Once-Weekly Insulin Icodec in Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Clinical Trials (ONWARDS Clinical Program)
Abstract
1. Background
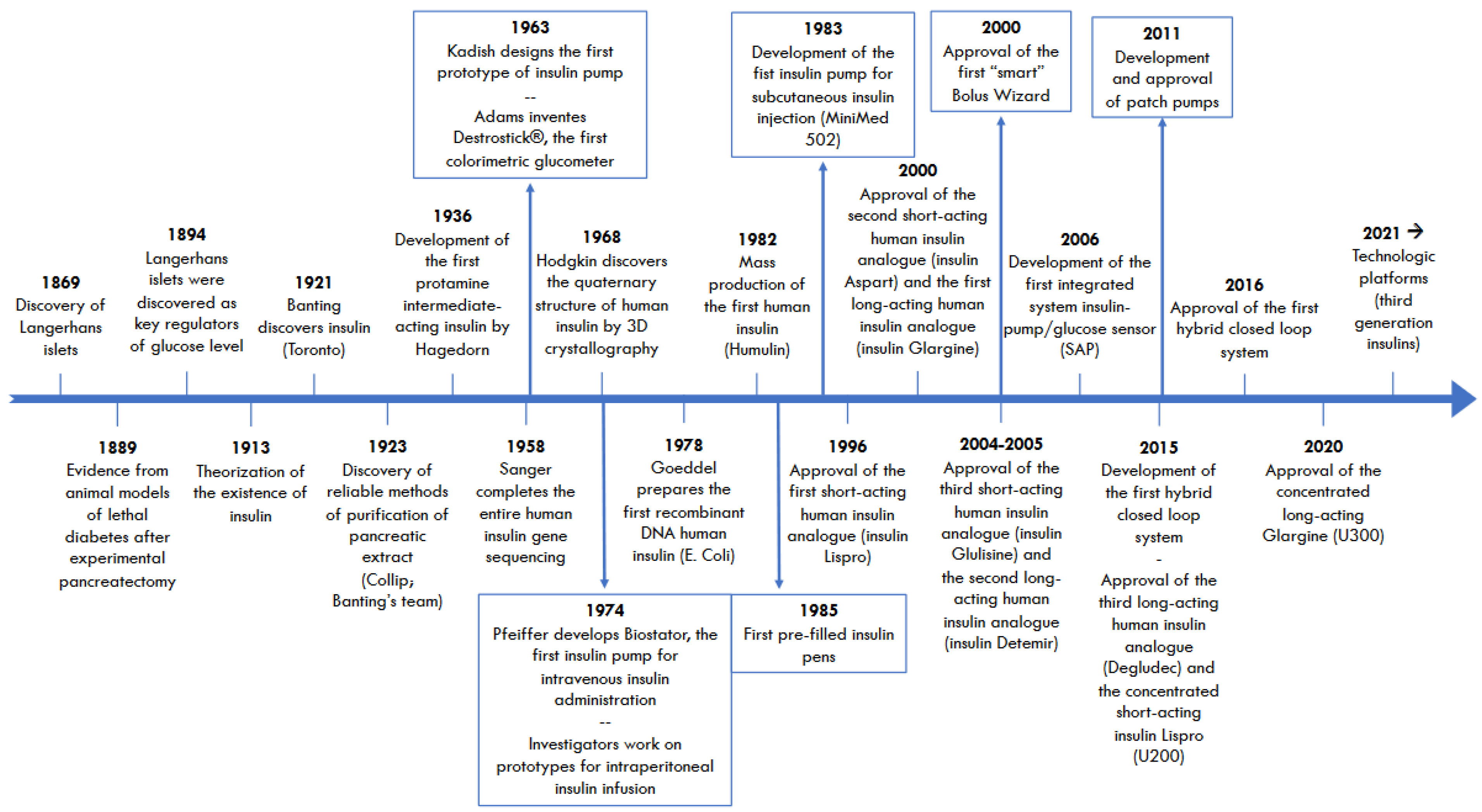
2. Progress in Once-Weekly Insulins
3. Efficacy and Safety of Insulin Icodec: The State of the Art
3.1. Phase 2 Trials
3.2. Phase 3 Trials
3.2.1. Overview of the ONWARDS Clinical Program
3.2.2. Procedures
Pretrial Antihyperglycemic Treatment
Starting Dose and Titration of Basal Analogs
Continuous Glucose Monitoring
Safety Endpoints
Satisfaction and Compliance Questionnaires
3.2.3. Methods
Searching, Screening, and Selection of Studies
Inclusion Criteria
Exclusion Criteria
Extraction and Synthesis: Comprehensive Details of RCTs
Extraction and Synthesis: Efficacy Endpoints
Extraction and Synthesis: Safety Endpoints
Intention-to-Treat Analysis
Participants Who Completed the Trials
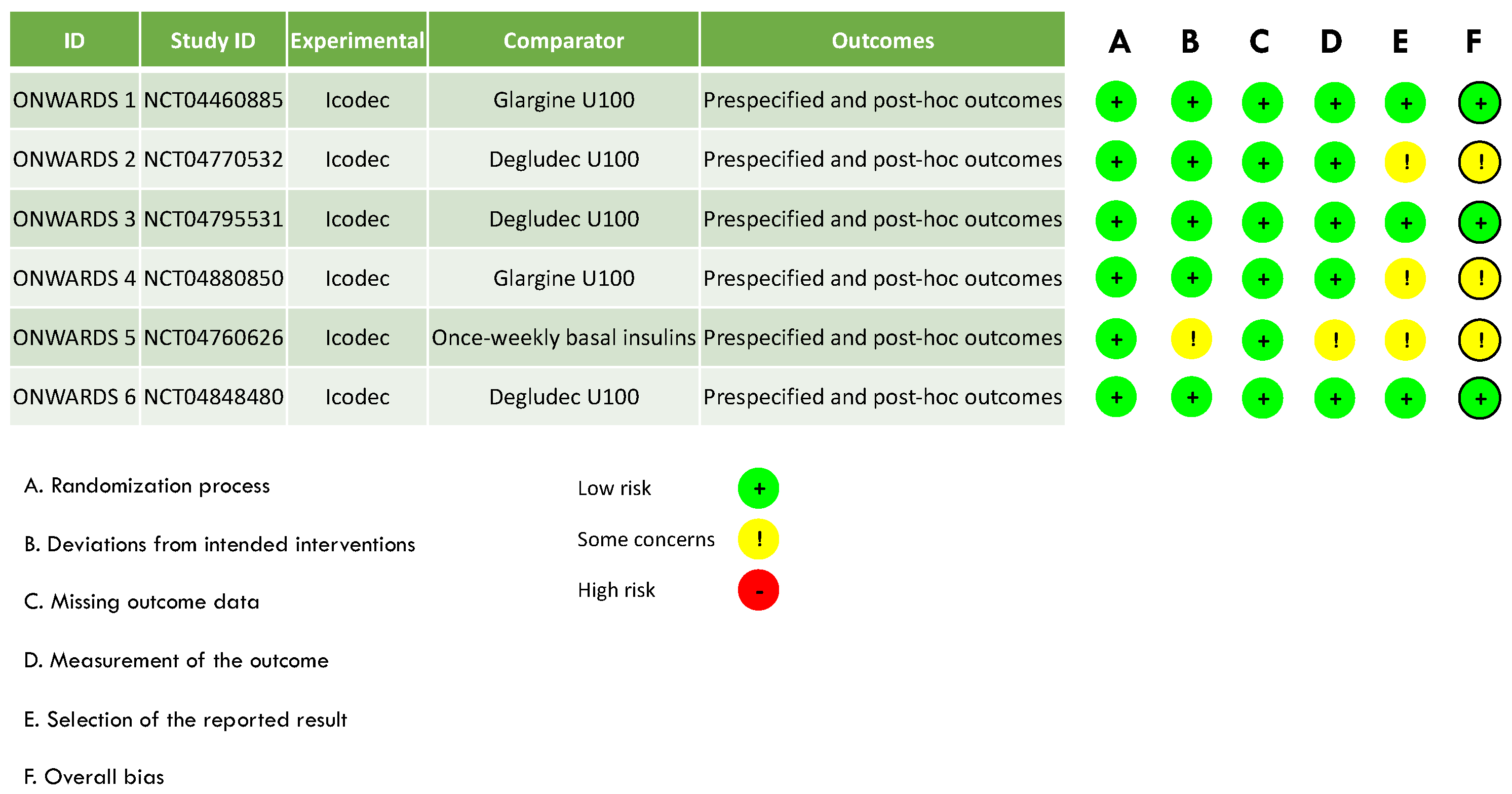
Assessment of the Risk of Bias and Publication Bias
Software for Statistics
3.2.4. Results
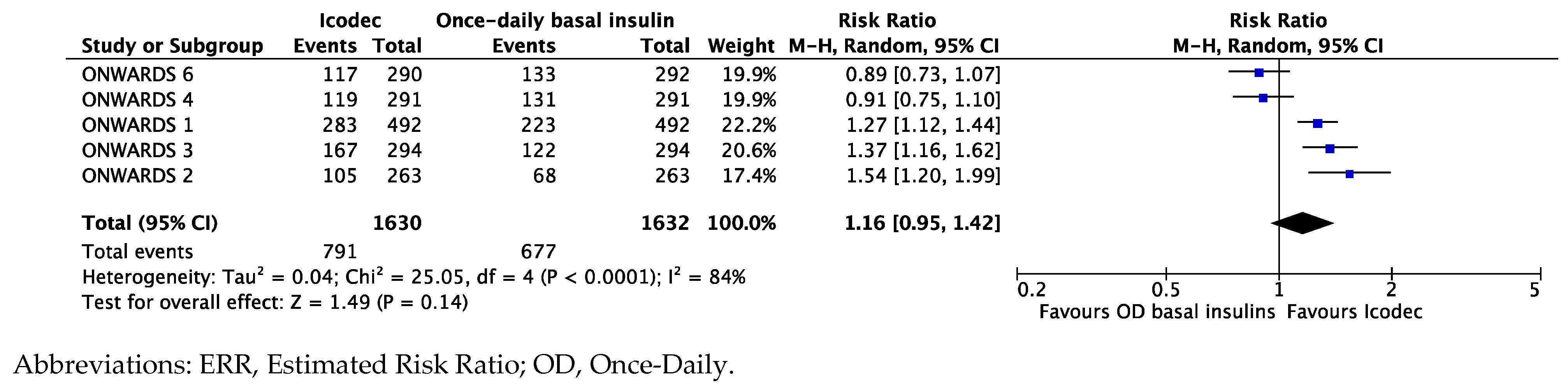
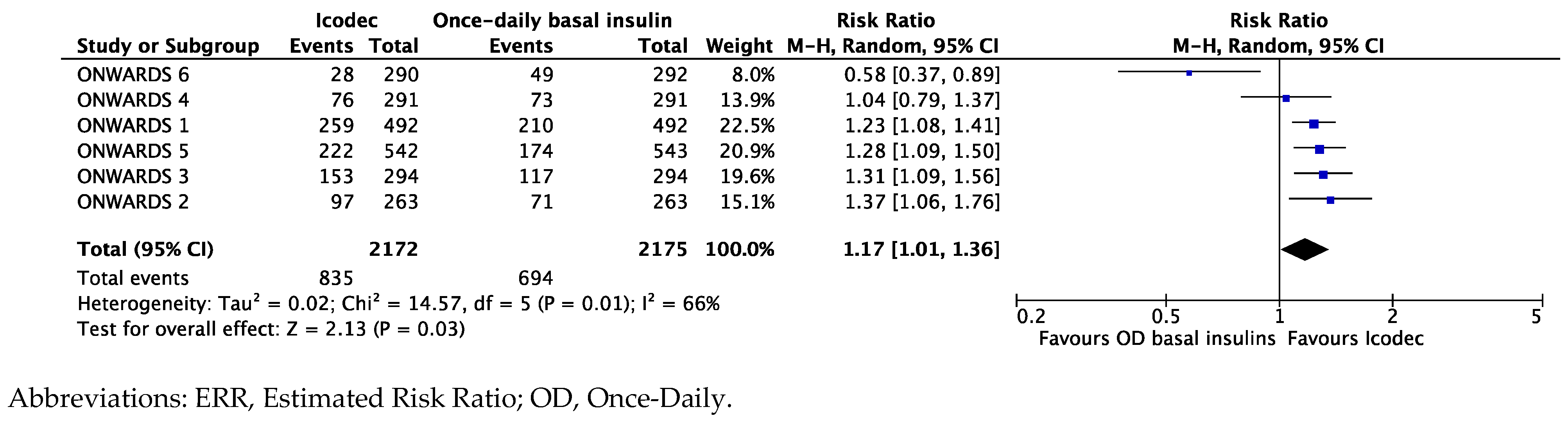
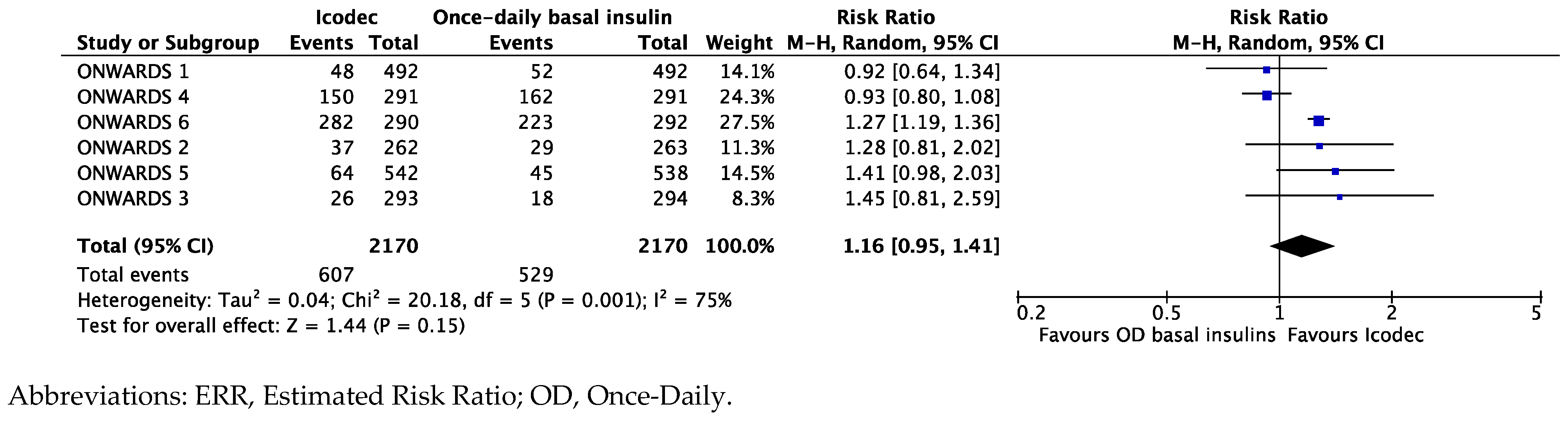
Synthesis of Data from the ONWARDS Clinical Program
Exploring the Heterogeneity of the Results
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CGM | Continuous glucose monitoring |
| CI | Confidence interval |
| ERR | Estimated risk ratio |
| ETD | Estimated treatment difference |
| FPG | Fasting plasma glucose |
| GLP-1RA | Glucagon-like peptide 1 receptor agonists |
| HbA1c | Glycated hemoglobin |
| MDI | Multiple daily injections |
| RCT | Randomized clinical trial |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| TAR | Time above range |
| TIR | Time in range |
References
- Jörgens, V.; Porta, M. Unveiling Diabetes—Historical Milestones in Diabetology. In Frontiers in Diabetes; Karger: Basel, Switzerland, 2020; 25p. [Google Scholar] [CrossRef]
- Banting, F.G.; Best, C.H.; Collip, J.B.; Campbell, W.R.; Fletcher, A.A. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can. Med. Assoc. J. 1922, 12, 141–146. [Google Scholar] [PubMed]
