Revisiting the Immunometabolic Basis for the Metabolic Syndrome from an Immunonutritional View
Abstract
1. Introduction
2. Data and Methodologies
2.1. Data Source
2.2. Methodology
3. Prevalence and Genetic Risk Factors for the MetS
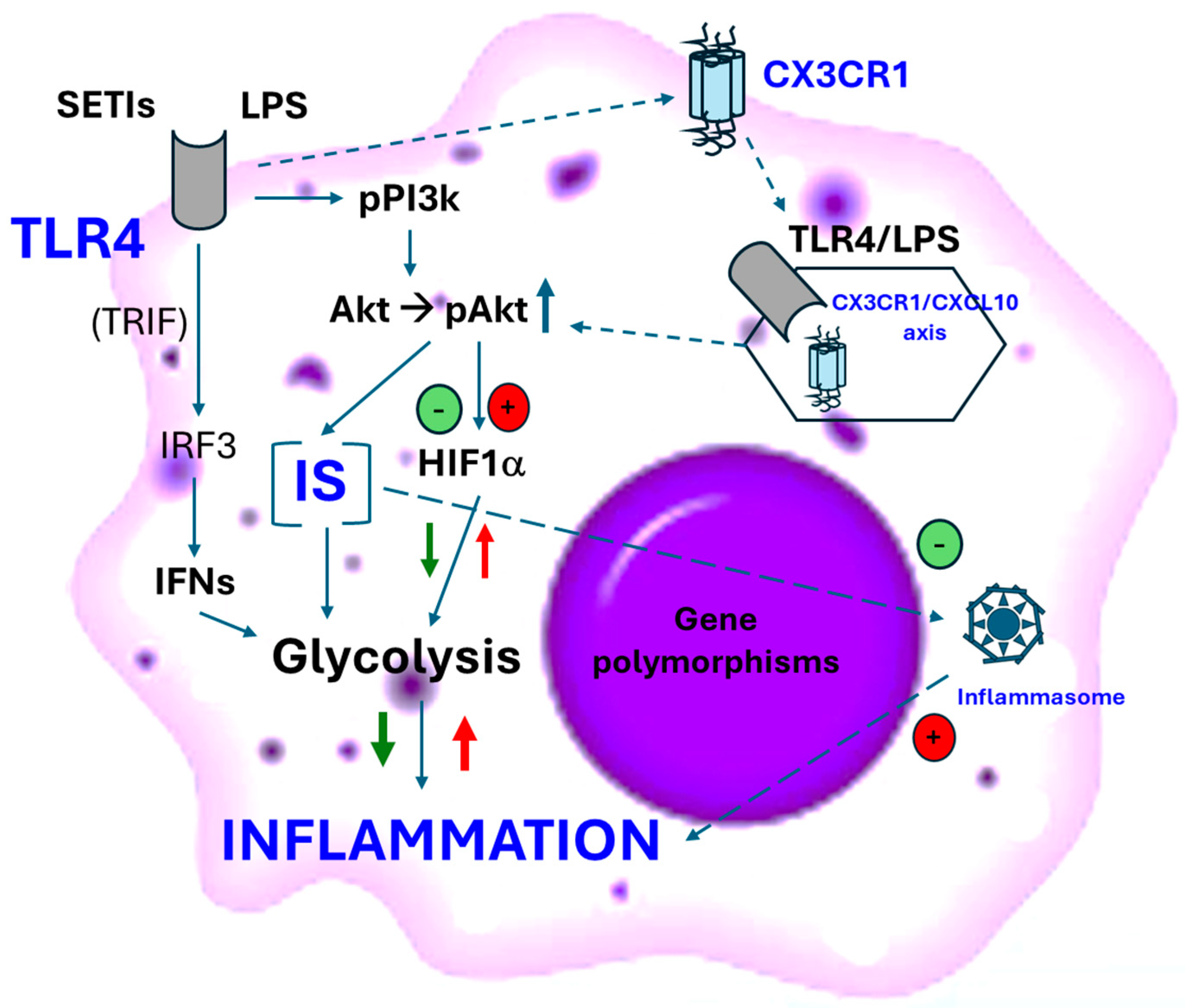
4. Innate Immunity’s Activity on the Insulin Signaling
5. Factors Determining the Innate Immune Adaptations in the MetS: Macrophage Polarization under Insulin Signaling
6. Concluding Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. A Global Brief of Hypertension: Silent Killer, Global Public Health Crisis; WHO/DCO/WHD/2013, World Health Day 2013; WHO: Geneva, Switzerland, 2013.
