Cholesterol Metabolism and Urinary System Tumors
Abstract
:1. Introduction
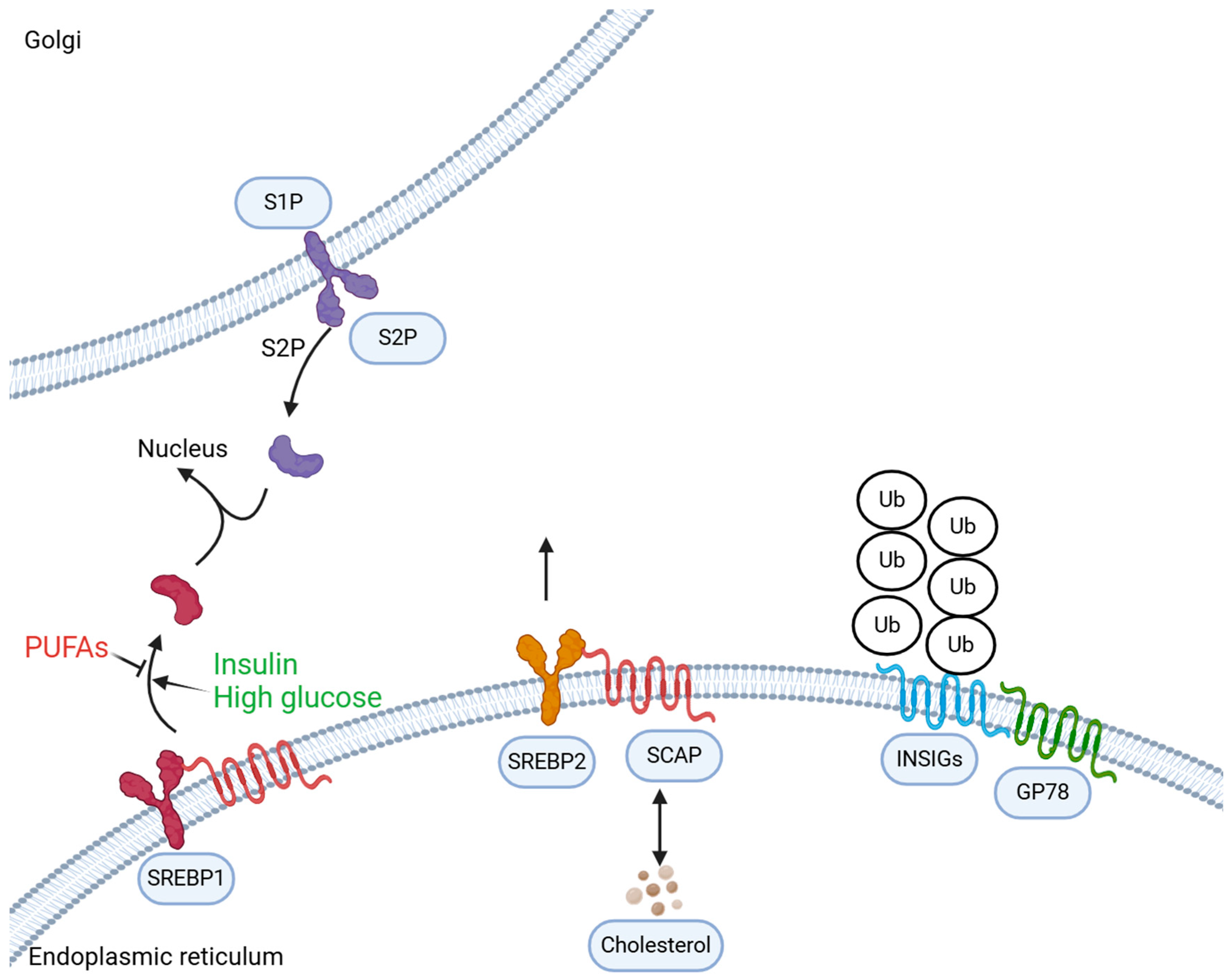
2. Normal Cholesterol Metabolism
3. Cholesterol Metabolism and Urinary System Tumors
3.1. Bladder Cancer
3.2. Prostate Cancer
3.3. Kidney Cancer
4. Targeting Cholesterol Metabolism for Cancer Therapy
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schulz, W.A.; Sørensen, K.D. Epigenetics of Urological Cancers. Int. J. Mol. Sci. 2019, 20, 4775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papavasileiou, G.; Tsilingiris, D.; Spyrou, N.; Vallianou, N.G.; Karampela, I.; Magkos, F.; Dalamaga, M. Obesity and main urologic cancers: Current systematic evidence, novel biological mechanisms, perspectives and challenges. Semin. Cancer Biol. 2023, 91, 70–98. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Jubber, I.; Ong, S.; Bukavina, L.; Black, P.C.; Compérat, E.; Kamat, A.M.; Kiemeney, L.; Lawrentschuk, N.; Lerner, S.P.; Meeks, J.J.; et al. Epidemiology of Bladder Cancer in 2023: A Systematic Review of Risk Factors. Eur. Urol. 2023, 84, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Emilio, S.; Luigi, V.; Riccardo, B.; Carlo, G. Lifestyle in urology: Cancer. Urologia 2019, 86, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Lucca, I.; Valerio, M.; Jichlinski, P.; Cerantola, Y. Inégalité des sexes face au cancer en urologie Gender disparities in urologic cancers. Rev. Med. Suisse 2015, 11, 2276–2278, 2280. [Google Scholar] [PubMed]
- Burgess, K.E.; DeRegis, C.J. Urologic Oncology. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Freifeld, Y.; Krabbe, L.M.; Clinton, T.N.; Woldu, S.L.; Margulis, V. Therapeutic strategies for upper tract urothelial carcinoma. Expert Rev. Anticancer Ther. 2018, 18, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.E. Bladder cancer. Curr. Opin. Oncol. 2007, 19, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Song, B.L.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol Review: A Metabolically Important Molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Cortes, V.A.; Busso, D.; Maiz, A.; Arteaga, A.; Nervi, F.; Rigotti, A. Physiological and pathological implications of cholesterol. Front. Biosci. Landmark Ed. 2014, 19, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Voisin, M.; de Medina, P.; Mallinger, A.; Dalenc, F.; Huc-Claustre, E.; Leignadier, J.; Serhan, N.; Soules, R.; Ségala, G.; Mougel, A.; et al. Identification of a tumor-promoter cholesterol metabolite in human breast cancers acting through the glucocorticoid receptor. Proc. Natl. Acad. Sci. USA 2017, 114, E9346–E9355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gabitova, L.; Gorin, A.; Astsaturov, I. Molecular pathways: Sterols and receptor signaling in cancer. Clin. Cancer Res. 2014, 20, 28–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, X.; Zhang, W.; Li, S.; Yang, H. Te role of cholesterol metabolism in cancer. Am. J. Cancer Res. 2019, 9, 219–227. [Google Scholar] [PubMed]