- Bliss, M. The history of insulin. Diabetes Care 1993, 16, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Stretton, A.O. The first sequence—Fred Sanger and insulin. Genetics 2002, 162, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Quianzon, C.C.; Cheikh, I. History of insulin. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 18701. [Google Scholar] [CrossRef] [PubMed]
- Goeddel, D.V.; Kleid, D.G.; Bolivar, F.; Heyneker, H.L.; Yansura, D.G.; Crea, R.; Hirose, T.; Kraszewski, A.; Itakura, K.; Riggs, A.D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc. Natl. Acad. Sci. USA 1979, 76, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.; Dyawanapelly, S.; Jain, R.; Dandekar, P. An overview of oral insulin delivery strategies (OIDS). Int. J. Biol. Macromol. 2022, 208, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Primavera, R.; Kevadiya, B.D.; Swaminathan, G.; Wilson, R.J.; De Pascale, A.; Decuzzi, P.; Thakor, A.S. Emerging Nano- and Micro-Technologies Used in the Treatment of Type-1 Diabetes. Nanomaterials 2020, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Jarosinski, M.A.; Dhayalan, B.; Rege, N.; Chatterjee, D.; Weiss, M.A. ‘Smart’ insulin-delivery technologies and intrinsic glucose-responsive insulin analogues. Diabetologia 2021, 64, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119, Erratum in Diabetes Res. Clin. Pract. 2023, 204, 110945. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, G.; Kim, B.S.; Han, K.D.; Kwon, S.Y.; Park, S.H.; Lee, Y.B.; Jin, S.M.; Kim, J.H. Insulin Fact Sheet in Type 1 and 2 Diabetes Mellitus and Trends of Antidiabetic Medication Use in Insulin Users with Type 2 Diabetes Mellitus: 2002 to 2019. Diabetes Metab. J. 2023, 47, 211–219. [Google Scholar] [CrossRef]
- Pitak, P.; Tasai, S.; Kumpat, N.; Na Songkla, P.; Fuangchan, A.; Krass, I.; Dhippayom, T. The prevalence of glycemic control in patients with type 2 diabetes treated with insulin: A systematic review and meta-analysis. Public Health 2023, 225, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Blonde, L.; Aschner, P.; Bailey, C.; Ji, L.; Leiter, L.A.; Matthaei, S.; Global