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease—Meta-analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Hernaez, R.; Eberhardt, M.S.; Bonekamp, S.; Kamel, I.; Guallar, E.; Koteish, A.; Brancati, F.L.; Clark, J.M. Prevalence of Nonalcoholic Fatty Liver Disease in the United States: The Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2013, 178, 38–45. [Google Scholar] [CrossRef]
- Asrih, M.; Jornayvaz, F.R. Metabolic Syndrome and Nonalcoholic Fatty Liver Disease: Is Insulin Resistance the Link? Mol. Cell Endocrinol. 2015, 418, 55–65. [Google Scholar] [CrossRef]
- Patel, B.M.; Goyal, R.K. Liver and Insulin Resistance: New Wine in Old Bottle!!! Eur. J. Pharmacol. 2019, 862, 172657. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Aronne, L.J.; Astrup, A.; de Cabo, R.; Cantley, L.C.; Friedman, M.I.; Heymsfield, S.B.; Johnson, J.D.; King, J.C.; Krauss, R.M.; et al. The Carbohydrate-Insulin Model: A Physiological Perspective on the Obesity Pandemic. Am. J. Clin. Nutr. 2021, 114, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Moro, K.; Kubota, T.; Kubota, N.; Kato, T.; Ohno, H.; Nakae, S.; Saito, H.; Koyasu, S. Innate Lymphoid Cells in the Induction of Obesity. Cell Rep. 2019, 28, 202–217.e7. [Google Scholar] [CrossRef] [PubMed]
- Cox, N.; Crozet, L.; Holtman, I.R.; Loyher, P.-L.; Lazarov, T.; White, J.B.; Mass, E.; Stanley, E.R.; Elemento, O.; Glass, C.K.; et al. Diet-Regulated Production of PDGFcc by Macrophages Controls Energy Storage. Science 1979 2021, 373, eabe9383. [Google Scholar] [CrossRef]
- Lu, P.; Sodhi, C.P.; Yamaguchi, Y.; Jia, H.; Prindle, T.; Fulton, W.B.; Vikram, A.; Bibby, K.J.; Morowitz, M.J.; Hackam, D.J. Intestinal Epithelial Toll-like Receptor 4 Prevents Metabolic Syndrome by Regulating Interactions between Microbes and Intestinal Epithelial Cells in Mice. Mucosal Immunol. 2018, 11, 727–740. [Google Scholar] [CrossRef]
- Mao, K.; Baptista, A.P.; Tamoutounour, S.; Zhuang, L.; Bouladoux, N.; Martins, A.J.; Huang, Y.; Gerner, M.Y.; Belkaid, Y.; Germain, R.N. Innate and Adaptive Lymphocytes Sequentially Shape the Gut Microbiota and Lipid Metabolism. Nature 2018, 554, 255–259. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Gut Microbiota as a Regulator of Energy Homeostasis and Ectopic Fat Deposition: Mechanisms and Implications for Metabolic Disorders. Curr. Opin. Lipidol. 2010, 21, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kanneganti, T.-D.; Dixit, V.D. Immunological Complications of Obesity. Nat. Immunol. 2012, 13, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha Rajkumar, P.B. The Impact of Obesity on Immune Response to Infection and Vaccine: An Insight into Plausible Mechanisms. Endocrinol. Metab. Syndr. 2013, 2, 1000113–1000122. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Zhou, H.; Li, Y.; Song, Y.-L. NOD1 Activation Induces Innate Immune Responses and Insulin Resistance in Human Adipocytes. Diabetes Metab. 2012, 38, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.L.; Tam, T.H.; Boroumand, P.; Prescott, D.; Costford, S.R.; Escalante, N.K.; Fine, N.; Tu, Y.; Robertson, S.J.; Prabaharan, D.; et al. Circulating NOD1 Activators and Hematopoietic NOD1 Contribute to Metabolic Inflammation and Insulin Resistance. Cell Rep. 2017, 18, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-J.; Liu, C.; Li, C.-L.; Song, Y.-L.; Tang, Y.-S.; Zhou, H.; Li, A.; Li, Y.; Weng, Y.; Zheng, F.-P. Increased NOD1, but Not NOD2, Activity in Subcutaneous Adipose Tissue from Patients with Metabolic Syndrome. Obesity 2015, 23, 1394–1400. [Google Scholar] [CrossRef]
- Ruggiero, A.D.; Vemuri, R.; Block, M.; DeStephanis, D.; Davis, M.; Chou, J.; Williams, A.; Brock, A.; Das, S.K.; Kavanagh, K. Macrophage Phenotypes and Gene Expression Patterns Are Unique in Naturally Occurring Metabolically Healthy Obesity. Int. J. Mol. Sci. 2022, 23, 12680. [Google Scholar] [CrossRef] [PubMed]