- Wang, Y.; Liu, C.; Hu, L. Cholesterol regulates cell proliferation and apoptosis of colorectal cancer by modulating miR-33a-PIM3 pathway. Biochem. Biophys. Res. Commun. 2019, 511, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.A.; de Souza, S.B.; da Silva Teixeira, L.R.; Okorokov, L.A.; Arnholdt, A.C.V.; Okorokova-Façanha, A.L.; Façanha, A.R. Tumor cell cholesterol depletion and V-ATPase inhibition as an inhibitory mechanism to prevent cell migration and invasiveness in melanoma. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhou, Z.S.; Shen, Y.; Xu, J.; Miao, H.H.; Xiong, Y.; Xu, F.; Li, B.L.; Luo, J.; Song, B.L. Inhibition of the sterol regulatory element-binding protein pathway suppresses hepatocellular carcinoma by repressing infammation in mice. Hepatology 2017, 65, 1936–1947. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The Role of Cholesterol in Cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, P.J.H.; Rideout, T. Lipids, sterols, and their metabolites. In Modern Nutrition in Health and Disease; Ross, A.C., Caballero, B., Cousins, J.R., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams and Wilkins: Baltimore, MD, USA, 2012. [Google Scholar]
- Sharpe, L.J.; Brown, A.J. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). J. Biol. Chem. 2013, 288, 18707–18715. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vance, D.E.; Vance, J.E. Biochemistry of Lipids, Lipoproteins, and Membranes; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Meaney, S. Epigenetic regulation of cholesterol homeostasis. Front. Genet. 2014, 5, 311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ye, J.; DeBose-Boyd, R.A. Regulation of cholesterol and fatty acid synthesis. Cold Spring Harb. Perspect. Biol. 2011, 3, a004754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuzu, O.F.; Gowda, R.; Noory, M.A.; Robertson, G.P. Modulating cancer cell survival by targeting intracellular cholesterol transport. Br. J. Cancer 2017, 117, 513–524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Erratum in CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33, Erratum in CA Cancer J. Clin. 2021, 71, 359. [Google Scholar] [CrossRef] [PubMed]
- Millán-Rodríguez, F.; Chéchile-Toniolo, G.; Salvador-Bayarri, J.; Palou, J.; Algaba, F.; Vicente-Rodríguez, J. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J. Urol. 2000, 164 Pt 1, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Holmäng, S.; Hedelin, H.; Anderström, C.; Johansson, S.L. The relationship among multiple recurrences, progression and prognosis of patients with stages Ta and T1 transitional cell cancer of the bladder followed for at least 20 years. J. Urol. 1995, 153, 1823–1826; discussion 1826–1827. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.C.; Sharma, A.; Panwar, M.S.; Radosevich, J.A. Is cholesterol a mediator of cold-induced cancer? Tumour Biol. 2016, 37, 9635–9648. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.V.; Correia, D.V.; Mensurado, S.; Nóbrega-Pereira, S.; deBarros, A.; Kyle-Cezar, F.; Tutt, A.; Hayday, A.C.; Norell, H.; Silva-Santos, B.; et al. Low-Density Lipoprotein Uptake Inhibits the Activation and Antitumor Functions of Human Vγ9Vδ2 T Cells. Cancer Immunol. Res. 2018, 6, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Liu, Y.; Dai, H.; Gao, T.; Zeng, L.; Sun, R.; Zheng, Z.; Yuan, J.; Xia, B.; Pan, Y. Association of metabolic syndrome and the risk of bladder cancer: A prospective cohort study. Front. Oncol. 