, O.A. Vitamin E against Oxidative Damage Caused by Formaldehyde in Frontal Cortex and Hippocampus: Biochemical and Histological Studies. J. Chem. Neuroanat. 2005, 29, 173–178. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Yang, X. Vitamin E Reduces the Extent of Mouse Brain Damage Induced by Combined Exposure to Formaldehyde and PM2.5. Ecotoxicol. Environ. Saf. 2019, 172, 33–39. [Google Scholar] [CrossRef]
- Mohammadi, S. Effect of Selenium on Neurotoxicity in Adult Male Mice Exposed to Formaldehyde. Electron. Physician 2014, 6, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, E.; Ozsoy, S.Y.; Donmez, N.; Ozsoy, B.; Yumuşak, N. The Protective Effect of L-Carnitine against Hippocampal Damage Due to Experimental Formaldehyde Intoxication in Rats. Biotech. Histochem. 2014, 89, 336–341. [Google Scholar] [CrossRef]
- Askaripour, M.; Hasanpour, A.; Hosseini, F.; Moshrefi, M.; Moshtaghi, G.; Hasannejad, M.; Rajabi, S.; Nematollahi-Mahani, S.N. The Effect of Aqueous Extract of Rosa Damascena on Formaldehyde-Induced Toxicity in Mice Testes. Pharm. Biol. 2018, 56, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, M.; Maghbool, M.; Seifalah-Zade, M.; Mahaldashtian, M.; Makoolati, Z.; Kouhpayeh, S.A.; Ghasemi, A.; Fereydouni, N. Effects of Common Fig (Ficus Carica) Leaf Extracts on Sperm Parameters and Testis of Mice Intoxicated with Formaldehyde. Evid. Based Complement. Altern. Med. 2016, 2016, 2539127. [Google Scholar] [CrossRef] [PubMed]
- Uluçam, E.; Bakar, E. The Effect of Proanthocyanidin on Formaldehyde-Induced Toxicity in Rat Testes. Turk. J. Med. Sci. 2016, 46, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-X.; Qiu, S.-D.; Zhang, J.; Tian, H.; Wang, H.-X. The Protective Effect of Vitamin E against Oxidative Damage Caused by Formaldehyde in the Testes of Adult Rats. Asian J. Androl. 2006, 8, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Al-Neklawy, A.F. Does Oral Spirulina Protect the Cornea from Formaldehyde Exposure? Application to Anatomy Laboratories. Clin. Anat. 2018, 31, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Farrukh, M.; Saleem, U.; Qasim, M.; Manan, M.; Shah, M.A. Sarcococca Saligna Extract Attenuates Formaldehyde-Induced Arthritis in Wistar Rats via Modulation of pro-Inflammatory and Inflammatory Biomarkers. Inflammopharmacology 2022, 30, 579–597. [Google Scholar] [CrossRef] [PubMed]
- Saygin, B.; Esrefoglu, M.; Bayindir, N.; Tok, O.E.; Selek, S.; Bulut, H.; Ozer, O.F.; Ozturk, A.; Yilmaz, O.; Meydan, S. Protection with Thymoquinone against Formaldehyde-Induced Neurotoxicity in the Rats. Bratisl. Lek. Listy 2018, 119, 726–730. [Google Scholar] [CrossRef]
- Zararsiz, I.; Sonmez, M.F.; Yilmaz, H.R.; Tas, U.; Kus, I.; Kavakli, A.; Sarsilmaz, M. Effects of V-3 Essential Fatty Acids against Formaldehyde-Induced Nephropathy in Rats. Toxicol. Ind. Health 2006, 22, 223–229. [Google Scholar] [CrossRef]
- Tajaddini, S.; Ebrahimi, S.; Behnam, B.; Bakhtiyari, M.; Joghataei, M.T.; Abbasi, M.; Amini, M.; Amanpour, S.; Koruji, M. Antioxidant Effect of Manganese on the Testis Structure and Sperm Parameters of Formalin-Treated Mice. Andrologia 2014, 46, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Köse, E.; Sarsılmaz, M.; Taş, U.; Kavaklı, A.; Türk, G.; Özlem Dabak, D.; Sapmaz, H.; Ögetürk, M. Rose Oil Inhalation Protects against Formaldehyde-Induced Testicular Damage in Rats: Effects of Rose Oil on FA-Intoxicated Testes. Andrologia 2012, 44, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Mierauskiene, J.; Lekevicius, R.; Lazutka, J.R. Anticlastogenic Effects of Aevitum Intake in a Group of Chemical Industry Workers. Hereditas 1993, 118, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Villar, V.M.; Soria, J.M. 3. Neuroprotective Actions of Curcumin. Available online: https://www.researchgate.net/profile/Jose-Miguel-Soria/publication/236191589_3_Neuroprotective_actions_of_curcumin/links/0deec516ea81e5b2ad000000/3-Neuroprotective-actions-of-curcumin.pdf (accessed on 30 May 2024).