- da Cunha, L.R.; Muniz-Junqueira, M.I.; dos Santos Borges, T.K. Impact of Polyphenols in Phagocyte Functions. J. Inflamm. Res. 2019, 12, 205–217. [Google Scholar] [CrossRef]
- Georgel, P.T.; Georgel, P. Where Epigenetics Meets Food Intake: Their Interaction in the Development/Severity of Gout and Therapeutic Perspectives. Front. Immunol. 2021, 12, 752359. [Google Scholar] [CrossRef]
- Singer, K.; Lumeng, C.N. The Initiation of Metabolic Inflammation in Childhood Obesity. J. Clin. Investig. 2017, 127, 65–73. [Google Scholar] [CrossRef]
- Nauta, A.J.; Ben Amor, K.; Knol, J.; Garssen, J.; van der Beek, E. Relevance of Pre- and Postnatal Nutrition to Development and Interplay between the Microbiota and Metabolic and Immune Systems. Am. J. Clin. Nutr. 2013, 98, 586S–593S. [Google Scholar] [CrossRef]
- Luo, Z.-C.; Xiao, L.; Nuyt, A.-M. Mechanisms of Developmental Programming of the Metabolic Syndrome and Related Disorders. World J. Diabetes 2010, 1, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Aumueller, E.; Jahn, D.; Hippe, B.; Brath, H.; Haslberger, A.G. Microbiota and Epigenetic Regulation of Inflammatory Mediators in Type 2 Diabetes and Obesity. Benef. Microbes 2014, 5, 33–43. [Google Scholar] [CrossRef]
- Bäckhed, F. Programming of Host Metabolism by the Gut Microbiota. Ann. Nutr. Metab. 2011, 58, 44–52. [Google Scholar] [CrossRef]
- Sáez-Lara, M.; Robles-Sanchez, C.; Ruiz-Ojeda, F.; Plaza-Diaz, J.; Gil, A. Effects of Probiotics and Synbiotics on Obesity, Insulin Resistance Syndrome, Type 2 Diabetes and Non-Alcoholic Fatty Liver Disease: A Review of Human Clinical Trials. Int. J. Mol. Sci. 2016, 17, 928. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Cardona Velásquez, S.; Guzmán Vivares, L.; Cardona-Arias, J.A. Caracterización de Ensayos Clínicos Relacionados Con El Tratamiento Del Síndrome Metabólico, 1980-2015. Endocrinol. Diabetes Nutr. 2017, 64, 82–91. [Google Scholar] [CrossRef]
- Elsaid, M.I.; Bridges, J.F.P.; Mumtaz, K.; Li, N.; Sobotka, L.; Rustgi, V.K.; Paskett, E.D. The Impact of Metabolic Syndrome Severity on Racial and Ethnic Disparities in Metabolic Dysfunction-Associated Steatotic Liver Disease. PLoS ONE 2024, 19, e0299836. [Google Scholar] [CrossRef]
- Bambha, K.; Belt, P.; Abraham, M.; Wilson, L.A.; Pabst, M.; Ferrell, L.; Unalp-Arida, A.; Bass, N. Ethnicity and Nonalcoholic Fatty Liver Disease. Hepatology 2012, 55, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Real, J.-M. Genetic Predispositions to Low-Grade Inflammation and Type 2 Diabetes. Diabetes Technol. Ther. 2006, 8, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.C.; González, C.; Pirola, C.J. Meta-analysis on the G- 308 A Tumor Necrosis Factor α Gene Variant and Phenotypes Associated with the Metabolic Syndrome. Obes. Res. 2005, 13, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; He, L.; Zhang, X. -308G/A Polymorphism of Tumor Necrosis Factor Alpha (TNF-α) Gene and Metabolic Syndrome Susceptibility: A Meta-Analysis. Sci. Rep. 2021, 11, 3840. [Google Scholar] [CrossRef] [PubMed]
- Barati, E.; Ghazizadeh, H.; Sadabadi, F.; Kazemi, E.; Ferns, G.A.; Avan, A.; Ghayour-Mobarhan, M. Association of the IL6 Gene Polymorphism with Component Features of Metabolic Syndrome in Obese Subjects. Biochem. Genet. 2019, 57, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Boeta-Lopez, K.; Duran, J.; Elizondo, D.; Gonzales, E.; Rentfro, A.; Schwarzbach, A.E.; Nair, S. Association of Interleukin-6 Polymorphisms with Obesity or Metabolic Traits in Young Mexican-Americans. Obes. Sci. Pract. 2018, 4, 85–96. [Google Scholar] [CrossRef]