2022, 12, 996440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seco, J.; King, C.C.; Camazzola, G.; Jansen, J.; Tirinato, L.; Marafioti, M.G.; Hanley, R.; Pagliari, F.; Beckman, S.P. Modulating Nucleus Oxygen Concentration by Altering Intramembrane Cholesterol Levels: Creating Hypoxic Nucleus in Oxic Conditions. Int. J. Mol. Sci. 2022, 23, 5077. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dianatinasab, M.; Wesselius, A.; Salehi-Abargouei, A.; Yu, E.Y.W.; Fararouei, M.; Brinkman, M.; van den Brandt, P.; White, E.; Weiderpass, E.; Le Calvez-Kelm, F.; et al. Dietary fats and their sources in association with the risk of bladder cancer: A pooled analysis of 11 prospective cohort studies. Int. J. Cancer 2022, 151, 44–55, Erratum in Int. J. Cancer 2023, 152, E4–E5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shih, H.J.; Lin, K.H.; Wen, Y.C.; Fan, Y.C.; Tsai, P.S.; Huang, C.J. Increased risk of bladder cancer in young adult men with hyperlipidemia: A population-based cohort study. Medicine 2021, 100, e28125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Zhou, Y.; Huang, M.; Wang, Z.; Liu, D.; Liu, J.; Fu, X.; Yang, S.; Shan, S.; Yang, L.; et al. DHCR7 promotes tumorigenesis via activating PI3K/AKT/mTOR signalling pathway in bladder cancer. Cell. Signal. 2023, 102, 110553. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, H.; Fang, W. Oncogenic roles of the cholesterol metabolite 25-hydroxycholesterol in bladder cancer. Oncol. Lett. 2020, 19, 3671–3676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.; Zheng, X.; Chen, J.; Zheng, L.; Ma, Z.; Chen, L.; Deng, M.; Tang, H.; Zhou, L.; Kang, T.; et al. NFYC-37 promotes tumor growth by activating the mevalonate pathway in bladder cancer.NFYC-37 promotes tumor growth by activating the mevalonate pathway in bladder cancer. Cell Rep. 2023, 42, 112963. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.R.; Tsai, Y.L.; Tsai, W.C.; Chen, T.M.; Chang, H.H.; Changchien, C.Y.; Wu, S.T.; Wang, H.H.; Chen, Y.; Lin, Y.H. Farnesoid X Receptor Overexpression Decreases the Migration, Invasion and Angiogenesis of Human Bladder Cancers via AMPK Activation and Cholesterol Biosynthesis Inhibition. Cancers 2022, 14, 4398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, L.; Sun, J.; Li, M.; Long, Y.; Zhang, D.; Guo, H.; Huang, R.; Yan, J. Oxidized Low-Density Lipoprotein Links Hypercholesterolemia and Bladder Cancer Aggressiveness by Promoting Cancer Stemness. Cancer Res. 2021, 81, 5720–5732. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Gencer, B.; Wiviott, S.D.; Park, J.G.; Fuchs, C.S.; Goessling, W.; Musliner, T.A.; Tershakovec, A.M.; Blazing, M.A.; Califf, R.; et al. Prospective Evaluation of Malignancy in 17,708 Patients Randomized to Ezetimibe Versus Placebo: Analysis From IMPROVE-IT. JACC CardioOncol. 2020, 2, 385–396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferro, M.; Lucarelli, G.; de Cobelli, O.; Dolce, P.; Terracciano, D.; Musi, G.; Porreca, A.; Busetto, G.M.; Del Giudice, F.; Soria, F.; et al. A risk-group classification model in patients with bladder cancer under neoadjuvant cisplatin-based combination chemotherapy. Future Oncol. 2021, 17, 3987–3994. [Google Scholar] [CrossRef] [PubMed]
