Deep Learning Radiomics Features of Mediastinal Fat and Pulmonary Nodules on Lung CT Images Distinguish Benignancy and Malignancy
Abstract
:1. Introduction
2. Materials and Methods
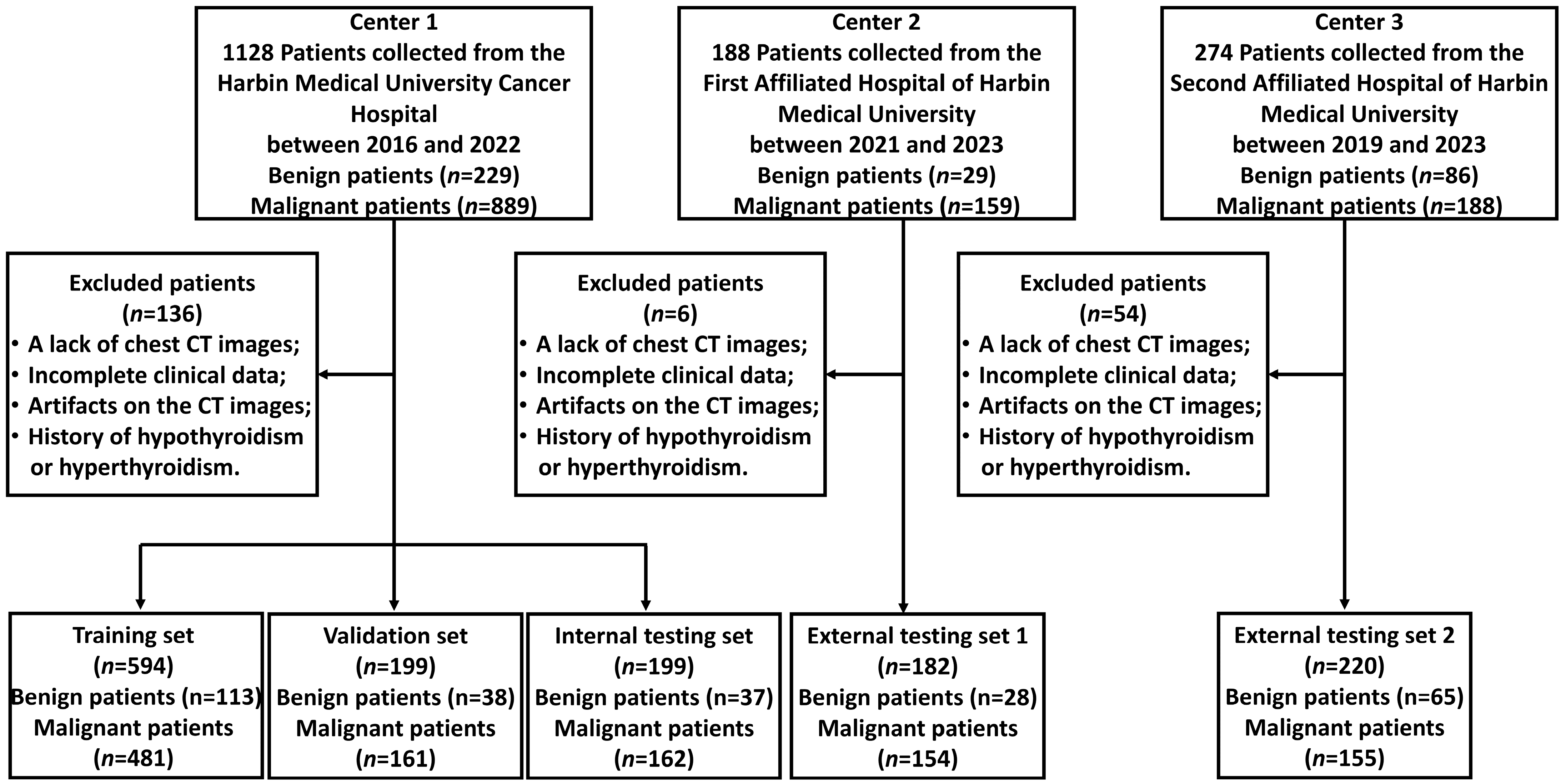
2.1. Participant Inclusion
2.2. CT Examination
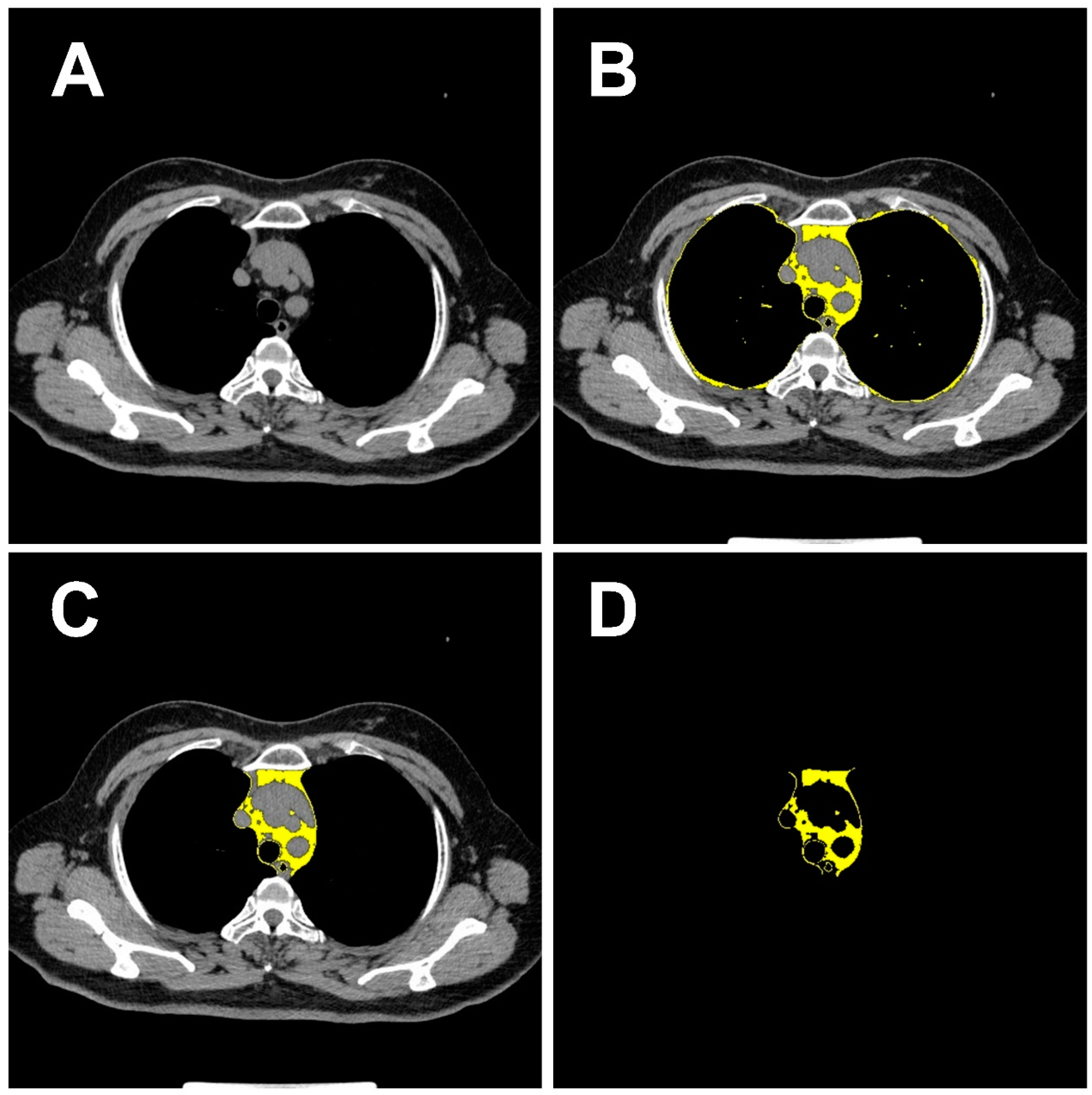
2.3. Imaging Data Acquisition and Processing
2.4. Radiomics Features Extraction of Pulmonary Nodules
2.5. Deep Learning Feature Extraction of Pulmonary Nodules and Mediastinal Fat
2.6. Score Building and Model Development
2.7. Models Performance Assessment
2.8. Statistical Analysis
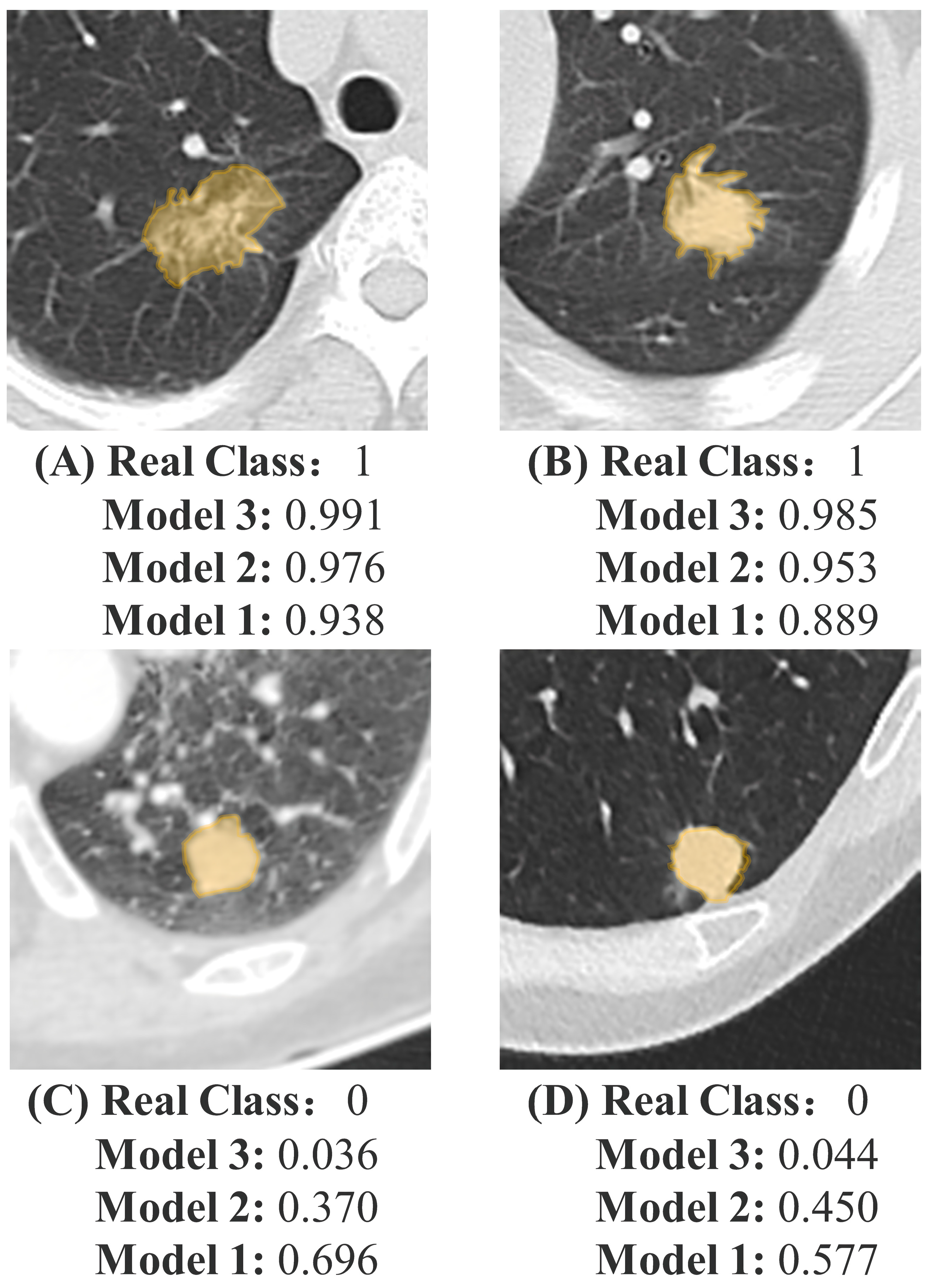
3. Results
3.1. Clinical Characteristics
3.2. Feature Selection and Score Building
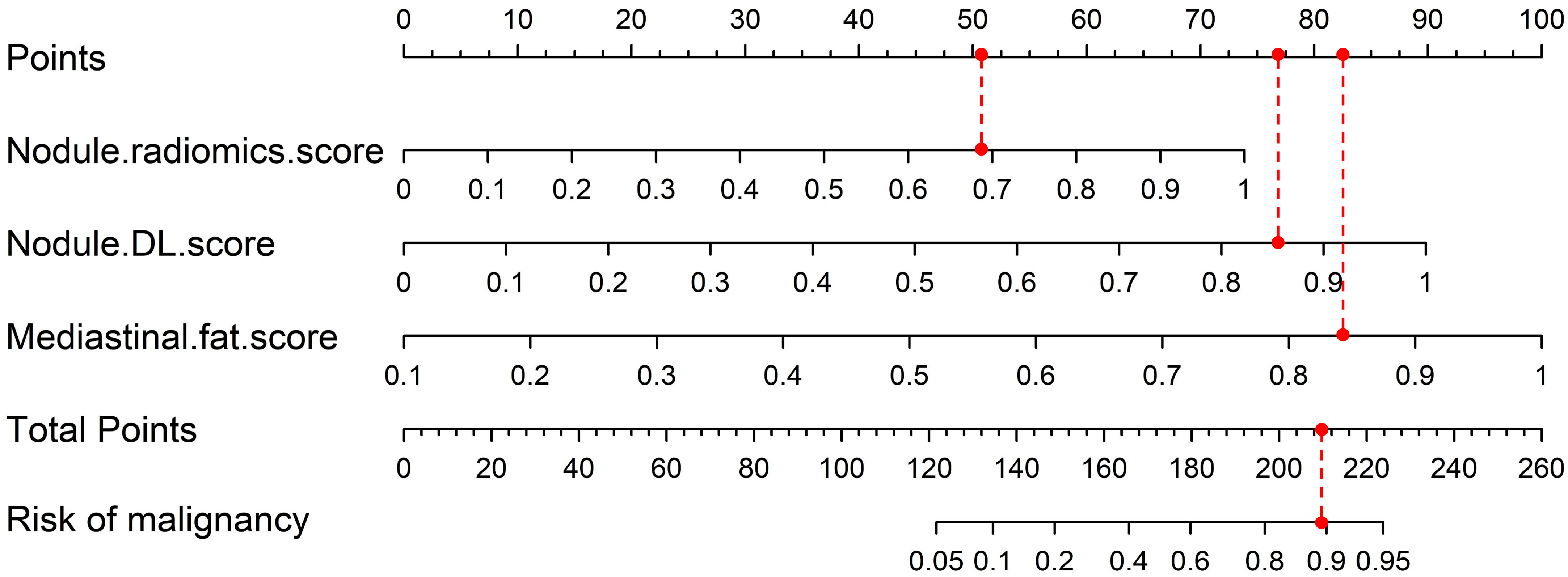
3.3. Nomogram Construction
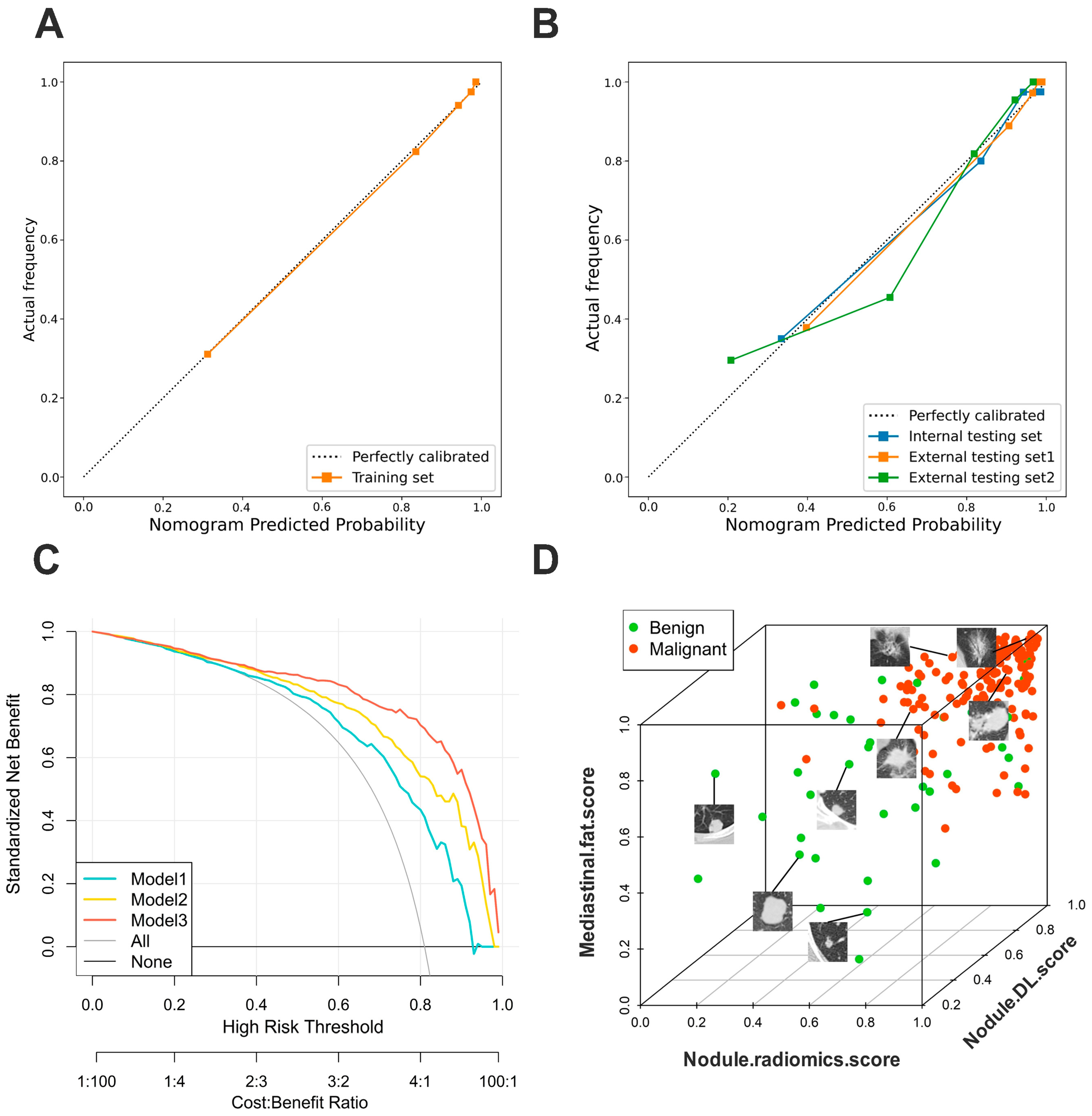
3.4. Validation of Nomogram Performance
3.5. Clinical Use
3.6. Incremental Predictive Value of Mediastinal Fat Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazzone, P.J.; Lam, L. Evaluating the patient with a pulmonary nodule: A review. JAMA 2022, 327, 264–273. [Google Scholar] [CrossRef]
- On behalf of the National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- Berlin, E.; Buckstein, M.; Yip, R.; Yankelevitz, D.F.; Rosenzweig, K.; Henschke, C.I.; I-ELCAP Investigators. Definitive radiation for stage I lung cancer in a screened population: Results from the I-ELCAP. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Callister, M.E.J.; Baldwin, D.R.; Akram, A.R.; Barnard, S.; Cane, P.; Draffan, J.; Franks, K.; Gleeson, F.; Graham, R.; Malhotra, P.; et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules: Accredited by NICE. Thorax 2015, 70 (Suppl. S2), ii1–ii54. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Oudkerk, M.; Liu, S.; Heuvelmans, M.A.; Walter, J.E.; Field, J.K. Lung cancer LDCT screening and mortality reduction—Evidence, pitfalls and future perspectives. Nat. Rev. Clin. Oncol. 2021, 18, 135–151. [Google Scholar] [CrossRef]
- Chansky, K.; Detterbeck, F.C.; Nicholson, A.G.; Rusch, V.W.; Vallières, E.; Groome, P.; Kennedy, C.; Krasnik, M.; Peake, M.; Shemanski, L.; et al. The IASLC lung cancer staging project: External validation of the revision of the TNM stage groupings in the eighth edition of the TNM classification of lung cancer. J. Thorac. Oncol. 2017, 12, 1109–1121. [Google Scholar] [CrossRef]
- McKee, B.J.; Regis, S.M.; McKee, A.B.; Flacke, S.; Wald, C. Performance of ACR Lung-RADS in a clinical CT lung screening program. J. Am. Coll. Radiol. 2016, 13, R25–R29. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, E.; Makowski, L.; DiGiovanni, J.; Kolonin, M.G. Cancer as a matter of fat: The crosstalk between adipose tissue and tumors. Trends Cancer 2018, 4, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-associated oxidative stress: Strategies finalized to improve redox state. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef]
- Luperini, B.C.O.; Almeida, D.C.; Porto, M.P.; Marcondes, J.P.D.C.; Prado, R.P.; Rasera, I.; Oliveira, M.R.M.; Salvadori, D.M.F. Gene polymorphisms and increased DNA damage in morbidly obese women. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2015, 776, 111–117. [Google Scholar] [CrossRef]