- Teixeira, A.A.; Quinto, B.M.R.; Dalboni, M.A.; Rodrigues, C.J.d.O.; Batista, M.C. Association of IL-6 Polymorphism -174G/C and Metabolic Syndrome in Hypertensive Patients. Biomed. Res. Int. 2015, 2015, 1927589. [Google Scholar] [CrossRef]
- Madeshiya, A.K.; Singh, S.; Dwivedi, S.; Konwar, R.; Natu, S.M.; Ghatak, A. Association of IL-10 Gene (−1082A>G, −819C>T and −592C>A) Polymorphism and Its Serum Level with Metabolic Syndrome of North Indian Subjects. J. Genet. 2017, 96, 53–64. [Google Scholar] [CrossRef]
- Fatima, S.S.; Jamil, Z.; Abidi, S.H.; Nadeem, D.; Bashir, Z.; Ansari, A. Interleukin-18 Polymorphism as an Inflammatory Index in Metabolic Syndrome: A Preliminary Study. World J. Diabetes 2017, 8, 304. [Google Scholar] [CrossRef]
- Presta, I.; Andreozzi, F.; Succurro, E.; Marini, M.A.; Laratta, E.; Lauro, R.; Hribal, M.L.; Perticone, F.; Sesti, G. IL-18 Gene Polymorphism and Metabolic Syndrome. Nutr. Metab. Cardiovasc. Dis. 2009, 19, e5–e6. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Ding, D.; Huang, J.; Qu, Y.; Wang, Y.; Huang, Q. Association of Genetic Variants in the Adiponectin Gene with Metabolic Syndrome: A Case-Control Study and a Systematic Meta-Analysis in the Chinese Population. PLoS ONE 2013, 8, e58412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Q.; Ren, D.; Bi, Y.; Yuan, R.; Li, D.; Wang, J.; Wang, R.; Zhang, L.; He, G.; Liu, B. Association and Functional Study between ADIPOQ and Metabolic Syndrome in Elderly Chinese Han Population. Aging 2020, 12, 25819–25827. [Google Scholar] [CrossRef]
- Yuan, H.-P.; Sun, L.; Li, X.-H.; Che, F.-G.; Zhu, X.-Q.; Yang, F.; Han, J.; Jia, C.-Y.; Yang, Z. Association of Adiponectin Polymorphism with Metabolic Syndrome Risk and Adiponectin Level with Stroke Risk: A Meta-Analysis. Sci. Rep. 2016, 6, 31945. [Google Scholar] [CrossRef]
- Zhou, J.M.; Zhang, M.; Wang, S.; Wang, B.Y.; Han, C.Y.; Ren, Y.C.; Zhang, L.; Zhang, H.Y.; Yang, X.Y.; Zhao, Y.; et al. Association of the ADIPOQ Rs2241766 and Rs266729 Polymorphisms with Metabolic Syndrome in the Chinese Population: A Meta-Analysis. Biomed. Environ. Sci. 2016, 29, 505–515. [Google Scholar] [CrossRef]
- Steinhardt, A.P.; Aranguren, F.; Tellechea, M.L.; Gómez Rosso, L.A.; Brites, F.D.; Martínez-Larrad, M.T.; Serrano-Ríos, M.; Frechtel, G.D.; Taverna, M.J. A Functional Nonsynonymous Toll-like Receptor 4 Gene Polymorphism Is Associated with Metabolic Syndrome, Surrogates of Insulin Resistance, and Syndromes of Lipid Accumulation. Metabolism 2010, 59, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Lorenz, E.; Reindl, M.; Wiedermann, C.J.; Oberhollenzer, F.; Bonora, E.; Willeit, J.; Schwartz, D.A. Toll-like Receptor 4 Polymorphisms and Atherogenesis. N. Engl. J. Med. 2002, 347, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kolek, M.J.; Carlquist, J.F.; Muhlestein, J.B.; Whiting, B.M.; Horne, B.D.; Bair, T.L.; Anderson, J.L. Toll–like Receptor 4 Gene Asp299Gly Polymorphism Is Associated with Reductions in Vascular Inflammation, Angiographic Coronary Artery Disease, and Clinical Diabetes. Am. Heart J. 2004, 148, 1034–1040. [Google Scholar] [CrossRef]
- Davis, F.M.; DenDekker, A.; Kimball, A.; Joshi, A.D.; El Azzouny, M.; Wolf, S.J.; Obi, A.T.; Lipinski, J.; Gudjonsson, J.E.; Xing, X.; et al. Epigenetic Regulation of TLR4 in Diabetic Macrophages Modulates Immunometabolism and Wound Repair. J. Immunol. 2020, 204, 2503–2513. [Google Scholar] [CrossRef]
- Trøseid, M.; Seljeflot, I.; Arnesen, H. The Role of Interleukin-18 in the Metabolic Syndrome. Cardiovasc. Diabetol. 2010, 9, 11. [Google Scholar] [CrossRef]