Partnership for Effective Diabetes Management. Gaps and barriers in the control of blood glucose in people with type 2 diabetes. Diabetes Vasc. Dis. Res. 2017, 14, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Rubino, D.M.; Greenway, F.L.; Khalid, U.; O’Neil, P.M.; Rosenstock, J.; Sørrig, R.; Wadden, T.A.; Wizert, A.; Garvey, W.T.; STEP 8 Investigators. Effect of Weekly Subcutaneous Semaglutide vs. Daily Liraglutide on Body Weight in Adults with Overweight or Obesity without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA 2022, 327, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M.; Povedano, S.T.; Forst, T.; González, J.G.; Atisso, C.; Sealls, W.; Fahrbach, J.L. Once-weekly dulaglutide vs. once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): A randomised, open-label, phase 3, non-inferiority trial. Lancet 2014, 384, 1349–1357, Erratum in Lancet 2014, 384, 1348. [Google Scholar] [CrossRef] [PubMed]
- Philis-Tsimikas, A.; Wysham, C.H.; Hardy, E.; Han, J.; Iqbal, N. Efficacy and tolerability of exenatide once weekly over 7 years in patients with type 2 diabetes: An open-label extension of the DURATION-1 study. J. Diabetes Complicat. 2019, 33, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; Disoteo, O.E.; De Geronimo, V.; De Tullio, A.; Giagulli, V.A.; Guastamacchia, E.; De Pergola, G.; Jirillo, E.; Triggiani, V. Is Tirzepatide the New Game Changer in Type 2 Diabetes? Endocrines 2024, 5, 72–86. [Google Scholar] [CrossRef]
- Blair, H.A. Insulin Icodec: First Approval. BioDrugs 2024, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Plum-Mörschel, L.; Andersen, L.R.; Hansen, S.; Hövelmann, U.; Krawietz, P.; Kristensen, N.R.; Lehrskov, L.L.; Haahr, H. Pharmacokinetic and Pharmacodynamic Characteristics of Insulin Icodec After Subcutaneous Administration in the Thigh, Abdomen or Upper Arm in Individuals with Type 2 Diabetes Mellitus. Clin. Drug Investig. 2023, 43, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.novonordisk.ca/content/dam/nncorp/ca/en/products/awiqli-en-consumer-information-12-march-2024.pdf (accessed on 7 July 2024).