- Bunnell, B.A.; Martin, E.C.; Matossian, M.D.; Brock, C.K.; Nguyen, K.; Collins-Burow, B.; Burow, M.E. The effect of obesity on adipose-derived stromal cells and adipose tissue and their impact on cancer. Cancer Metastasis Rev. 2022, 41, 549–573. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.A.; Encarnação, C.; Franco, V.A.; Botelho, L.G.X.; Rodrigues, G.P.; Ramos-Andrade, I.; Barja-Fidalgo, C.; Renovato-Martins, M. Adipose tissue-derived extracellular vesicles and the tumor microenvironment: Revisiting the hallmarks of cancer. Cancers 2021, 13, 3328. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, adipose tissue and vascular dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Xing, Y.; Li, Z.; Jiang, S.; Xiang, W.; Sun, X. Analysis of pre-invasive pulmonary adenocarcinoma lesions on thin-section computerized tomography. Clin. Respir. J. 2015, 9, 289–296. [Google Scholar] [CrossRef]
- Ren, C.; Xu, M.; Zhang, J.; Zhang, F.; Song, S.; Sun, Y.; Wu, K.; Cheng, J. Classification of solid pulmonary nodules using a machine-learning nomogram based on 18F-FDG PET/CT radiomics integrated clinicobiological features. Ann. Transl. Med. 2022, 10, 1265. [Google Scholar] [CrossRef]
- Li, M.; Ruan, Y.; Feng, Z.; Sun, F.; Wang, M.; Zhang, L. Preoperative CT-based radiomics combined with nodule type to predict the micropapillary pattern in pulmonary adenocarcinoma of size 2 cm or less: A multicenter study. Front. Oncol. 2021, 11, 788424. [Google Scholar]
- Zhou, J.H.; Yao, Z.X.; Zheng, Z.; Yang, J.; Wang, R.; Fu, S.J.; Pan, X.F.; Liu, Z.H.; Wu, K. G-MDSCs-derived exosomal miRNA-143-3p promotes proliferation via targeting of ITM2B in pulmonary cancer. OncoTargets Ther. 2020, 13, 9701. [Google Scholar] [CrossRef]