- Ernst, O.; Glucksam-Galnoy, Y.; Bhatta, B.; Athamna, M.; Ben-Dror, I.; Glick, Y.; Gerber, D.; Zor, T. Exclusive Temporal Stimulation of IL-10 Expression in LPS-Stimulated Mouse Macrophages by CAMP Inducers and Type I Interferons. Front. Immunol. 2019, 10, 1788. [Google Scholar] [CrossRef] [PubMed]
- Abraham, L.J.; Kroeger, K.M. Impact of the -308 TNF Promoter Polymorphism on the Transcriptional Regulation of the TNF Gene: Relevance to Disease. J. Leukoc. Biol. 1999, 66, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Razquin, C.; Martínez, J.A.; Martínez-González, M.A.; Salas-Salvadó, J.; Estruch, R.; Marti, A. A 3-Year Mediterranean-Style Dietary Intervention May Modulate the Association between Adiponectin Gene Variants and Body Weight Change. Eur. J. Nutr. 2010, 49, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.W.; Koch, T.C.L.; Watzl, B.; Dietrich, H.; Will, F.; Bub, A. Moderate Effects of Apple Juice Consumption on Obesity-Related Markers in Obese Men: Impact of Diet–Gene Interaction on Body Fat Content. Eur. J. Nutr. 2012, 51, 841–850. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Primo, D.; Izaola, O.; Gomez Hoyos, E.; Lopez Gomez, J.J.; Ortola, A.; Aller, R. Role of the Variant in Adiponectin Gene Rs266729 on Weight Loss and Cardiovascular Risk Factors after a Hypocaloric Diet with the Mediterranean Pattern. Nutrition 2019, 60, 1–5. [Google Scholar] [CrossRef] [PubMed]
- de Luis, D.A.; Primo, D.; Izaola, O.; Gómez, E.; Bachiller, R. Serum Lipid and Adiponectin Improvements after a Mediterranean Dietary Pattern in Non-G-Allele Carriers of the Variant Rs3774261. Lifestyle Genom. 2020, 13, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Delgado, F.; Alcala-Diaz, J.F.; Garcia-Rios, A.; Delgado-Lista, J.; Ortiz-Morales, A.; Rangel-Zuñiga, O.; Tinahones, F.J.; Gonzalez-Guardia, L.; Malagon, M.M.; Bellido-Muñoz, E.; et al. Polymorphism at the TNF-alpha Gene Interacts with Mediterranean Diet to Influence Triglyceride Metabolism and Inflammation Status in Metabolic Syndrome Patients: From the CORDIOPREV Clinical Trial. Mol. Nutr. Food Res. 2014, 58, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Wieser, V.; Adolph, T.E.; Grander, C.; Grabherr, F.; Enrich, B.; Moser, P.; Moschen, A.R.; Kaser, S.; Tilg, H. Adipose Type I Interferon Signalling Protects against Metabolic Dysfunction. Gut 2018, 67, 157–165. [Google Scholar] [CrossRef]
- Guarda, G.; Braun, M.; Staehli, F.; Tardivel, A.; Mattmann, C.; Förster, I.; Farlik, M.; Decker, T.; Du Pasquier, R.A.; Romero, P.; et al. Type I Interferon Inhibits Interleukin-1 Production and Inflammasome Activation. Immunity 2011, 34, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, J.; Kong, X.; Tenta, M.; Wang, X.; Kang, S.; Rosen, E.D. Interferon Regulatory Factor 4 Regulates Obesity-Induced Inflammation Through Regulation of Adipose Tissue Macrophage Polarization. Diabetes 2013, 62, 3394–3403. [Google Scholar] [CrossRef]
- Memon, R.A.; Feingold, K.R.; Moser, A.H.; Doerrler, W.; Grunfeld, C. In Vivo Effects of Interferon-Alpha and Interferon-Gamma on Lipolysis and Ketogenesis. Endocrinology 1992, 131, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, G.; Sivasami, P.; Ramirez, R.N.; Zhang, Y.; Benoist, C.; Mathis, D. Interferon-α-Producing Plasmacytoid Dendritic Cells Drive the Loss of Adipose Tissue Regulatory T Cells during Obesity. Cell Metab. 2021, 33, 1610–1623.e5. [Google Scholar] [CrossRef] [PubMed]
- Bassaganya-Riera, J.; Misyak, S.; Guri, A.J.; Hontecillas, R. PPAR γ Is Highly Expressed in F4/80hi Adipose Tissue Macrophages and Dampens Adipose-Tissue Inflammation. Cell Immunol. 2009, 258, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M.; Palmieri, E.M.; Gonzalez-Cotto, M.; Bettencourt, I.A.; Megill, E.L.; Snyder, N.W.; McVicar, D.W. Itaconic Acid Underpins Hepatocyte Lipid Metabolism in Non-Alcoholic Fatty Liver Disease in Male Mice. Nat. Metab. 2023, 5, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, H.J.; Song, J.-H.; Shin, Y.K.; Abd El-Aty, A.M.; Ramadan, A.; Hacimuftuoglu, A.; Jeong, J.H.; Jung, T.W. Dimethyl Itaconate Attenuates Palmitate-Induced Insulin Resistance in Skeletal Muscle Cells through the AMPK/FGF21/PPARδ-Mediated Suppression of Inflammation. Life Sci. 2021, 287, 120129. [Google Scholar] [CrossRef] [PubMed]