- Symvoulidis, P.; Tsioutis, C.; Zamboglou, C.; Agouridis, A.P. The Effect of Statins on the Incidence and Prognosis of Bladder Cancer: A Systematic Review and Meta-Analysis. Curr. Oncol. 2023, 30, 6648–6665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedman, G.D.; Flick, E.D.; Udaltsova, N.; Chan, J.; Quesenberry, C.P., Jr.; Habel, L.A. Screening statins for possible carcinogenic risk: Up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol. Drug Saf. 2008, 17, 27–36, Erratum in Pharmacoepidemiol. Drug Saf. 2008, 17, 751. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate cancer. Lancet 2021, 398, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Gravis, G.; Boher, J.M.; Chen, Y.H.; Liu, G.; Fizazi, K.; Carducci, M.A.; Oudard, S.; Joly, F.; Jarrard, D.M.; Soulie, M.; et al. Burden of Metastatic Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. Eur. Urol. 2018, 73, 847–855. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Francini, E.; Gray, K.P.; Xie, W.; Shaw, G.K.; Valença, L.; Bernard, B.; Albiges, L.; Harshman, L.C.; Kantoff, P.W.; Taplin, M.E.; et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC). Prostate 2018, 78, 889–895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magura, L.; Blanchard, R.; Hope, B.; Beal, J.R.; Schwartz, G.G.; Sahmoun, A.E. Hypercholesterolemia and prostate cancer: A hospital-based case-control study. Cancer Causes Control 2008, 19, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Clipp, S.L.; Helzlsouer, K.J.; Platz, E.A. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control 2010, 21, 61–68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Platz, E.A.; Till, C.; Goodman, P.J.; Parnes, H.L.; Figg, W.D.; Albanes, D.; Neuhouser, M.L.; Klein, E.A.; Thompson, I.M., Jr.; Kristal, A.R. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2807–2813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shafique, K.; McLoone, P.; Qureshi, K.; Leung, H.; Hart, C.; Morrison, D.S. Cholesterol and the risk of grade-specific prostate cancer incidence: Evidence from two large prospective cohort studies with up to 37 years’ follow up. BMC Cancer 2012, 12, 25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lethongsavarn, V.; Pinault, M.; Diedhiou, A.; Guimaraes, C.; Guibon, R.; Bruyère, F.; Mathieu, R.; Rioux-Leclercq, N.; Multigner, L.; Brureau, L.; et al. Tissue cholesterol metabolism and prostate cancer aggressiveness: Ethno-geographic variations. Prostate 2021, 81, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Leon, C.G.; Locke, J.A.; Adomat, H.H.; Etinger, S.L.; Twiddy, A.L.; Neumann, R.D.; Nelson, C.C.; Guns, E.S.; Wasan, K.M. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate 2010, 70, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hughes-Fulford, M. Human prostate cancer cells lack feedback regulation of low-density lipoprotein receptor and its regulator, SREBP2. Int. J. Cancer 2001, 91, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Taylor, M.G.; Robinet, P.; Smith, J.D.; Schweitzer, J.; Sehayek, E.; Falzarano, S.M.; Magi-Galluzzi, C.; Klein, E.A.; Ting, A.H. Dysregulation of cholesterol homeostasis in human prostate cancer through loss of ABCA1. Cancer Res. 2013, 73, 1211–1218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, J.; Wu, Y.; Mohler, J.L.; Ip, C. The transcriptomics of de novo androgen biosynthesis in prostate cancer cells following androgen reduction. Cancer Biol. Ther. 2010, 9, 1033–1042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papadopoulos, V.; Amri, H.; Li, H.; Boujrad, N.; Vidic, B.; Garnier, M. Targeted disruption of the peripheral-type benzodiazepine receptor gene inhibits steroidogenesis in the R2C Leydig tumor cell line. J. Biol. Chem. 1997, 272, 32129–32135. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Sankanagoudar, S.; Dogra, P.; Chandra, N.C. Interlink between cholesterol & cell cycle in prostate carcinoma. Indian J. Med. Res. 2017, 146, S38–S44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalogirou, C.; Linxweiler, J.; Schmucker, P.; Snaebjornsson, M.T.; Schmitz, W.; Wach, S.; Krebs, M.; Hartmann, E.; Puhr, M.; Müller, A.; et al. MiR-205-driven downregulation of cholesterol biosynthesis through SQLE-inhibition identifies therapeutic vulnerability in aggressive prostate cancer. Nat. Commun. 2021, 12, 5066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hryniewicz-Jankowska, A.; Augoff, K.; Sikorski, A.F. The role of cholesterol and cholesterol-driven membrane raft domains in prostate cancer. Exp. Biol. Med. 2019, 244, 1053–1061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, L.; Song, Z.; Hu, J.; Liu, L.; Hou, Y.; Zhang, X.; Yang, X.; Chen, K. ACSS3 represses prostate cancer progression through downregulating lipid droplet-associated protein PLIN3. Theranostics 2021, 11, 841–860. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gan, S.S.; Ye, J.Q.; Wang, L.; Qu, F.J.; Chu, C.M.; Tian, Y.J.; Yang, W.; Cui, X.G. Inhibition of PCSK9 protects against radiation-induced damage of prostate cancer cells. Onco Targets Ther. 2017, 10, 2139–2146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, S.; Yarmolinsky, J.; Gill, D.; Bull, C.J.; Perks, C.M.; PRACTICAL Consortium; Davey Smith, G.; Gaunt, T.R.; Richardson, T.G. Association between genetically proxied PCSK9 inhibition and prostate cancer risk: A Mendelian randomisation study. PLoS Med. 2023, 20, e1003988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, L.; Ding, H.; Jia, Y.; Shi, M.; Guo, D.; Yang, P.; Wang, Y.; Liu, F.; Zhang, Y.; Zhu, Z. Associations of genetically proxied inhibition of HMG-CoA reductase, NPC1L1, and PCSK9 with breast cancer and prostate cancer. Breast Cancer Res. 2022, 24, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Sun, B.; Wei, L.; Jian, X.; Shan, K.; He, Q.; Huang, F.; Ge, X.; Gao, X.; Feng, N.; et al. Cholesterol and saturated fatty acids synergistically promote the malignant progression of prostate cancer. Neoplasia 2022, 24, 6–97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Maso, M.; Augustin, L.S.A.; Jenkins, D.J.A.; Carioli, G.; Turati, F.; Grisoni, B.; Crispo, A.; La Vecchia, C.; Serraino, D.; Polesel, J. Adherence to a cholesterol-lowering diet and the risk of prostate cancer. Food Funct. 2022, 13, 5730–5738. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.B.; Chung, L.W.; Huang, W.C. Anti-cancer efficacy of SREBP inhibitor, alone or in combination with docetaxel, in prostate cancer harboring p53 mutations. Oncotarget 2015, 6, 41018–41032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Chen, Y.T.; Hu, P.; Huang, W.C. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol. Cancer Ther. 2014, 13, 855–866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gholkar, A.A.; Cheung, K.; Williams, K.J.; Lo, Y.C.; Hamideh, S.A.; Nnebe, C.; Khuu, C.; Bensinger, S.J.; Torres, J.Z. Fatostatin Inhibits Cancer Cell Proliferation by Affecting Mitotic Microtubule Spindle Assembly and Cell Division. J. Biol. Chem. 2016, 291, 17001–17008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, M.; Zhang, J.; Sampieri, K.; Clohessy, J.G.; Mendez, L.; Gonzalez-Billalabeitia, E.; Liu, X.S.; Lee, Y.R.; Fung, J.; Katon, J.M.; et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat. Genet. 2018, 50, 206–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyata, S.; Inoue, J.; Shimizu, M.; Sato, R. Xanthohumol Improves Diet-induced Obesity and Fatty Liver by Suppressing Sterol Regulatory Element-binding Protein (SREBP) Activation. J. Biol. Chem. 2015, 290, 20565–20579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, J.J.; Li, J.G.; Qi, W.; Qiu, W.W.; Li, P.S.; Li, B.L.; Song, B.L. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 2011, 13, 44–56, Erratum in Cell Metab. 2021, 33, 222. [Google Scholar] [CrossRef] [PubMed]
- Vettenranta, A.; Murtola, T.J.; Raitanen, J.; Raittinen, P.; Talala, K.; Taari, K.; Stenman, U.H.; Tammela, T.L.J.; Auvinen, A. Outcomes of Screening for Prostate Cancer Among Men Who Use Statins. JAMA Oncol. 2022, 8, 61–68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joshua, A.M.; Armstrong, A.; Crumbaker, M.; Scher, H.I.; de Bono, J.; Tombal, B.; Hussain, M.; Sternberg, C.N.; Gillessen, S.; Carles, J.; et al. Statin and metformin use and outcomes in patients with castration-resistant prostate cancer treated with enzalutamide: A meta-analysis of AFFIRM, PREVAIL and PROSPER. Eur. J. Cancer 2022, 170, 285–295. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- An, Y.; Sun, J.X.; Xu, M.Y.; Liu, C.Q.; Xu, J.Z.; Zhong, X.Y.; Hu, J.; Xia, Q.D.; Hu, H.L.; Wang, S.G. Statin Use Is Associated with Better Prognosis of Patients with Prostate Cancer after Definite Therapies: A Systematic Review and Meta-Analysis of Cohort Studies. J. Oncol. 2022, 2022, 9275466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prabhu, N.; Kapur, N.; Catalona, W.; Leikin, R.; Helenowski, I.; Jovanovich, B.; Gurley, M.; Okwuosa, T.M.; Kuzel, T.M. Statin use and risk of prostate cancer biochemical recurrence after radical prostatectomy. Urol. Oncol. 2021, 39, 130.e9–130.e15. [Google Scholar] [CrossRef] [PubMed]
- Craig, E.L.; Stopsack, K.H.; Evergren, E.; Penn, L.Z.; Freedland, S.J.; Hamilton, R.J.; Allott, E.H. Statins and prostate cancer-hype or hope? The epidemiological perspective. Prostate Cancer Prostatic Dis. 2022, 25, 641–649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldberg, H.; Mohsin, F.K.; Saskin, R.; Kulkarni, G.S.; Berlin, A.; Kenk, M.; Wallis, C.J.D.; Klaassen, Z.; Chandrasekar, T.; Ahmad, A.E.; et al. The Suggested Unique Association Between the Various Statin Subgroups and Prostate Cancer. Eur. Urol. Focus 2021, 7, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Naito, S.; Makhov, P.; Astsaturov, I.; Golovine, K.; Tulin, A.