- Kumar, D.; Wong, A.; Clausi, D.A. Lung nodule classification using deep features in CT images. In Proceedings of the 12th Conference on Computer and Robot Vision, Halifax, NS, Canada, 3–5 June 2015; pp. 133–138. [Google Scholar]
- Liu, G.; Liu, F.; Gu, J.; Mao, X.; Xie, X.; Sang, J. An attention-based deep learning network for pulmonary nodule malignancy discrimination. Front. Neurosci. 2022, 16, 1106937. [Google Scholar]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, L.; Polosukhin, I. Attention is all you need. Adv. Neural Inf. Process. Syst. 2017, 30. [Google Scholar] [CrossRef]
- Gupta, S.; Punn, N.S.; Sonbhadra, S.K.; Agarwal, S. MAG-Net: Multi-task attention guided network for brain tumor segmentation and classification. In Proceedings of the Big Data Analytics: 9th International Conference, Tokyo, Japan, 15–18 December 2021; Proceedings 9. Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 3–15. [Google Scholar]
- Lai, K.D.; Nguyen, T.T.; Le, T.H. Detection of pulmonary nodules on CT images based on the convolutional neural network with attention mechanism. Ann. Emerg. Technol. Comput. 2021, 5, 78–89. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; MacDonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets-Tan, R.G.; Fillion-Robin, J.C.; Pieper, S.; Aerts, H.J. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef]
- Asaki, T.; Konishi, M.; Miyake, A.; Kato, S.; Tomizawa, M.; Itoh, N. Roles of fibroblast growth factor 10 (Fgf10) in adipogenesis in vivo. Mol. Cell. Endocrinol. 2004, 218, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Thijs, W.; Dehnavi, R.A.; Hiemstra, P.S.; de Roos, A.; Melissant, C.F.; Janssen, K.; Tamsma, J.T.; Rabe, K.F. Association of lung function measurements and visceral fat in men with metabolic syndrome. Respir. Med. 2014, 108, 351–357. [Google Scholar] [CrossRef]