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverrí, J. Renoprotective Effect of the Antioxidant Curcumin: Recent Findings. Redox Biol. 2013, 1, 448–456. [Google Scholar] [CrossRef]
- Luca, A.; Alexa, T.; Dondaş, A.; Andron, G.; Bădescu, M.; Alexa, I.D.; Bohotin, C. Pain Modulation by Curcumin and Ascorbic Acid in Mice. Rev. Med. Chir. Soc. Med. Nat. IASI 2014, 118, 346–351. [Google Scholar] [PubMed]
- Bavarsad, K.; Barreto, G.E.; Hadjzadeh, M.-A.-R.; Sahebkar, A. Protective Effects of Curcumin Against Ischemia-Reperfusion Injury in the Nervous System. Mol. Neurobiol. 2019, 56, 1391–1404. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Wang, H.; Jiao, D.; Geng, Y.; Wu, Q.; Yan, H.; Li, C. Curcumin Restrains Oxidative Stress of After Intracerebral Hemorrhage in Rat by Activating the Nrf2/HO-1 Pathway. Front. Pharmacol. 2022, 13, 889226. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Lei, M. Mechanisms Underlying Curcumin-Induced Neuroprotection in Cerebral Ischemia. Front. Pharmacol. 2022, 13, 893118. [Google Scholar] [CrossRef] [PubMed]
- Perumpail, B.J.; Li, A.A.; John, N.; Sallam, S.; Shah, N.D.; Kwong, W.; Cholankeril, G.; Kim, D.; Ahmed, A. The Role of Vitamin E in the Treatment of NAFLD. Diseases 2018, 6, 86. [Google Scholar] [CrossRef]
- Jaworek, A.K.; Szepietowski, J.C.; Hałubiec, P.; Wojas-Pelc, A.; Jaworek, J. Melatonin as an Antioxidant and Immunomodulator in Atopic Dermatitis—A New Look on an Old Story: A Review. Antioxidants 2021, 10, 1179. [Google Scholar] [CrossRef]
- Przybylska, S.; Tokarczyk, G. Lycopene in the Prevention of Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 1957. [Google Scholar] [CrossRef] [PubMed]
- Isaev, N.K.; Chetverikov, N.S.; Stelmashook, E.V.; Genrikhs, E.E.; Khaspekov, L.G.; Illarioshkin, S.N. Thymoquinone as a Potential Neuroprotector in Acute and Chronic Forms of Cerebral Pathology. Biochem. Mosc. 2020, 85, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Zaidi, A.; Anwar, H.; Kizilbash, N. Mechanism of the Antidiabetic Action of Nigella Sativa and Thymoquinone: A Review. Front. Nutr. 2023, 10, 1126272. [Google Scholar] [CrossRef] [PubMed]
- Kassab, R.B.; Lokman, M.S.; Daabo, H.M.A.; Gaber, D.A.; Habotta, O.A.; Hafez, M.M.; Zhery, A.S.; Moneim, A.E.A.; Fouda, M.S. Ferulic Acid Influences Nrf2 Activation to Restore Testicular Tissue from Cadmium-induced Oxidative Challenge, Inflammation, and Apoptosis in Rats. J. Food Biochem. 2020, 44, e13505. [Google Scholar] [CrossRef] [PubMed]
- Dragan, M.; Stan, C.D.; Iacob, A.T.; Dragostin, O.; Profire, L. Ferulic acid: Potential therapeutic applications. Med. Surg. J. 2018, 122, 388–395. [Google Scholar]
- Mousa, A.M.; Aldebasi, Y.H. L-Carnosine Mitigates Interleukin-1α-Induced Dry Eye Disease in Rabbits via Its Antioxidant, Anti-Inflammatory, Antiapoptotic, and Antifibrotic Effects. Cutan. Ocul. Toxicol. 2021, 40, 241–251. [Google Scholar] [CrossRef]
- Lungu, I.I.; Huzum, B.; Humulescu, I.A.; Cioancă, O.; Morariu, D.; Șerban, I.-L.; Hăncianu, M. Flavonoids as Promising Therapeutic and Dietary Agents. Med. Surg. J. 2020, 124, 151–156. [Google Scholar]
- Kumari, S.; Goyal, A.; Garg, M. Phytochemistry and Pharmacological Update on Tetraterpenoids. NPJ 2021, 11, 617–628. [Google Scholar] [CrossRef]
