The Role of Fecal Microbiota Transplantation (FMT) in the Management of Metabolic Diseases in Humans: A Narrative Review
Abstract
:1. Introduction
2. Methods of Literature Search and Review Criteria
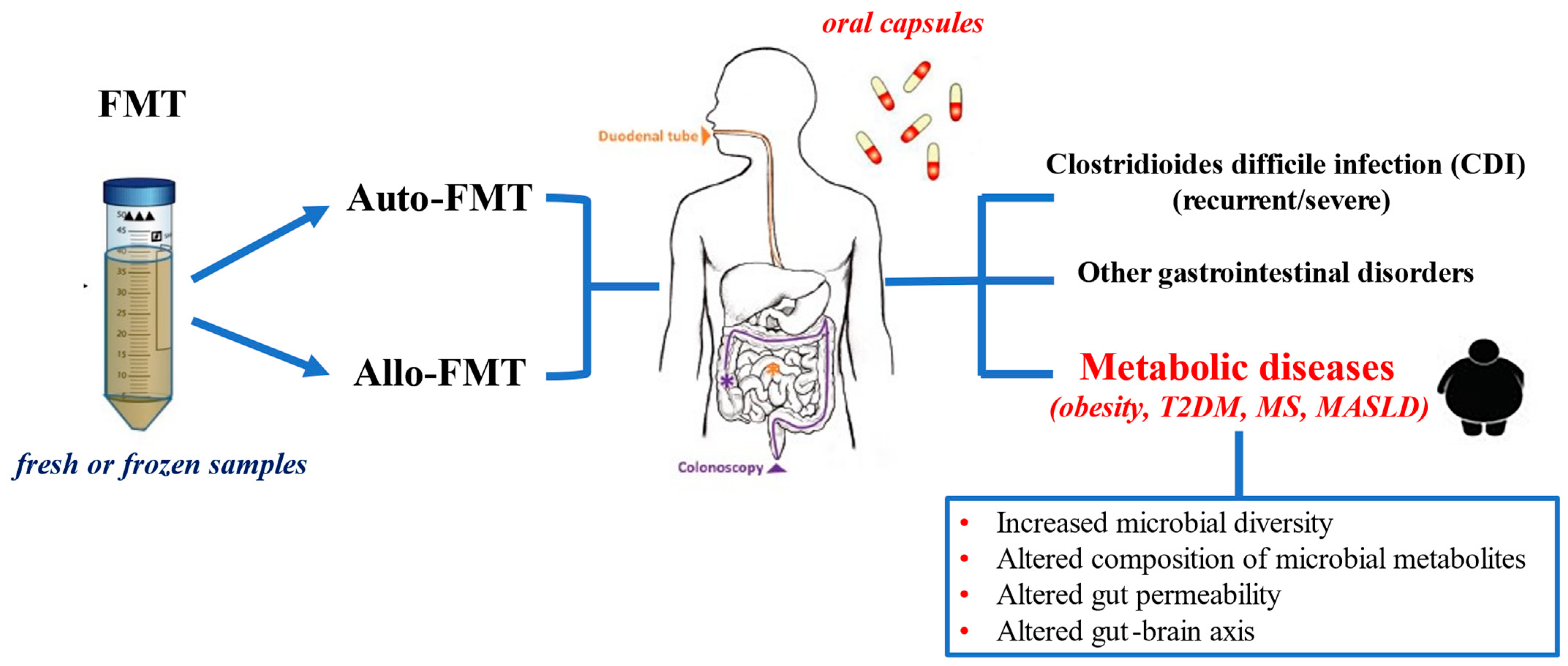
3. Underlying Principles and Delivering Methods of FMT
4. Clinical Applications of FMT in Human Diseases
5. The Role of FMT in Obesity
5.1. Experimental Data (Animal Models)
5.2. Clinical Data (Humans)
6. The Role of FMT in T2DM and Metabolic Syndrome
6.1. FMT with Dietary Intervention in T2DM
6.2. FMT in Metabolic Syndrome
6.3. FMT in Metabolic Dysfunction Associated Steatotic Liver Disease
7. Limitations, Critical Perspectives, Areas for Future Research
8. Summary and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Costello, K.R.; Schones, D.E. Chromatin Modifications in Metabolic Disease: Potential Mediators of Long-Term Disease Risk. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, e1416. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Dalamaga, M.; Liatis, S. Update on the Obesity Epidemic: After the Sudden Rise, Is the Upward Trajectory Beginning to Flatten? Curr. Obes. Rep. 2023, 12, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and Cardiovascular Disease: Revisiting an Old Relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Golay, A.; Ybarra, J. Link between Obesity and Type 2 Diabetes. Best Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and Treatment of Type 2 Diabetes: Perspectives on the Past, Present, and Future. Lancet Lond. Engl. 2014, 383, 1068–1083. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Spinos, T.; Spinou, Μ.; Brinia, Μ.-E.; Mitsopoulou, D.; Katsilambros, N. Defining the Optimal Dietary Approach for Safe, Effective and Sustainable Weight Loss in Overweight and Obese Adults. Healthc. Basel Switz. 2018, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Angelidi, A.M.; Belanger, M.J.; Kokkinos, A.; Koliaki, C.C.; Mantzoros, C.S. Novel Noninvasive Approaches to the Treatment of Obesity: From Pharmacotherapy to Gene Therapy. Endocr. Rev. 2022, 43, 507–557. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Tamboli, C.P.; Neut, C.; Desreumaux, P.; Colombel, J.F. Dysbiosis in Inflammatory Bowel Disease. Gut 2004, 53, 1–4. [Google Scholar] [CrossRef]