- Elliot, J.G.; Donovan, G.M.; Wang, K.C.; Green, F.H.; James, A.L.; Noble, P.B. Fatty airways: Implications for obstructive disease. Eur. Respir. J. 2019, 54, 1900857. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Caruso, D.; Tuccinardi, D.; Risi, R.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Tarallo, M.; Strigari, L.; Manfrini, S.; et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 2020, 111, 154319. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef]
- Seo, B.R.; Bhardwaj, P.; Choi, S.; Gonzalez, J.; Andresen Eguiluz, R.C.; Wang, K.; Mohanan, S.; Morris, P.G.; Du, B.; Zhou, X.K.; et al. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci. Transl. Med. 2015, 7, ra130–ra301. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Prieur, X.; Mok, C.Y.; Velagapudi, V.R.; Núñez, V.; Fuentes, L.; Montaner, D.; Ishikawa, K.; Camacho, A.; Barbarroja, N.; O’Rahilly, S.; et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes 2011, 60, 797–809. [Google Scholar] [CrossRef]
- Li, B.; Sun, S.; Li, J.J.; Yuan, J.P.; Sun, S.R.; Wu, Q. Adipose tissue macrophages: Implications for obesity-associated cancer. Mil. Med. Res. 2023, 10, 1. [Google Scholar] [CrossRef]
- Zoico, E.; Rizzatti, V.; Darra, E.; Budui, S.L.; Franceschetti, G.; Vinante, F.; Pedrazzani, C.; Guglielmi, A.; De Manzoni, G.; Mazzali, G.; et al. Morphological and functional changes in the peritumoral adipose tissue of colorectal cancer patients. Obesity 2017, 25, S87–S94. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Yang, Y.; Sun, X.; Lim, S.; Xie, S.; Guo, Z.; Xiong, W.; Kuroda, M.; Sakaue, H.; Hosaka, K.; et al. Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature 2022, 608, 421–428. [Google Scholar] [CrossRef]