- Serbulea, V.; Upchurch, C.M.; Schappe, M.S.; Voigt, P.; DeWeese, D.E.; Desai, B.N.; Meher, A.K.; Leitinger, N. Macrophage Phenotype and Bioenergetics Are Controlled by Oxidized Phospholipids Identified in Lean and Obese Adipose Tissue. Proc. Natl. Acad. Sci. USA 2018, 115, E6254–E6263. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, H.; Tao, Y.; Jie, H.; Zhao, J.; Zang, J.; Li, H.; Wang, Y.; Wang, T.; Zhao, H.; et al. CX3CR1hi Macrophages Sustain Metabolic Adaptation by Relieving Adipose-Derived Stem Cell Senescence in Visceral Adipose Tissue. Cell Rep. 2023, 42, 112424. [Google Scholar] [CrossRef]
- Li, G.; Yu, H.; Liu, N.; Zhang, P.; Tang, Y.; Hu, Y.; Zhang, Y.; Pan, C.; Deng, H.; Wang, J.; et al. Overexpression of CX3CR1 in Adipose-Derived Stem Cells Promotes Cell Migration and Functional Recovery After Experimental Intracerebral Hemorrhage. Front. Neurosci. 2019, 13, 462. [Google Scholar] [CrossRef]
- Nagashimada, M.; Sawamoto, K.; Ni, Y.; Kitade, H.; Nagata, N.; Xu, L.; Kobori, M.; Mukaida, N.; Yamashita, T.; Kaneko, S.; et al. CX3CL1-CX3CR1 Signaling Deficiency Exacerbates Obesity-Induced Inflammation and Insulin Resistance in Male Mice. Endocrinology 2021, 162, bqab064. [Google Scholar] [CrossRef]
- Ni, Y.; Zhuge, F.; Ni, L.; Nagata, N.; Yamashita, T.; Mukaida, N.; Kaneko, S.; Ota, T.; Nagashimada, M. CX3CL1/CX3CR1 Interaction Protects against Lipotoxicity-Induced Nonalcoholic Steatohepatitis by Regulating Macrophage Migration and M1/M2 Status. Metabolism 2022, 136, 155272. [Google Scholar] [CrossRef]
- Garcia Tejedor, A.; Haros, C.M.; Laparra Llopis, J.M. Chenopodium Quinoa’s Ingredients Improve Control of the Hepatic Lipid Disturbances Derived from a High-Fat Diet. Foods 2023, 12, 3321. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.C.C.; de Souza, B.R.; Reis, I.B.; de Arruda Camargo, G.C.; de Oliveira, G.; de Barros Frazão Salmazo, M.I.; Gonçalves, J.M.; de Castro Roston, J.R.; Caria, P.H.F.; da Silva Santos, A.; et al. OncoTherad® (MRB-CFI-1) Nanoimmunotherapy: A Promising Strategy to Treat Bacillus Calmette–Guérin-Unresponsive Non-Muscle-Invasive Bladder Cancer: Crosstalk among T-Cell CX3CR1, Immune Checkpoints, and the Toll-Like Receptor 4 Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 17535. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.-Y.; Fang, S.-P.; Zhou, M.; Luo, J.; Wei, J.; Wen, X.-P.; Yan, X.-D.; Zou, Z. TLR4-Dependent Internalization of CX3CR1 Aggravates Sepsis-Induced Immunoparalysis. Am. J. Transl. Res. 2016, 8, 5696–5705. [Google Scholar] [PubMed]
- Daryabor, G.; Atashzar, M.R.; Kabelitz, D.; Meri, S.; Kalantar, K. The Effects of Type 2 Diabetes Mellitus on Organ Metabolism and the Immune System. Front. Immunol. 2020, 11, 1582. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Arrese, M.; Cabrera, D.; Kalergis, A.M.; Feldstein, A.E. Innate Immunity and Inflammation in NAFLD/NASH. Dig. Dis. Sci. 2016, 61, 1294–1303. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory Mechanisms Linking Obesity and Metabolic Disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Datz, C.; Felder, T.K.; Niederseer, D.; Aigner, E. Iron Homeostasis in the Metabolic Syndrome. Eur. J. Clin. Investig. 2013, 43, 215–224. [Google Scholar] [CrossRef]