- Kalra, S.; Bhattacharya, S.; Kapoor, N. Counseling for Insulin Icodec: A Proposed Practitioner’s Guide. Diabetes Ther. 2024, 15, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Haahr, H.; Cieslarová, B.; Hingst, J.R.; Jiang, S.; Kristensen, N.R.; Kupčová, V.; Nørgreen, L.; Wagner, F.H.; Ignatenko, S. The Effect of Various Degrees of Renal or Hepatic Impairment on the Pharmacokinetic Properties of Once-weekly Insulin Icodec. Clin. Pharmacokinet. 2024, 63, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, F.; Refsgaard, H.H.F.; Gram-Nielsen, S.; Madsen, P.; Nishimura, E.; Münzel, M.; Brand, C.L.; Stidsen, C.E.; Claussen, C.H.; Wulff, E.M.; et al. Molecular engineering of safe and efficacious oral basal insulin. Nat. Commun. 2020, 11, 3746, Erratum in Nat. Commun. 2020, 11, 4232. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, T.B.; Hubálek, F.; Hjørringgaard, C.U.; Tagmose, T.M.; Nishimura, E.; Stidsen, C.E.; Porsgaard, T.; Fledelius, C.; Refsgaard, H.H.F.; Gram-Nielsen, S.; et al. Molecular Engineering of Insulin Icodec, the First Acylated Insulin Analog for Once-weekly Administration in Humans. J. Med. Chem. 2021, 64, 8942–8950. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, E.; Pridal, L.; Glendorf, T.; Hansen, B.F.; Hubálek, F.; Kjeldsen, T.; Kristensen, N.R.; Lützen, A.; Lyby, K.; Madsen, P.; et al. Molecular and pharmacological characterization of insulin icodec: A new basal insulin analog designed for Once-weekly dosing. BMJ Open Diabetes Res. Care 2021, 9, e002301. [Google Scholar] [CrossRef] [PubMed]
- Moyers, J.S.; Hansen, R.J.; Day, J.W.; Dickinson, C.D.; Zhang, C.; Ruan, X.; Ding, L.; Brown, R.M.; Baker, H.E.; Beals, J.M. Preclinical Characterization of LY3209590, a Novel Weekly Basal Insulin Fc-Fusion Protein. J. Pharmacol. Exp. Ther. 2022, 382, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Kazda, C.M.; Bue-Valleskey, J.M.; Chien, J.; Zhang, Q.; Chigutsa, E.; Landschulz, W.; Wullenweber, P.; Haupt, A.; Dahl, D. Novel Once-weekly Basal Insulin Fc Achieved Similar Glycemic Control with a Safety Profile Comparable to Insulin Degludec in Patients With Type 1 Diabetes. Diabetes Care 2023, 46, 1052–1059. [Google Scholar] [CrossRef]
- Bue-Valleskey, J.M.; Kazda, C.M.; Ma, C.; Chien, J.; Zhang, Q.; Chigutsa, E.; Landschulz, W.; Haupt, A.; Frias, J.P. Once-weekly Basal Insulin Fc Demonstrated Similar Glycemic Control to Once-Daily Insulin Degludec in Insulin-Naive Patients with Type 2 Diabetes: A Phase 2 Randomized Control Trial. Diabetes Care 2023, 46, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Frias, J.; Chien, J.; Zhang, Q.; Chigutsa, E.; Landschulz, W.; Syring, K.; Wullenweber, P.; Haupt, A.; Kazda, C. Safety and efficacy of Once-weekly basal insulin Fc in people with type 2 diabetes previously treated with basal insulin: A multicentre, open-label, randomised, phase 2 study. Lancet Diabetes Endocrinol. 2023, 11, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Bergenstal, R.M.; Philis-Tsimikas, A.; Wysham, C.; Carr, M.C.; Bue-Valleskey, J.M.; Botros, F.T.; Blevins, T.; Rosenstock, J. Once-weekly insulin efsitora alfa: Design and rationale for the QWINT phase 3 clinical development programme. Diabetes Obes. Metab. 2024, 26, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Bajaj, H.S.; Janež, A.; Silver, R.; Begtrup, K.; Hansen, M.V.; Jia, T.; Goldenberg, R.; NN1436-4383 Investigators. Once-weekly Insulin for Type 2 Diabetes without Previous Insulin Treatment. N. Engl. J. Med. 2020, 383, 2107–2116. [Google Scholar] [CrossRef]
- Lingvay, I.; Buse, J.B.; Franek, E.; Hansen, M.V.; Koefoed, M.M.; Mathieu, C.; Pettus, J.; Stachlewska, K.; Rosenstock, J. A Randomized, Open-Label Comparison of Once-weekly Insulin Icodec Titration Strategies vs. Once-Daily Insulin Glargine U100. Diabetes Care 2021, 44, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, H.S.; Bergenstal, R.M.; Christoffersen, A.; Davies, M.J.; Gowda, A.; Isendahl, J.; Lingvay, I.; Senior, P.A.; Silver, R.J.; Trevisan, R.; et al. Switching to Once-weekly Insulin Icodec vs. Once-Daily Insulin Glargine U100 in Type 2 Diabetes Inadequately Controlled on Daily Basal Insulin: A Phase 2 Randomized Controlled Trial. Diabetes Care 2021, 44, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, J.; Bain, S.C.; Gowda, A.; Jódar, E.; Liang, B.; Lingvay, I.; Nishida, T.; Trevisan, R.; Mosenzon, O.; ONWARDS 1 Trial Investigators. Weekly Icodec vs. Daily Glargine U100 in Type 2 Diabetes without Previous Insulin. N. Engl. J. Med. 2023, 389, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Philis-Tsimikas, A.; Asong, M.; Franek, E.; Jia, T.; Rosenstock, J.; Stachlewska, K.; Watada, H.; Kellerer, M. Switching to Once-weekly insulin icodec vs. once-daily insulin degludec in individuals with basal insulin-treated type 2 diabetes (ONWARDS 2): A phase 3a, randomised, open label, multicentre, treat-to-target trial. Lancet Diabetes Endocrinol. 