- Laveneziana, P.; Palange, P.; on behalf of the ERS Research Seminar Faculty. Physical activity, nutritional status and systemic inflammation in COPD. Eur. Respir. J. 2012, 40, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, H.J.; Son, K.R.; Chun, E.J.; Lim, K.Y.; Goo, J.M.; Im, J.G.; Heo, J.N.; Song, J.W. CT analysis of the anterior mediastinum in idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia. Korean J. Radiol. 2006, 7, 173–179. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]

| Characteristic | Training Set | Validation Set | p Value | Internal Testing Set | External Testing Set 1 | External Testing Set 2 |
|---|---|---|---|---|---|---|
| Number of patients | 594 | 199 | 199 | 182 | 220 | |
| Age (yr, mean ± s.d) | 57.3 ± 9.1 | 57.2 ± 9.3 | 0.899 | 57.3 ± 9.5 | 57.9 ± 9.8 | 57.2 ± 9.8 |
| Sex | 0.840 | |||||
| Female | 360(60.6) | 119(59.8) | 125(62.8) | 116(63.7) | 132(60.0) | |
| Male | 234(39.4) | 80(40.2) | 74(37.2) | 66(36.3) | 88(40.0) | |
| Smoking | 0.752 | |||||
| Yes | 178(30.0) | 62(31.2) | 65(32.7) | 36(19.8) | 43(19.5) | |
| No | 416(70.0) | 137(68.8) | 134(67.3) | 146(80.2) | 177(80.5) | |
| Pathological type | 0.982 | |||||
| Malignant nodule | 481 | 161 | 162 | 154 | 155 | |
| Adenocarcinoma | 449(93.5) | 156(96.9) | 155(95.7) | 150(97.4) | 144(92.9) | |
| Squamous carcinoma | 26(5.4) | 4(2.5) | 6(3.7) | 2(1.3) | 9(5.8) | |
| Other Malignant histological types | 6(1.2) | 1(0.6) | 1(0.6) | 2(1.3) | 2(1.3) | |