- Pereira, M.; Chen, T.-D.; Buang, N.; Olona, A.; Ko, J.-H.; Prendecki, M.; Costa, A.S.H.; Nikitopoulou, E.; Tronci, L.; Pusey, C.D.; et al. Acute Iron Deprivation Reprograms Human Macrophage Metabolism and Reduces Inflammation In Vivo. Cell Rep. 2019, 28, 498–511.e5. [Google Scholar] [CrossRef]
- Martinelli, N.; Traglia, M.; Campostrini, N.; Biino, G.; Corbella, M.; Sala, C.; Busti, F.; Masciullo, C.; Manna, D.; Previtali, S.; et al. Increased Serum Hepcidin Levels in Subjects with the Metabolic Syndrome: A Population Study. PLoS ONE 2012, 7, e48250. [Google Scholar] [CrossRef]
- Ghadimi, D.; Yoness Hassan, M.F.; Fölster-Holst, R.; Röcken, C.; Ebsen, M.; de Vrese, M.; Heller, K.J. Regulation of Hepcidin/Iron-Signalling Pathway Interactions by Commensal Bifidobateria Plays an Important Role for the Inhibition of Metaflammation-Related Biomarkers. Immunobiology 2020, 225, 151874. [Google Scholar] [CrossRef] [PubMed]
- Rueda Huélamo, M.A.; Martínez Perlado, A.; Consoli, V.; García-Tejedor, A.; Haros, C.M.; Laparra Llopis, J.M. Improvement of Hepatic Innate Immunity in Chemically-Injured Livers to Develop Hepatocarcinoma by a Serine Type-Protease Inhibitors Enriched Extract from Chenopodium quinoa. Food Funct. 2024, 15, 3600–3614. [Google Scholar] [CrossRef] [PubMed]
- Laparra, J.; Fotschki, B.; Haros, C. Immunonutritional Consequences of Different Serine-Type Protease Inhibitors in a C57BL/6 Hepatocarcinoma Model. Oncotarget 2019, 10, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Bishehsari, F.; Voigt, R.M.; Keshavarzian, A. Circadian Rhythms and the Gut Microbiota: From the Metabolic Syndrome to Cancer. Nat. Rev. Endocrinol. 2020, 16, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Vijay-Kumar, M.; Aitken, J.D.; Carvalho, F.A.; Cullender, T.C.; Mwangi, S.; Srinivasan, S.; Sitaraman, S.V.; Knight, R.; Ley, R.E.; Gewirtz, A.T. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-Like Receptor 5. Science 1979 2010, 328, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; Papandreou, C.; Arcelin, P.; Garcia, D.; Palau-Galindo, A.; Gutiérrez-Tordera, L.; Folch, À.; Bulló, M. Examining the Interaction of the Gut Microbiome with Host Metabolism and Cardiometabolic Health in Metabolic Syndrome. Nutrients 2021, 13, 4318. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Colonna, M. Innate Lymphoid Cells: A Potential Link between Microbiota and Immune Responses against Cancer. Semin. Immunol. 2019, 41, 101271. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms Underlying the Resistance to Diet-Induced Obesity in Germ-Free Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef]
- Jeon, Y.G.; Kim, Y.Y.; Lee, G.; Kim, J.B. Physiological and Pathological Roles of Lipogenesis. Nat. Metab. 2023, 5, 735–759. [Google Scholar] [CrossRef]
- Lackey, D.E.; Olefsky, J.M. Regulation of Metabolism by the Innate Immune System. Nat. Rev. Endocrinol. 2016, 12, 15–28. [Google Scholar] [CrossRef]
- Ni, Y.; Ni, L.; Zhuge, F.; Xu, L.; Fu, Z.; Ota, T. Adipose Tissue Macrophage Phenotypes and Characteristics: The Key to Insulin Resistance in Obesity and Metabolic Disorders. Obesity 2020, 28, 225–234. [Google Scholar] [CrossRef]
- Rivers, S.L.; Klip, A.; Giacca, A. NOD1: An Interface Between Innate Immunity and Insulin Resistance. Endocrinology 2019, 160, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Vandanmagsar, B.; Youm, Y.-H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 Inflammasome Instigates Obesity-Induced Inflammation and Insulin Resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-W.; Hung, L.-C.; Chen, Y.-C.; Wang, W.-H.; Lin, C.-Y.; Tzeng, H.-H.; Suen, J.-L.; Chen, Y.-H. Insulin Reduces Inflammation by Regulating the Activation of the NLRP3 Inflammasome. Front. Immunol. 2021, 11, 587229. [Google Scholar] [CrossRef]