2023, 11, 414–425, Erratum in Lancet Diabetes Endocrinol. 2023, 11, e9. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Asong, M.; Desouza, C.; Gourdy, P.; Kar, S.; Vianna, A.; Vilsbøll, T.; Vinther, S.; Mu, Y. Once-weekly Insulin Icodec vs Once-Daily Insulin Degludec in Adults with Insulin-Naive Type 2 Diabetes: The ONWARDS 3 Randomized Clinical Trial. JAMA 2023, 330, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Ásbjörnsdóttir, B.; Bajaj, H.S.; Lane, W.; Matos, A.L.S.A.; Murthy, S.; Stachlewska, K.; Rosenstock, J. Switching to Once-weekly insulin icodec vs. once-daily insulin glargine U100 in individuals with basal-bolus insulin-treated type 2 diabetes (ONWARDS 4): A phase 3a, randomised, open-label, multicentre, treat-to-target, non-inferiority trial. Lancet 2023, 401, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, H.S.; Aberle, J.; Davies, M.; Donatsky, A.M.; Frederiksen, M.; Yavuz, D.G.; Gowda, A.; Lingvay, I.; Bode, B. Once-weekly Insulin Icodec with Dosing Guide App vs. Once-Daily Basal Insulin Analogues in Insulin-Naive Type 2 Diabetes (ONWARDS 5): A Randomized Trial. Ann. Intern. Med. 2023, 176, 1476–1485, Erratum in Ann. Intern. Med. 2023, 176, 1688. [Google Scholar] [CrossRef] [PubMed]
- Russell-Jones, D.; Babazono, T.; Cailleteau, R.; Engberg, S.; Irace, C.; Kjaersgaard, M.I.S.; Mathieu, C.; Rosenstock, J.; Woo, V.; Klonoff, D.C. Once-weekly insulin icodec vs. once-daily insulin degludec as part of a basal-bolus regimen in individuals with type 1 diabetes (ONWARDS 6): A phase 3a, randomised, open-label, treat-to-target trial. Lancet 2023, 402, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Philis-Tsimikas, A.; Bajaj, H.S.; Begtrup, K.; Cailleteau, R.; Gowda, A.; Lingvay, I.; Mathieu, C.; Russell-Jones, D.; Rosenstock, J. Rationale and design of the phase 3a development programme (ONWARDS 1-6 trials) investigating Once-weekly insulin icodec in diabetes. Diabetes Obes. Metab. 2023, 25, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Agiostratidou, G.; Anhalt, H.; Ball, D.; Blonde, L.; Gourgari, E.; Harriman, K.N.; Kowalski, A.J.; Madden, P.; McAuliffe-Fogarty, A.H.; McElwee-Malloy, M.; et al. Standardizing Clinically Meaningful Outcome Measures beyond HbA1c for Type 1 Diabetes: A Consensus Report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017, 40, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Pamporis, K.; Popovic, D.S.; Stachteas, P.; Bougioukas, K.I.; Fragakis, N.; Rizzo, M. Efficacy and safety of Once-weekly vs. once-daily basal insulin analogues in the treatment of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Obes. Metab. 2023, 25, 3648–3661. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro ESilva, R.; de Miranda Gauza, M.; Guisso, M.E.S.; da Silva, J.O.N.; Kohara, S.K. Once-weekly Insulin Icodec vs. Once-Daily Insulin Glargine U100 for type 2 diabetes: A systematic review and meta-analysis of phase 2 randomized controlled trials. Arch. Endocrinol. Metab. 2023, 67, e000614. [Google Scholar] [CrossRef]
- Dutta, D.; Nagendra, L.; Bhat, S.; Mohindra, R.; Surana, V.; Misra, A. Optimal use of once weekly icodec insulin in type-2 diabetes: An updated meta-analysis of phase-2 and phase-3 randomized controlled trials. Diabetes Metab. Syndr. 2023, 17, 102877. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiao, W.; Liang, Z.; Li, S.; Tang, Q. Efficacy and safety of Once-weekly basal insulin vs. once-daily basal insulin in patients with type 2 diabetes: A systematic review and meta-analysis. Medicine 2023, 102, e36308. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Suvarna, R. Efficacy and safety of Once-weekly insulin icodec in type 2 diabetes: A meta-analysis of ONWARDS phase 3 randomized controlled trials. Diabetes Obes. Metab. 2024, 26, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Shafiq, A.; Javaid, H.; Jain, H.; Nashwan, A.J.; Tul-Ain, Q.; Basit, J. Clinical Outcomes with Once-weekly Insulin Icodec vs. Once-Daily Insulin Glargine U100 in Insulin-Naïve and Previously Insulin-Treated Individuals with Type 2 Diabetes: A Meta-Analysis of Randomised Controlled Trials. Endocrinol. Diabetes Metab. 2024, 7, e00480. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, H.S.; Ásbjörnsdóttir, B.; Bari, T.J.; Begtrup, K.; Vilsbøll, T.; Rosenstock, J. Once-weekly insulin icodec compared with daily basal insulin analogues in type 2 diabetes: Participant-level meta-analysis of the ONWARDS 1-5 trials. Diabetes Obes. Metab. 2024, 26, 3810–3820. [Google Scholar] [CrossRef] [PubMed]