| Benign nodule | 113 | 38 | 37 | 28 | 65 | |
| Hyperplasia | 38(33.6) | 12(31.6) | 9(24.3) | 8(28.6) | 10(15.4) | |
| Hamartoma | 21(18.6) | 4(10.5) | 10(27.0) | 5(17.8) | 11(16.9) | |
| Other Benign histological types | 54(47.8) | 22(57.9) | 18(48.7) | 15(53.6) | 44(67.7) |
| Intercept and Variables | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | OR (95%CI) | p Value | β | OR (95%CI) | p Value | β | OR (95%CI) | p Value | |
| Intercept | −3.107 | - | <0.001 | −6.220 | - | <0.001 | −10.608 | - | <0.001 |
| Nodule.radiomic.score | 5.977 | 394.278 (45.183–3440.535) | <0.001 | 4.880 | 131.644 (14.599–1187.028) | <0.001 | 4.263 | 71.006 (5.539–910.308) | 0.001 |
| Nodule.DL.score | - | - | - | 5.305 | 201.255 (20.273–1997.909) | <0.001 | 5.182 | 178.108 (13.557–2339.988) | <0.001 |
| Mediastinal.fat.score | - | - | - | - | - | - | 6.411 | 608.687 (29.190–12,692.520) | <0.001 |
| Internal Testing Set | External Testing Set1 | External Testing Set2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Overall | |||||||||
| Brier | 0.112 | 0.090 | 0.079 | 0.118 | 0.107 | 0.068 | 0.176 | 0.159 | 0.133 |
| R2 | 0.299 | 0.443 | 0.560 | 0.154 | 0.258 | 0.591 | 0.201 | 0.308 | 0.484 |
| Discrimination | |||||||||
| C-index | 0.766 | 0.840 | 0.903 | 0.747 | 0.829 | 0.942 | 0.732 | 0.794 | 0.880 |
| Specificity | 0.676 | 0.784 | 0.919 | 0.679 | 0.857 | 0.929 | 0.708 | 0.708 | 0.908 |
| Sensitivity | 0.765 | 0.741 | 0.765 | 0.786 | 0.714 | 0.864 | 0.671 | 0.703 | 0.755 |
| Discrimination slope | 0.254 | 0.385 | 0.476 | 0.105 | 0.190 | 0.500 | 0.156 | 0.239 | 0.379 |
| Calibration | |||||||||