- Aitken, R.J.; Roman, S.D. Antioxidant Systems and Oxidative Stress in the Testes. Oxid. Med. Cell Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D.; Collin, F. Melatonin: Action as Antioxidant and Potential Applications in Human Disease and Aging. Toxicology 2010, 278, 55–67. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, J.; Liu, H.; Wang, J.; Lu, W. Melatonin Inhibits Apoptosis and Oxidative Stress of Mouse Leydig Cells via a SIRT1-Dependent Mechanism. Molecules 2019, 24, 3084. [Google Scholar] [CrossRef]
- Frungieri, M.; Calandra, R.; Rossi, S. Local Actions of Melatonin in Somatic Cells of the Testis. Int. J. Mol. Sci. 2017, 18, 1170. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, J.; Liu, H.; Wang, J.; Lu, W. Melatonin Inhibits Apoptosis in Mouse Leydig Cells via the Retinoic Acid-Related Orphan Nuclear Receptor α/P53 Pathway. Life Sci. 2020, 246, 117431. [Google Scholar] [CrossRef]
- Traber, M.G.; Bruno, R.S. Vitamin E. In Present Knowledge in Nutrition; Elsevier: Amsterdam, The Netherlands, 2020; pp. 115–136. ISBN 978-0-323-66162-1. [Google Scholar]
- Javed, H.; Mohamed Fizur, N.M.; Jha, N.K.; Ashraf, G.M.; Ojha, S. Neuroprotective Potential and Underlying Pharmacological Mechanism of Carvacrol for Alzheimer’s and Parkinson’s Diseases. CN 2023, 21, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Beall, J.R.; Ulsamer, A.G. Formaldehyde and Hepatotoxicity: A Review. J. Toxicol. Environ. Health 1984, 14, 1–21. [Google Scholar] [CrossRef]
- Gottschling, L.M.; Beaulieu, H.J.; Melvin, W.W. Monitoring of Formic Acid in Urine of Humans Exposed to Low Levels of Formaldehyde. Am. Ind. Hyg. Assoc. J. 1984, 45, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Boeniger, M.F. Formate in Urine as a Biological Indicator of Formaldehyde Exposure: A Review. Am. Ind. Hyg. Assoc. J. 1987, 48, 900–908. [Google Scholar] [CrossRef]
- Ma, T.H.; Harris, M.M. Review of the Genotoxicity of Formaldehyde. Mutat. Res. 1988, 196, 37–59. [Google Scholar] [CrossRef]
- Pazdrak, K.; Górski, P.; Krakowiak, A.; Ruta, U. Changes in Nasal Lavage Fluid Due to Formaldehyde Inhalation. Int. Arch. Occup. Environ. Health 1993, 64, 515–519. [Google Scholar] [CrossRef]
- Nilsson, J.A.; Zheng, X.; Sundqvist, K.; Liu, Y.; Atzori, L.; Elfwing, A.; Arvidson, K.; Grafström, R.C. Toxicity of Formaldehyde to Human Oral Fibroblasts and Epithelial Cells: Influences of Culture Conditions and Role of Thiol Status. J. Dent. Res. 1998, 77, 1896–1903. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.D.; Cho, S.H. Formaldehyde Exposure Levels and Serum Antibodies to Formaldehyde-Human Serum Albumin of Korean Medical Students. Arch. Environ. Health 1999, 54, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.J.; Ness, R.; Tyl, R.W.; Krivanek, N.; Esmen, N.A.; Hall, T.A. A Review of Adverse Pregnancy Outcomes and Formaldehyde Exposure in Human and Animal Studies. Regul. Toxicol. Pharmacol. 2001, 34, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Lovschall, H.; Eiskjaer, M.; Arenholt-Bindslev, D. Formaldehyde Cytotoxicity in Three Human Cell Types Assessed in Three Different Assays. Toxicol. Vitr. 2002, 16, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Heck, H.D.; Casanova, M. The Implausibility of Leukemia Induction by Formaldehyde: A Critical Review of the Biological Evidence on Distant-Site Toxicity. Regul. Toxicol. Pharmacol. 2004, 40, 92–106. [Google Scholar] [CrossRef]