- Hövelmann, U.; Engberg, S.; Heise, T.; Kristensen, N.R.; Nørgreen, L.; Zijlstra, E.; Ribel-Madsen, R. Pharmacokinetic and pharmacodynamic properties of Once-weekly insulin icodec in individuals with type 1 diabetes. Diabetes Obes. Metab. 2024, 26, 1941–1949. [Google Scholar] [CrossRef] [PubMed]


| NCT03751657 [27] | NCT03951805 [28] | NCT03922750 [29] | |
|---|---|---|---|
| Study design | 26-week double-blind, RCT | 16-week open-label, randomized, treat-to-target, titration trial with glucose monitoring | 16-week open-label, randomized, treat-to-target, switching trial with glucose monitoring |
| Population | T2D | T2D | T2D |
| Intervention | Once-weekly insulin Icodec | Once-weekly insulin Icodec Titration A (80–130 mg/dL = adjustment ± 21 IU/week); Titration B (80–130 mg/dL = ±28 IU/week); Titration C (70–108 mg/dL = ±28 IU/week) | Once-weekly insulin Icodec A) with loading dose (a 100% increase from the initial dose) B) without loading dose |
| Comparator | Once-daily insulin Glargine U100 | Once-daily insulin Glargine U100 Titration (80–130 mg/dL = ±4 IU/day) | Once-daily insulin Glargine U100 |
| Baseline characteristics | 247 insulin-naïve participants, mean HbA1c 8%, metformin ± DPP-IV inhibitors | 205 insulin-naïve participants, mean HbA1c 8.1%, any oral antihyperglycemic agents | 154 insulin users (10–50 IU/day): Detemir, Degludec U100, Glargine U100, Glargine U300, mean HbA1c 7.9% |
| Main findings | Mean change from baseline in HbA1c: −1.33% Icodec vs. −1.15% Glargine U100 (p = 0.08) Hypoglycemia (levels 2 and 3): 0.53 events per patient-year Icodec vs. 0.46 events per patient-year Glargine U100 (RR 1.09; 95%CI, 0.45 to 2.65) | Mean change in TIR (baseline to 15–16 weeks) Icodec A: from 57.0% to 76.6% Icodec B: from 55.2% to 83% Icodec C: from 51.0% to 80.9% Glargine U100: from 55.3% to 75.9% Level 2 hypoglycemia (<54 mg/dL, events per patient-year of exposure) Icodec A: 0.05 Icodec B: 0.15 Icodec C: 0.38 Glargine U100: 0.00 No level 3 hypos were observed. | Mean change in TIR (baseline to 15–16 weeks) Icodec A: from 58.9% to 72.9% Icodec B: from 54.5% to 66.0% Glargine U100: from 58.7% to 65.0% Mean change in HbA1c Icodec A: from 7.9% to 7.1% Icodec B: from 7.9% to 7.4% Glargine U100: from 7.9% to 7.4% Level 1 and 2 hypos were similar (among the 3 groups), and no level 3 hypos were registered |
| ONWARDS 1 [30] (NCT04460885) | ONWARDS 2 [31] (NCT04770532) | ONWARDS 3 [32] (NCT04795531) | ONWARDS 4 [33] (NCT04880850) | ONWARDS 5 [34] (NCT04760626) | ONWARDS 6 [35] (NCT04848480) | |
|---|---|---|---|---|---|---|
| Sponsored | Yes | Yes | Yes | Yes | Yes | Yes |
| Population | Insulin-naïve T2D | Basal insulin-treated T2D | Insulin-naïve T2D | Basal bolus-treated T2D | Insulin-naïve T2D | T1D |
| Inclusion criteria | Age ≥ 18 yrs, baseline HbA1c 7–11%, baseline BMI ≤ 40 kg/m2 | Age ≥ 18 yrs, baseline HbA1c 7–10% | Age ≥ 18 yrs, baseline HbA1c 7–11%, baseline BMI ≤ 40 kg/m2 | Age ≥ 18 yrs, baseline HbA1c 7–10% | Age ≥ 18 yrs, baseline HbA1c > 7%, for whom insulin treatment is required | HbA1c < 10% At least 1 year of basal-bolus regimen |
| Study design | Randomized, open-label, treat-to-target phase 3a trial | Randomized, open-label, active-controlled, multicentric, treat-to-target phase 3a trial | Randomized, double-masked, double-dummy, active-controlled, treat-to-target phase 3a trial | Randomized, open-label, multicentric, treat-to-target, non-inferiority trial | Randomized, open-label, multinational trial | Randomized, multicenter, open-label, active-controlled, parallel-group, treat-to-target, phase 3a trial |
| Study duration, weeks | 78 (52 + 26 of extension safety phase) + a 5-week follow-up | 26 + a 5-week follow-up | 26 + a 5-week follow-up | 26 + a 5-week follow-up | 52 + a 5-week follow-up | 52 (26 + 26 of extension safety phase) + a 5-week follow-up |
| Pretrial antihyperglycemic drugs | Any non-insulin drugs allowed | Once or twice-daily basal insulins ± non insulin antihyperglycemic agents | Any non-insulin drugs allowed | Any basal-bolus regimen ± non insulin antihyperglycemic agents (>90 days) | Any non-insulin drugs allowed | Basal-bolus regimen (any analogues allowed) |
| Handling of pretrial antihyperglycemic drugs at the randomization | Pretrial drugs confirmed, except secretagogues | Pretrial drugs confirmed, except secretagogues | Pretrial drugs confirmed at the same dose, including secretagogues (initial dose was reduced by 50%) | Pretrial drugs confirmed, except secretagogues | Pretrial drugs confirmed at the same dose, including secretagogues (initial dose was reduced by 50%) | Pretrial prandial insulins were switched to insulin Aspart |