- Rheinheimer, J.; de Souza, B.M.; Cardoso, N.S.; Bauer, A.C.; Crispim, D. Current Role of the NLRP3 Inflammasome on Obesity and Insulin Resistance: A Systematic Review. Metabolism 2017, 74, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity Is Associated with Macrophage Accumulation in Adipose Tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Kim, J.; Bachmann, R.A.; Chen, J. Chapter 21 Interleukin-6 and Insulin Resistance. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2009; Volume 80, pp. 613–633. [Google Scholar]
- Russell, D.G.; Huang, L.; VanderVen, B.C. Immunometabolism at the Interface between Macrophages and Pathogens. Nat. Rev. Immunol. 2019, 19, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Vazirian, F.; Darroudi, S.; Rahimi, H.R.; Latifi, M.; Shakeri, B.; Abolbashari, S.; Mohammadpour, A.H.; Esmaily, H.; Mouhebati, M.; Samadi, S.; et al. Non-HDL Cholesterol and Long-Term Follow-up Outcomes in Patients with Metabolic Syndrome. Lipids Health Dis. 2023, 22, 165. [Google Scholar] [CrossRef]
- Lee, M.-S.; Bensinger, S.J. Reprogramming Cholesterol Metabolism in Macrophages and Its Role in Host Defense against Cholesterol-Dependent Cytolysins. Cell Mol. Immunol. 2022, 19, 327–336. [Google Scholar] [CrossRef]
- Ioannidis, I.; Ye, F.; McNally, B.; Willette, M.; Flaño, E. Toll-Like Receptor Expression and Induction of Type I and Type III Interferons in Primary Airway Epithelial Cells. J. Virol. 2013, 87, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Irie, E.; Ishihara, R.; Mizushima, I.; Hatai, S.; Hagihara, Y.; Takada, Y.; Tsunoda, J.; Iwata, K.; Matsubara, Y.; Yoshimatsu, Y.; et al. Enrichment of Type I Interferon Signaling in Colonic Group 2 Innate Lymphoid Cells in Experimental Colitis. Front. Immunol. 2022, 13, 982827. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chu, C.; Teng, F.; Bessman, N.J.; Goc, J.; Santosa, E.K.; Putzel, G.G.; Kabata, H.; Kelsen, J.R.; Baldassano, R.N.; et al. Innate Lymphoid Cells Support Regulatory T Cells in the Intestine through Interleukin-2. Nature 2019, 568, 405–409. [Google Scholar] [CrossRef] [PubMed]

| Gene | SNP | Related Disease | Reference |
|---|---|---|---|
| TNF-α | rs1800629 | MS, hypertension, insulin resistance | [35,36] |
| IL-6 | rs1800795; rs1800796 | MS, diabetes, obesity | [37,38,39] |
| IL-10 | rs1800896; rs1800872; rs1800871 | MS and diabetes | [40] |
| IL-18 | rs1946518; rs5744292 | MS and inflammation | [41,42] |
| ADIPOQ | rs2241766; rs266729 rs3774261; rs6773957 rs1501299 | MS | [43,44,45,46] |
| TLR-4 | rs4986790 | MS; insulin resistance | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apaza, C.J.; Cerezo, J.F.; García-Tejedor, A.; Giménez-Bastida, J.A.; Laparra-Llopis, J.M. Revisiting the Immunometabolic Basis for the Metabolic Syndrome from an Immunonutritional View. Biomedicines 2024, 12, 1825. https://doi.org/10.3390/biomedicines12081825
Apaza CJ, Cerezo JF, García-Tejedor A, Giménez-Bastida JA, Laparra-Llopis JM. Revisiting the Immunometabolic Basis for the Metabolic Syndrome from an Immunonutritional View. Biomedicines. 2024; 12(8):1825. https://doi.org/10.3390/biomedicines12081825
Chicago/Turabian StyleApaza, César Jeri, Juan Francisco Cerezo, Aurora García-Tejedor, Juan Antonio Giménez-Bastida, and José Moisés Laparra-Llopis. 2024. "Revisiting the Immunometabolic Basis for the Metabolic Syndrome from an Immunonutritional View" Biomedicines 12, no. 8: 1825. https://doi.org/10.3390/biomedicines12081825
APA StyleApaza, C. J., Cerezo, J. F., García-Tejedor, A., Giménez-Bastida, J. A., & Laparra-Llopis, J. M. (2024). Revisiting the Immunometabolic Basis for the Metabolic Syndrome from an Immunonutritional View. Biomedicines, 12(8), 1825. https://doi.org/10.3390/biomedicines12081825