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. LDL cholesterol counteracts the antitumour effect of tyrosine kinase inhibitors against renal cell carcinoma. Br. J. Cancer 2017, 116, 1203–1207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Tan, M.; Ge, J.; Zhang, P.; Zhong, J.; Tao, L.; Wang, Q.; Tong, X.; Qiu, J. Lysosomal acid lipase promotes cholesterol ester metabolism and drives clear cell renal cell carcinoma progression. Cell Prolif. 2018, 51, e12452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.; Liu, X.; Liu, S.; Cao, Q. Cholesterol promotes the migration and invasion of renal carcinoma cells by regulating the KLF5/miR-27a/FBXW7 pathway. Biochem. Biophys. Res. Commun. 2018, 502, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Stone, L. Cutting cholesterol curbs clear cell RCC. Nat. Rev. Urol. 2021, 18, 509. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Zhou, H.; Lv, D.; Wen, J.; Gong, Q.; Rong, Y.; Kang, Y.; Jia, M.; Shuang, W. Preoperative serum low-density lipoprotein cholesterol is an independent prognostic factor in patients with renal cell carcinoma after nephrectomy. Lipids Health Dis. 2023, 22, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohno, Y.; Nakashima, J.; Nakagami, Y.; Gondo, T.; Ohori, M.; Hatano, T.; Tachibana, M. Clinical implications of preoperative serum total cholesterol in patients with clear cell renal cell carcinoma. Urology 2014, 83, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Seo, S.I.; Lee, H.M.; Oh, J.J.; Lee, S.C.; Hong, S.K.; Lee, S.E.; et al. Preoperative Cholesterol Level Is Associated With Worse Pathological Outcomes and Postoperative Survival in Localized Renal Cell Carcinoma Patients: A Propensity Score-Matched Study. Clin. Genitourin. Cancer 2017, 15, e935–e941. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Fujiwara, Y.; Nagai, R.; Yoshida, M.; Ueda, S. Expression of two isozymes of acyl-coenzyme A: Cholesterol acyltransferase-1 and -2 in clear cell type renal cell carcinoma. Int. J. Urol. 2008, 15, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.C.; Bashraheel, F.K.; Byun, S.S.; Kwak, C.; Hwang, E.C.; Kang, S.H.; Chung, J.; Kim, T.H.; Kim, Y.J.; Hong, S.H. Gender- and cholesterol-specific predictive value of body mass index in renal cell carcinoma: A multicenter study. Asia Pac. J. Clin. Oncol. 2019, 15, e36–e42. [Google Scholar] [CrossRef] [PubMed]
- Riscal, R.; Bull, C.J.; Mesaros, C.; Finan, J.M.; Carens, M.; Ho, E.S.; Xu, J.P.; Godfrey, J.; Brennan, P.; Johansson, M.; et al. Cholesterol Auxotrophy as a Targetable Vulnerability in Clear Cell Renal Cell Carcinoma. Cancer Discov. 2021, 11, 3106–3125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drabkin, H.A.; Gemmill, R.M. Cholesterol and the development of clear-cell renal carcinoma. Curr. Opin. Pharmacol. 2012, 12, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Cytochrome P450 CYP1A1: Wider roles in cancer progression and prevention. BMC Cancer 2009, 9, 187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Larsen, S.B.; Dehlendorff, C.; Skriver, C.; Dalton, S.O.; Jespersen, C.G.; Borre, M.; Brasso, K.; Nørgaard, M.; Johansen, C.; Sørensen, H.T.; et al. Postdiagnosis Statin Use and Mortality in Danish Patients With Prostate Cancer. J. Clin. Oncol. 2017, 35, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xiao, X. Targeting ACAT1 in cancer: From threat to treatment. Front. Oncol. 2024, 14, 1395192. [Google Scholar] [CrossRef]