| Comorbidities | NA | NA | Arterial hypertension (65%), hepatic steatosis (12.5%), coronary artery disease (10%), renal impairment (8.5%) | NA | Arterial hypertension (70%), hepatic steatosis (9.8%), coronary artery disease (8.5%) | NA |
| Intervention | Once-weekly insulin Icodec | Once-weekly insulin Icodec | Once-weekly insulin Icodec + once-daily placebo | Once-weekly insulin Icodec + insulin Aspart | Once-weekly insulin Icodec | Once-weekly insulin Icodec + insulin Aspart |
| Comparators | Once-daily insulin Glargine U100 | Once-daily insulin Degludec U100 | Once-daily insulin Degludec U100 | Once-daily insulin Glargine U100 + insulin Aspart | Once-daily basal insulins (Glargine U100 or Glargine U300 or Degludec U100) | Once-daily insulin Degludec U100 + insulin Aspart |
| Sample size: n | Icodec: 492 Glargine: 492 | Icodec: 263 Degludec: 263 | Icodec: 294 Degludec: 294 | Icodec: 292 Glargine: 291 | Icodec: 542 OD Basal: 543 | Icodec: 290 Degludec: 292 |
| Completed the “in-trial” period: % | Icodec: 96.5% Glargine: 97.4% | Icodec: 97.7% Degludec: 96.2% | Icodec: 95.9% Degludec: 96.2% | Icodec: 94% Glargine: 92% | Icodec: 89.1% OD Basal: 90.8% | Icodec: 90% Glargine: 95% |
| Primary Outcome | Mean change from baseline to study completion in HbA1c | Mean change from baseline to study completion in HbA1c | Mean change from baseline to study completion in HbA1c | Mean change from baseline to study completion in HbA1c | Mean change from baseline to study completion in HbA1c | Mean change from baseline to study completion (week 26) in HbA1c |
| Secondary outcomes | Mean change from baseline to study completion in FPG, TIR, weekly insulin dose, body weight | Mean change from baseline to study completion in FPG, TIR, weekly insulin dose, body weight, diabetes satisfaction | Mean change from baseline to study completion in FPG, weekly insulin dose, body weight | Mean change from baseline to study completion in FPG, TIR, weekly insulin dose, body weight | Mean change from baseline to study completion in diabetes satisfaction and compliance, weekly insulin dose and body weight | Mean change from baseline to study completion in FPG, TIR, HbA1c (week 52), body weight, diabetes satisfaction |
| Safety outcomes | Adverse events, hypoglycemic episodes (levels 1, 2, and 3) | Adverse events, hypoglycemic episodes (levels 1, 2, and 3), daytime and nocturnal hypos | Adverse events, hypoglycemic episodes (levels 1, 2, and 3) | Adverse events, hypoglycemic episodes (levels 1, 2, and 3) | Adverse events, hypoglycemic episodes (levels 1, 2, and 3), daytime and nocturnal hypos | Adverse events, hypoglycemic episodes (levels 1, 2, and 3), daytime and nocturnal hypos |
| Age, yrs: mean ± sd | Icodec: 59.1 ± 10.1 Glargine: 58.9 ± 9.9 | Icodec: 62.3 ± 9.8 Degludec: 62.6 ± 8.4 | Icodec: 58 ± 10 Degludec: 59 ± 10 | Icodec: 59.7 ± 10.1 Glargine: 59.9 ± 9.9 | Icodec: 59.1 ± 10.8 OD Basal: 59.4 ± 10.2 | Icodec: 44.1 ± 14.1 Degludec: 44.3 ± 14.1 |
| Diabetes duration, yrs: mean ± sd | Icodec: 11.6 ± 6.7 Glargine: 11.5 ± 6.8 | Icodec: 16.5 ± 8.4 Degludec: 16.9 ± 7.9 | Icodec: 10.5 Degludec: 10.7 | Icodec: 18 ± 9.1 Glargine: 16.3 ± 7.7 | Icodec: 11.9 ± 6.9 OD Basal: 12 ± 7.6 | Icodec: 20 ± 13.2 Degludec: 19 ± 12.9 |
| Baseline HbA1c, %: mean ± sd | Icodec: 8.5 ± 1 Glargine: 8.4 ± 1 | Icodec: 8.17 ± 0.77 Degludec: 8.1 ± 0.77 | Icodec: 8.55 ± 1.11 Degludec: 8.48 ± 1.01 | Icodec: 8.29 ± 0.86 Glargine: 8.31 ± 0.9 | Icodec: 8.96 ± 1.6 OD Basal: 8.88 ± 1.5 | Icodec: 7.59 ± 0.96 Degludec: 7.63 ± 0.93 |
| Final HbA1c, %: mean ± sd | Icodec: 6.93 ± 1.33 Glargine: 7.12 ± 1.11 | Icodec: 7.2 ± 0.81 Degludec: 7.42 ± 0.97 | Icodec: 7 ± 1.09 Degludec: 7.2 ± 0.98 | Icodec: 7.14 ± 0.85 Glargine: 7.12 ± 0.85 | Icodec: 7.24 ± 2.01 OD Basal: 7.61 ± 2.7 | Icodec: 7.15 ± 1.1 Degludec: 7.1 ± 1.1 |
| Baseline FPG, mg/dL: mean ± sd | Icodec: 185.3 ± 49 Glargine: 185.7 ± 51.7 | Icodec: 155.2 ± 47 Degludec: 150.7 ± 40.9 | Icodec: 187 ± 54 Degludec: 176 ± 46 | Icodec: 165.6 ± 54 Glargine: 172.8 ± 63 | Icodec: NA OD Basal: NA | Icodec: 179 ± 74 Degludec: 172 ± 72 |
| Final FPG, mg/dL: mean ± sd | Icodec: 125.2 ± 37 Glargine: 125.4 ± 37.3 | Icodec: 129.1 ± 29.3 Degludec: 117.7 ± 26 | Icodec: 127 ± NA Degludec: 127 ± NA | Icodec: 137 ± 41 Glargine: 132 ± 39 | Icodec: NA OD Basal: NA | Icodec: 163.9 ± NA Degludec: 138.3 ± NA |
| Baseline BMI, kg/m2: mean ± sd | Icodec: 30 ± 4.8 Glargine: 30.1 ± 5.1 | Icodec: 29.5 ± 5.2 Degludec: 29.2 ± 4.9 | Icodec: 29.9 ± 5.2 Degludec: 29.2 ± 5.1 | Icodec: 30.5 ± 5 Glargine: 30 ± 5 | Icodec: 32.6 ± 7 OD Basal: 33 ± 6.9 | Icodec: 26.8 ± 5 Degludec: 26.2 ± 4.5 |