- Costa, S.; Coelho, P.; Costa, C.; Silva, S.; Mayan, O.; Santos, L.S.; Gaspar, J.; Teixeira, J.P. Genotoxic Damage in Pathology Anatomy Laboratory Workers Exposed to Formaldehyde. Toxicology 2008, 252, 40–48. [Google Scholar] [CrossRef]
- Speit, G.; Schmid, O.; Neuss, S.; Schütz, P. Genotoxic Effects of Formaldehyde in the Human Lung Cell Line A549 and in Primary Human Nasal Epithelial Cells. Environ. Mol. Mutagen. 2008, 49, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Duong, A.; Steinmaus, C.; McHale, C.M.; Vaughan, C.P.; Zhang, L. Reproductive and Developmental Toxicity of Formaldehyde: A Systematic Review. Mutat. Res. 2011, 728, 118–138. [Google Scholar] [CrossRef]
- Kim, K.-H.; Jahan, S.A.; Lee, J.-T. Exposure to Formaldehyde and Its Potential Human Health Hazards. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2011, 29, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Chen, J.; Li, J.; Sun, M.; Mo, W.; Liu, K.; Meng, Y.; Liu, Y.; Wang, F.; He, R.; et al. The Protective Effect of Geniposide on Human Neuroblastoma Cells in the Presence of Formaldehyde. BMC Complement. Altern. Med. 2013, 13, 152. [Google Scholar] [CrossRef]
- Costa, S.; Carvalho, S.; Costa, C.; Coelho, P.; Silva, S.; Santos, L.S.; Gaspar, J.F.; Porto, B.; Laffon, B.; Teixeira, J.P. Increased Levels of Chromosomal Aberrations and DNA Damage in a Group of Workers Exposed to Formaldehyde. Mutagenesis 2015, 30, 463–473. [Google Scholar] [CrossRef]
- Fenech, M.; Nersesyan, A.; Knasmueller, S. A Systematic Review of the Association between Occupational Exposure to Formaldehyde and Effects on Chromosomal DNA Damage Measured Using the Cytokinesis-Block Micronucleus Assay in Lymphocytes. Mutat. Res. Rev. Mutat. Res. 2016, 770, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Edling, C.; Hellquist, H.; Odkvist, L. Occupational Exposure to Formaldehyde and Histopathological Changes in the Nasal Mucosa. Br. J. Ind. Med. 1988, 45, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, C.; Viegas, S.; Carolino, E.; Prista, J.; Gomes, M.C.; Brito, M. Genotoxicity Biomarkers in Occupational Exposure to Formaldehyde—The Case of Histopathology Laboratories. Mutat. Res. 2011, 721, 15–20. [Google Scholar] [CrossRef]
- Conolly, R.B.; Kimbell, J.S.; Janszen, D.B.; Miller, F.J. Dose Response for Formaldehyde-Induced Cytotoxicity in the Human Respiratory Tract. Regul. Toxicol. Pharmacol. 2002, 35, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Kum, C.; Sekkin, S.; Kiral, F.; Akar, F. Effects of Xylene and Formaldehyde Inhalations on Renal Oxidative Stress and Some Serum Biochemical Parameters in Rats. Toxicol. Ind. Health 2007, 23, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Wang, W.; Luo, W.; Lv, J.; Li, H.; Luo, H.; Jia, J.; He, R. Urine Formaldehyde Predicts Cognitive Impairment in Post-Stroke Dementia and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Ribas, G.S.; Vargas, C.R.; Wajner, M. L-Carnitine Supplementation as a Potential Antioxidant Therapy for Inherited Neurometabolic Disorders. Gene 2014, 533, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Isaev, N.K.; Genrikhs, E.E.; Stelmashook, E.V. Antioxidant Thymoquinone and Its Potential in the Treatment of Neurological Diseases. Antioxidants 2023, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, K.H. Neurobehavioral Impairment and Seizures from Formaldehyde. Arch. Environ. Health 1994, 49, 37–44. [Google Scholar] [CrossRef]