- Lee, H.J.; Li, J.; Vickman, R.E.; Li, J.; Liu, R.; Durkes, A.C.; Elzey, B.D.; Yue, S.; Liu, X.; Ratliff, T.L.; et al. Cholesterol Esterification Inhibition Suppresses Prostate Cancer Metastasis by Impairing the Wnt/β-catenin Pathway. Mol. Cancer Res. 2018, 16, 974–985. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.S.; Li, J.; Tai, J.N.; Ratliff, T.L.; Park, K.; Cheng, J.X. Avasimibe encapsulated in human serum albumin blocks cholesterol esterification for selective cancer treatment. ACS Nano 2015, 9, 2420–2432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, G.; Wang, Q.; Xu, Y.; Li, J.; Zhang, H.; Qi, G.; Xia, Q. Targeting the transcription factor receptor LXR to treat clear cell renal cell carcinoma: Agonist or inverse agonist? Cell Death Dis. 2019, 10, 416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kong, Y.; Cheng, L.; Mao, F.; Zhang, Z.; Zhang, Y.; Farah, E.; Bosler, J.; Bai, Y.; Ahmad, N.; Kuang, S.; et al. Inhibition of cholesterol biosynthesis overcomes enzalutamide resistance in castration-resistant prostate cancer (CRPC). J. Biol. Chem. 2018, 293, 14328–14341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Reagent | Target | Mechanism | Cancer Type | References | |
|---|---|---|---|---|---|
| Targeting cholesterol biosynthesis | Statins | HMGCR | Decreased cancer mortality and longer survival, according to retrospective clinical analysis | Prostate cancer | [98] |
| Targeting cholesterol esterification | Avasimibe (15 mg/kg, PO) | ACAT1 | Impaired Wnt–β-catenin signalling through decreased Wnt3a secretion; decreased metastasis; according to preclinical animal models with in vitro analysis (non-obese diabetic/severe combined immunodeficiency mice) | PC-3M prostate cancer model | [100] |
| Avasimin (10 mg/kg, IV) | Increased cell apoptosis with no clear cytotoxicity; according to preclinical animal models (BALB/c mice) | PC3 prostate and HCT116 CRC models | [101] | ||
| Targeting LXR signaling | LXR623 (30 mg/kg, PO) | LXR | Decreased cellular cholesterol of cancer cells; according to preclinical animal models (C57BL/6 mice) | Clear cell renal cell carcinoma model | [102] |
| SR9243 (30 mg/kg, PO) | Repression of lipogenesis and glycolysis of cancer cells; induction of cell apoptosis; according to preclinical animal models (C57BL/6 mice) | Prostate cancer | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Ye, Z.; Ning, J.; Wang, P.; Zhou, X.; Li, W.; Cheng, F. Cholesterol Metabolism and Urinary System Tumors. Biomedicines 2024, 12, 1832. https://doi.org/10.3390/biomedicines12081832
Yang S, Ye Z, Ning J, Wang P, Zhou X, Li W, Cheng F. Cholesterol Metabolism and Urinary System Tumors. Biomedicines. 2024; 12(8):1832. https://doi.org/10.3390/biomedicines12081832
Chicago/Turabian StyleYang, Songyuan, Zehua Ye, Jinzhuo Ning, Peihan Wang, Xiangjun Zhou, Wei Li, and Fan Cheng. 2024. "Cholesterol Metabolism and Urinary System Tumors" Biomedicines 12, no. 8: 1832. https://doi.org/10.3390/biomedicines12081832