| H-L test (p) | 0.770 | 0.280 | 0.725 | 0.478 | 0.652 | 0.651 | 0.824 | 0.784 | 0.446 |
| Clinical usefulness | |||||||||
| Accuracy | 0.869 | 0.894 | 0.910 | 0.824 | 0.863 | 0.901 | 0.732 | 0.777 | 0.789 |
| Training Set | Internal Testing Set | |||||
|---|---|---|---|---|---|---|
| NRI | 95%CI | p Value | NRI | 95%CI | p Value | |
| Model 2 vs. Model 1 | 0.140 | 0.063–0.217 | <0.001 | 0.177 | 0.029–0.325 | 0.019 |
| Model 3 vs. Model 2 | 0.144 | 0.057–0.231 | <0.001 | 0.243 | 0.104–0.383 | <0.001 |
| IDI | 95%CI | p Value | IDI | 95%CI | p Value | |
| Model 2 vs. Model 1 | 0.150 | 0.109–0.190 | <0.001 | 0.132 | 0.058–0.205 | <0.001 |
| Model 3 vs. Model 2 | 0.130 | 0.089–0.171 | <0.001 | 0.090 | 0.030–0.151 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Xuan, Q.; Liu, P.; An, Y.; Huang, W.; Miao, S.; Wang, Q.; Liu, Z.; Wang, R. Deep Learning Radiomics Features of Mediastinal Fat and Pulmonary Nodules on Lung CT Images Distinguish Benignancy and Malignancy. Biomedicines 2024, 12, 1865. https://doi.org/10.3390/biomedicines12081865
Qi H, Xuan Q, Liu P, An Y, Huang W, Miao S, Wang Q, Liu Z, Wang R. Deep Learning Radiomics Features of Mediastinal Fat and Pulmonary Nodules on Lung CT Images Distinguish Benignancy and Malignancy. Biomedicines. 2024; 12(8):1865. https://doi.org/10.3390/biomedicines12081865
Chicago/Turabian StyleQi, Hongzhuo, Qifan Xuan, Pingping Liu, Yunfei An, Wenjuan Huang, Shidi Miao, Qiujun Wang, Zengyao Liu, and Ruitao Wang. 2024. "Deep Learning Radiomics Features of Mediastinal Fat and Pulmonary Nodules on Lung CT Images Distinguish Benignancy and Malignancy" Biomedicines 12, no. 8: 1865. https://doi.org/10.3390/biomedicines12081865