- Rana, I.; Rieswijk, L.; Steinmaus, C.; Zhang, L. Formaldehyde and Brain Disorders: A Meta-Analysis and Bioinformatics Approach. Neurotox. Res. 2021, 39, 924–948. [Google Scholar] [CrossRef]
- Stroup, N.E.; Blair, A.; Erikson, G.E. Brain Cancer and Other Causes of Death in Anatomists. J. Natl. Cancer Inst. 1986, 77, 1217–1224. [Google Scholar] [PubMed]
- Costa, S.; Pina, C.; Coelho, P.; Costa, C.; Silva, S.; Porto, B.; Laffon, B.; Teixeira, J.P. Occupational Exposure to Formaldehyde: Genotoxic Risk Evaluation by Comet Assay and Micronucleus Test Using Human Peripheral Lymphocytes. J. Toxicol. Environ. Health Part. A 2011, 74, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Smith, M.T.; Tang, X.; Guo, W.; Vermeulen, R.; Ji, Z.; Hu, W.; Hubbard, A.E.; Shen, M.; McHale, C.M.; et al. Chromosome-Wide Aneuploidy Study of Cultured Circulating Myeloid Progenitor Cells from Workers Occupationally Exposed to Formaldehyde. Carcinogenesis 2015, 36, 160–167. [Google Scholar] [CrossRef]
- Adamović, D.; Čepić, Z.; Adamović, S.; Stošić, M.; Obrovski, B.; Morača, S.; Vojinović Miloradov, M. Occupational Exposure to Formaldehyde and Cancer Risk Assessment in an Anatomy Laboratory. Int. J. Environ. Res. Public Health 2021, 18, 11198. [Google Scholar] [CrossRef] [PubMed]
- Aung, W.-Y.; Sakamoto, H.; Sato, A.; Yi, E.-E.-P.-N.; Thein, Z.-L.; Nwe, M.-S.; Shein, N.; Linn, H.; Uchiyama, S.; Kunugita, N.; et al. Indoor Formaldehyde Concentration, Personal Formaldehyde Exposure and Clinical Symptoms during Anatomy Dissection Sessions, University of Medicine 1, Yangon. Int. J. Environ. Res. Public Health 2021, 18, 712. [Google Scholar] [CrossRef] [PubMed]
- Karátson, A.; Buzogány, I.; Wágner, G.; Rácz, L. Sclerosing Peritonitis Caused by Disinfectant in a Patient under Peritoneal Dialysis. Int. Urol. Nephrol. 1991, 23, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Restani, P.; Galli, C.L. Oral Toxicity of Formaldehyde and Its Derivatives. Crit. Rev. Toxicol. 1991, 21, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Matthews, I.P.; Gibson, C.; Samuel, A.H. Sterilisation of Implantable Devices. Clin. Mater. 1994, 15, 191–215. [Google Scholar] [CrossRef]
- Kusy, R.P.; Whitley, J.Q. Degradation of Plastic Polyoxymethylene Brackets and the Subsequent Release of Toxic Formaldehyde. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 420–427. [Google Scholar] [CrossRef]
- Jennings, M.B.; Ricketti, J.; Guadara, J.; Nach, W.; Goodwin, S. Treatment for Simple Plantar Verrucae: Monochloroacetic Acid and 10% Formaldehyde versus 10% Formaldehyde Alone. JAPMA 2006, 96, 53–58. [Google Scholar] [CrossRef]
- Fujita, M.; Ueda, T.; Handa, T. Generation of Formaldehyde by Pharmaceutical Excipients and Its Absorption by Meglumine. Chem. Pharm. Bull. 2009, 57, 1096–1099. [Google Scholar] [CrossRef][Green Version]