| Baseline body weight, kg: mean ± sd | Icodec: 85.2 ± 17.7 Glargine: 84.3 ± 17.6 | Icodec: 83.7 ± 18.4 Degludec: 81.5 ± 17.1 | Icodec: 85.8 ± 20.1 Degludec: 83.2 ± 18.2 | Icodec: 85.5 ± 17.6 Glargine: 83.1 ± 17.3 | Icodec: 93.2 ± 22.5 OD Basal: 94.3 ± 21.5 | Icodec: 78.6 ± 17.6 Degludec: 77.1 ± 16.8 |
| Final body weight, kg: mean ± sd | Icodec: 87.03 ± 0.21 Glargine: 86.57 ± 0.21 | NA | Icodec: 87.3 ± NA Degludec: 86.8 ± NA | Icodec: 88.2 ± NA Glargine: 85.3 ± NA | Icodec: 96 OD Basal: 95.2 | Icodec: 79.9 ± NA Degludec: 78.1 ± NA |
| Starting dose of basal insulin (IU/week) | Icodec: 70 Glargine: 70 | 1:1 ratio with pretrial basal insulins | Icodec: 70 Degludec: 70 | 1:1 ratio with pretrial basal insulins | Icodec: 70 Degludec: 70 | 1:1 ratio with pretrial basal insulins |
| Weekly insulin dose at the study completion: n (IU) | Icodec: 214 Glargine: 222 | Icodec: 268 Degludec: 244 | Icodec: 204 Degludec: 186 | Icodec: 305 Glargine: 279 | Icodec: 227 OD Basal: 185 | Icodec: 132 Degludec: 161 |
| Treat-to-target approach | Yes, 80–130 mg/dL | Yes, 80–130 mg/dL | Yes, 80–130 mg/dL | Yes, 80–130 mg/dL | NA | Yes, 80–130 mg/dL |
| Titration of basal insulin | Icodec: ±20 per week Glargine: ±3 per day | NA | Icodec: ±20 per week Degludec: ±3 per day | Icodec: ±20 per week Glargine: ±3 per day | Icodec: algorithmic-assisted titration Degludec: at the discretion of investigators | Icodec: ±20 per week Degludec: ±3 per day Aspart: dose adjustment (week 0 to 8) or carbohydrate-counting |
| Frequency of insulin dose adjustment | Once a week | Once a week | Once a week | Once a week | Once a week | Once a week |
| Additional metrics/tools | Yes, double-blind CGM (weeks 48–52) | Yes, double-blind CGM (weeks 22–26) | None | Yes, double-blind CGM (weeks 22–26) | ICOBOT engine for guiding Icodec titration only | Yes, open CGM (whole study, but not used for insulin titration) |
| Satisfaction questionnaire | None | Yes, DTSQ | None | None | Yes, DTSQ, TRIM-D | Yes, DTSQ |
| Main Outcomes with Significant Heterogenicity (I2 >60%) | Icodec in the Context of Basal Regimen (Subgroup 1-ONWARDS 1, 2, 3, and 5) | Icodec in the Context of Basal-Bolus Regimen (Subgroup 2-ONWARDS 4 and 6) |
|---|---|---|
| ETD [95%CI], I2 in HbA1c | −0.22% [−0.29; −0.14], I2 0% Favors Icodec | 0.03% [−0.08; 0.13], I2 0% |
| ERR [95%CI], I2 in probability to achieve HbA1c < 7% * | 1.33 [1.21; 1.47], I2 2% Favors Icodec | 0.90 [0.79; 1.02], I2 0% |
| ERR [95%CI], I2 in probability to achieve HbA1c < 7% without experiencing clinically relevant or severe hypoglycemia | 1.28 [1.17; 1.39], I2 0% Favors Icodec | 0.79 [0.44; 1.42], I2 81% |
| ETD [95%CI], I2 in TIR ** | 4.55% [2.01; 7.08], I2 0% Favors Icodec | −0.88% [−2.97; 1.20], I2 28% |
| ETD [95%CI], I2 in FPG *** | 0.1 mg/dL [−3.01; 3.20], I2 0% | 8.05 mg/dL [−12.99; 29], I2 91% |
| ETD [95%CI], I2 in TAR ** | −5.14% [−7.27; −3.01], I2 0% Favors Icodec | 0.07% [−2; 2.15], I2 0% |
| ERR [95%CI], I2 in the probability of experiencing combined level 2 or 3 hypoglycemic events **** | 1.12 [0.98; 1.15], I2 0% | 1.09 [0.76; 1.57], I2 95% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisco, G.; De Tullio, A.; De Geronimo, V.; Giagulli, V.A.; Guastamacchia, E.; Piazzolla, G.; Disoteo, O.E.; Triggiani, V. Once-Weekly Insulin Icodec in Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Clinical Trials (ONWARDS Clinical Program). Biomedicines 2024, 12, 1852. https://doi.org/10.3390/biomedicines12081852
Lisco G, De Tullio A, De Geronimo V, Giagulli VA, Guastamacchia E, Piazzolla G, Disoteo OE, Triggiani V. Once-Weekly Insulin Icodec in Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Clinical Trials (ONWARDS Clinical Program). Biomedicines. 2024; 12(8):1852. https://doi.org/10.3390/biomedicines12081852
Chicago/Turabian StyleLisco, Giuseppe, Anna De Tullio, Vincenzo De Geronimo, Vito Angelo Giagulli, Edoardo Guastamacchia, Giuseppina Piazzolla, Olga Eugenia Disoteo, and Vincenzo Triggiani. 2024. "Once-Weekly Insulin Icodec in Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Clinical Trials (ONWARDS Clinical Program)" Biomedicines 12, no. 8: 1852. https://doi.org/10.3390/biomedicines12081852
APA StyleLisco, G., De Tullio, A., De Geronimo, V., Giagulli, V. A., Guastamacchia, E., Piazzolla, G., Disoteo, O. E., & Triggiani, V. (2024). Once-Weekly Insulin Icodec in Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Clinical Trials (ONWARDS Clinical Program). Biomedicines, 12(8), 1852. https://doi.org/10.3390/biomedicines12081852