- Kaden, D.A.; Mandin, C.; Nielsen, G.D.; Wolkoff, P. Formaldehyde. In WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Kuritzky, L.A.; Pratt, M. Systemic Allergic Contact Dermatitis After Formaldehyde-Containing Influenza Vaccination. J. Cutan. Med. Surg. 2015, 19, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Postolache, P.; Nemes, R.-M.; Serban, R.I.; Rad, R.M.; Stratulat, I.S. Electronic cigarette—A way of smoking cessation? Med. Surg. J. 2015, 119, 510–516. [Google Scholar]
- Muralidharan, A.; Li, C.; Wang, L.; Li, X. Immunopathogenesis Associated with Formaldehyde-Inactivated RSV Vaccine in Preclinical and Clinical Studies. Expert. Rev. Vaccines 2017, 16, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.D.; Larsen, S.T.; Wolkoff, P. Re-Evaluation of the WHO (2010) Formaldehyde Indoor Air Quality Guideline for Cancer Risk Assessment. Arch. Toxicol. 2017, 91, 35–61. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kitamura, T.; Miyata, Y.; Miyaji, K. Mitral Valve Necrosis After Cardiac Surgery Using Gelatin-Resorcinol-Formaldehyde Glue. Ann. Thorac. Surg. 2017, 103, e435–e436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Astill, J.; Alkie, T.; Yitbarek, A.; Taha-Abdelaziz, K.; Shojadoost, B.; Petrik, J.J.; Nagy, É.; Sharif, S. A Comparison of Toll-Like Receptor 5 and 21 Ligands as Adjuvants for a Formaldehyde Inactivated H9N2 Avian Influenza Virus Vaccine in Chickens. Viral Immunol. 2018, 31, 605–612. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Ozpinar, A.; Ozpinar, A.; Perez, J.L.; Elmaci, İ. Methenamine’s Journey of 160 Years: Repurposal of an Old Urinary Antiseptic for Treatment and Hypoxic Radiosensitization of Cancers and Glioblastoma. Clin. Exp. Pharma Physio 2019, 46, 407–412. [Google Scholar] [CrossRef]
- Shouval, D. Immunization against Hepatitis A. Cold Spring Harb. Perspect. Med. 2019, 9, a031682. [Google Scholar] [CrossRef]
- López-Sánchez, L.; Miralles, P.; Salvador, A.; Merino-Sanjuán, M.; Merino, V. In Vitro Skin Penetration of Bronidox, Bronopol and Formaldehyde from Cosmetics. Regul. Toxicol. Pharmacol. 2021, 122, 104888. [Google Scholar] [CrossRef]
- Jahromi, B.; Razeghi, M.; Dastgheib, L.; Fazelzadeh, A.; Miri, A.; Vakili, S.; Foruhari, S.; Sabetian, S. Formaldehyde 5% in Flexible Collodion Compared to Cryotherapy for Treatment of Female Genital Warts: A Randomized Clinical Trial. Indian J. Dermatol. 2022, 67, 478. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, S.; Wu, S.H.; Chen, G.Z.; Huang, S.T.; Lin, C.M.; Lee, Y.C.; Chen, C.H. Development of Ratiometric Electrochemical Molecular Switches to Assay Endogenous Formaldehyde in Live Cells, Whole Blood and Creatinine in Saliva. Biosens. Bioelectron. 2021, 171, 112720. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Sheshukova, E.V.; Bialik, T.E.; Komarova, T.V. Human Endogenous Formaldehyde as an Anticancer Metabolite: Its Oxidation Downregulation May Be a Means of Improving Therapy. BioEssays 2018, 40, 1800136. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, W.; Zhang, J.; Zhao, H.; Cui, J.; Wu, J.; Shi, A. Dual Effects of Endogenous Formaldehyde on the Organism and Drugs for Its Removal. J. Appl. Toxicol. 2023, 44, 798–817. [Google Scholar] [CrossRef]

| Theme | Keywords and Boolean Descriptors |
|---|---|
| Oxidative Stress | “Oxidative Stress” AND “Antioxidants” |
| Formaldehyde | “Formaldehyde” |
| Histology | “Histology” OR “Histopathology” |
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Type of reference | Full-text articles, research articles | No full text, books/chapters, conference articles, review |
| Language | English language | Non-English language |
| Type of study | In vivo studies | In vitro studies |
| Outcome | Studies focused on antioxidant effects | Studies not related to antioxidant effect |
| Organ | Animal Model/ References | Antioxidant and Administration in Relation to FA | Outcome in Comparison with FA Administration by Biochemical and Histopathological Analysis |
|---|---|---|---|
| Liver | Mouse [37] | pumpkin seed oil 4 mL/kg p.o. + FA 10 mg/kg i.p. vit E 4 IU/kg p.o. + 10 mg/kg FA i.p., daily, 28 days |
|
| Brain | Rat [28] | lycopene 10 mg/kg p.o., daily, 12 weeks + FA (10 or 20 ppm) inhalation, 6 h, 5 days/week |
|
| Mouse [37] | pumpkin seed oil (4 mL/kg) p.o. + FA 10 mg/kg i.p. vit E 4 IU/kg p.o. + 10 mg/kg FA i.p., daily, 28 days |
| |
| Rat [34] | Matricaria chamomilla (200 mg/kg, 500 mg/kg) 1 h after FA 10 mg/kg i.p., 30 days |
| |
| Lung | Pregnant rat and offspring [1] |
|
|
| Kidney | Mouse [37] | pumpkin seed oil p.o. + FA i.p. vit. E p.o. + FA i.p., daily, 28 days |
|
| Organ | Animal Model/ Reference | Antioxidant and Administration in Relation to FA | Outcome in Comparison with FA Administration by Biochemical and Histopathological Analysis |
|---|---|---|---|
| Liver | Rat [26] | carvacrol 20 or 40 mg/kg i.p. once every 48 h + FA inhalation (10 ppm/8 h) 5 days/week, daily 4 weeks |
|
| Rat [40] | ferulic acid 50 mg/kg i.p. + FA 10 mg/kg i.p., daily, 10 days |
| |
| Rat [41] | proanthocyanidin 100 mg/kg i.g. + 10 mg/kg FA i.p. vitamin E 30 mg/kg i.g. + 10 mg/kg FA i.p., daily, 14 days |
| |
| Rat [5] | vitamin E 300 mg/kg i.m. + FA 10 mg/kg i.p., daily, 10 days |
| |
| Rat [7] | carnosine 100 mg/kg p.o. daily + FA 5.27 ± 0.24 or 10.02 ± 0.16 or 15.2 ± 0.19 ppm inhalation, 5 days/week, 4 weeks |
| |
| Rat [4] | carnosine 150 mg/kg/day p.o. + FA 10 ppm inhalation 8 h/day, 5 days/week, 4 weeks |
| |
| Brain | Rat [52] | thymoquinone in corn oil 20 mg/kg i.g. + FA 10 mg/kg diluted in 10% i.p., daily, 15 days |
|
| Rat [42] | vitamin E 300 mg/kg i.m. + FA 10 mg/kg i.p., daily, 10 days |
| |
| Mouse [43] | vitamin E 50 mg/kg i.g. + 0.155 mg/kg/day FA + 0.193 mg/kg/day PM2.5 intranasal instillation, daily, 7 days |
| |
| Rat [8] | curcumin 100 mg/kg i.g.+ FA 9 mg/kg i.p., daily, 2 weeks |
| |
| Rat [38] | melatonin 25 mg/kg i.p. + FA 10 mg/kg i.p., daily, 14 days |
| |
| Mouse [44] | selenium 0.1, 0.2, 0.4, 0.8 mg/kg i.p. + FA 10 mg/kg i.p., daily, 14 days |
| |
| Rat [31] | omega-3 fatty acids 400 mg/ kg i.g. + FA 10 mg/ kg i.p., daily, 14 days |
| |
| Rat [45] | L-carnitine 0.5, 1 g/kg i.p. + FA 10 mg/kg FA diluted with 10% PBS i.p., daily, 14 days |
| |
| Heart | Pregnant mouse [3] | vitamin E 0.1 μg i.p. + FA 0.5, 1, 1.5 mg/kg FA 40% (w/w) in aqueous solution i.p., daily, 21 days |
|
| Lung | Rats [7] | carnosine 100 mg/kg p.o. daily + FA 5.27 ± 0.24 or 10.02 ± 0.16 or 15.2 ± 0.19 ppm inhalation, 5 days/week, 4 weeks |
|
| Testis | Rat [35] | Matricaria chamomilla 200 mg/kg or 500 mg/kg i.p. + FA 10 mg/kg i.p. daily, 30 days |
|
| Mouse [46] | Rosa damascena 10, 20 or 40 mg/kg p.o. + FA 10% 10 mg/kg of i.p., daily, 40 days |
| |
| Mouse [47] | Ficus carica 200 mg/kg p.o. daily + 10 mg/kg FA (1/10) i.p. twice/day, 14 days |
| |
| Rat [48] | proanthocyanidin 100 mg/kg i.g. + FA 1/10 diluted 10 mg/kg i.p., daily, 14 days |
| |
| Rat [49] | vitamin E 30 mg/kg/day p.o. + FA 10 mg/m3 (12 h/day) by inhalation daily, 2 weeks |
| |
| Cornea | Rat [2] | Nigella sativa oil 40 mg/kg i.g. + FA 10% 2 h/day inhalation, 5 days/week, for 2 weeks |
|
| Rat [50] | spirulina dissolved in distilled water p.o. of 400 mg/kg daily + 10% FA inhalation for 2 h/day 5 days per week, 2 weeks |
| |
| Joints | Rat with rheumatoid arthritis [51] | Sarcococca saligna 250, 500, 1000 mg/kg p.o. + FA 0.1 mL 2% subplantar daily, 28 days |
|
| Kidney | Rat [39] | proanthocyanidin 100 mg/kg p.o. + FA 10 mg/kg 1:10 with NS i.p. vitamin E 30 mg/kg i.g. FA 10 mg/kg 1:10 with NS i.p., daily, 14 days |
|
| Rat [31] | melatonin 25 mg/kg i.p. + FA 10 mg/kg i.p., daily, 14 days |
| |
| Rat [53] | omega-3 fatty acids 400 mg/kg i.g. + FA 10 mg/kg i.p., daily, 14 days |
| |
| Rat [4] | carnosine 150 mg/kg/day p.o. + FA 10 ppm for 8 h/day, 5 days/week by inhalation, 4 weeks |
|
| Organ | Animal Model/ Reference | Antioxidant and Administration in Relation to FA | Outcome in Comparison with FA Administration by Biochemical and Histopathological Analysis |
|---|---|---|---|
| Brain | Mouse [29] | Epigallocatechin-3-gallate 20, 100, 500 mg/kg p.o. 1 h after 3 mg/m3 FA inhalation for 8 h, daily, 14 days |
|
| Lung | Rat [27] | Bronco-T p.o. after 1 h FA 40% vapor environment Salbutamol p.o. 1 h after FA 40% vapor environment |
|
| Testis | Rat [55] | Rose oil inhalation 1 mL/1 h after FA 10 ppm/1 h inhalation, 35 days |
|
| Rat [32] | Melatonin 25 mg/kg i.p. 1 h after FA 10 mg/kg, i.p., daily, 1 month |
| |
| Mouse [54] | Manganese chloride 5 mg kg/day i.p. a week after FA 10 mg/kg twice per day i.p., 2 weeks |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungureanu, L.B.; Ghiciuc, C.M.; Amalinei, C.; Ungureanu, C.; Petrovici, C.G.; Stănescu, R.Ș. Antioxidants as Protection against Reactive Oxygen Stress Induced by Formaldehyde (FA) Exposure: A Systematic Review. Biomedicines 2024, 12, 1820. https://doi.org/10.3390/biomedicines12081820
Ungureanu LB, Ghiciuc CM, Amalinei C, Ungureanu C, Petrovici CG, Stănescu RȘ. Antioxidants as Protection against Reactive Oxygen Stress Induced by Formaldehyde (FA) Exposure: A Systematic Review. Biomedicines. 2024; 12(8):1820. https://doi.org/10.3390/biomedicines12081820
Chicago/Turabian StyleUngureanu, Loredana Beatrice, Cristina Mihaela Ghiciuc, Cornelia Amalinei, Carmen Ungureanu, Cristina Gabriela Petrovici, and Raluca Ștefania Stănescu. 2024. "Antioxidants as Protection against Reactive Oxygen Stress Induced by Formaldehyde (FA) Exposure: A Systematic Review" Biomedicines 12, no. 8: 1820. https://doi.org/10.3390/biomedicines12081820
APA StyleUngureanu, L. B., Ghiciuc, C. M., Amalinei, C., Ungureanu, C., Petrovici, C. G., & Stănescu, R. Ș. (2024). Antioxidants as Protection against Reactive Oxygen Stress Induced by Formaldehyde (FA) Exposure: A Systematic Review. Biomedicines, 12(8), 1820. https://doi.org/10.3390/biomedicines12081820