- Walker, A.W.; Lawley, T.D. Therapeutic Modulation of Intestinal Dysbiosis. Pharmacol. Res. 2013, 69, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Machiels, K.; Joossens, M.; Sabino, J.; De Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Van Immerseel, F.; Verbeke, K.; et al. A Decrease of the Butyrate-Producing Species Roseburia Hominis and Faecalibacterium Prausnitzii Defines Dysbiosis in Patients with Ulcerative Colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Brahe, L.K.; Astrup, A.; Larsen, L.H. Can We Prevent Obesity-Related Metabolic Diseases by Dietary Modulation of the Gut Microbiota? Adv. Nutr. Bethesda Md 2016, 7, 90–101. [Google Scholar] [CrossRef]
- He, M.; Shi, B. Gut Microbiota as a Potential Target of Metabolic Syndrome: The Role of Probiotics and Prebiotics. Cell Biosci. 2017, 7, 54. [Google Scholar] [CrossRef]
- Wortelboer, K.; Nieuwdorp, M.; Herrema, H. Fecal Microbiota Transplantation beyond Clostridioides Difficile Infections. EBioMedicine 2019, 44, 716–729. [Google Scholar] [CrossRef]
- Fuschillo, G.; Celentano, V.; Rottoli, M.; Sciaudone, G.; Gravina, A.G.; Pellegrino, R.; Marfella, R.; Romano, M.; Selvaggi, F.; Pellino, G. Influence of Diabetes Mellitus on Inflammatory Bowel Disease Course and Treatment Outcomes. A Systematic Review with Meta-Analysis. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2023, 55, 580–586. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial Regulation of Organismal Energy Homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.-C.; Feng, D.D.; Chen, C.; Lee, H.-G.; et al. Acetate and Propionate Short Chain Fatty Acids Stimulate Adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Wu, C.-Y. The Gut Microbiome in Obesity. J. Formos. Med. Assoc. 2019, 118, S3–S9. [Google Scholar] [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef]
- Allin, K.H.; Nielsen, T.; Pedersen, O. MECHANISMS IN ENDOCRINOLOGY: Gut Microbiota in Patients with Type 2 Diabetes Mellitus. Eur. J. Endocrinol. 2015, 172, R167–R177. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Yan, S.; Yang, Y.; Chen, J.; Li, T.; Gao, X.; Yan, H.; Wang, Y.; Wang, J.; Wang, S.; et al. A Metagenome-Wide Association Study of the Gut Microbiome and Metabolic Syndrome. Front. Microbiol. 2021, 12, 682721. [Google Scholar] [CrossRef] [PubMed]
- Rittiphairoj, T.; Pongpirul, K.; Janchot, K.; Mueller, N.T.; Li, T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Adv. Nutr. Bethesda Md 2021, 12, 722–734. [Google Scholar] [CrossRef]
- Kasińska, M.A.; Drzewoski, J. Effectiveness of Probiotics in Type 2 Diabetes: A Meta-Analysis. Pol. Arch. Med. Wewn. 2015, 125, 803–813. [Google Scholar] [CrossRef]
- Akbari, V.; Hendijani, F. Effects of Probiotic Supplementation in Patients with Type 2 Diabetes: Systematic Review and Meta-Analysis. Nutr. Rev. 2016, 74, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Tiderencel, K.A.; Hutcheon, D.A.; Ziegler, J. Probiotics for the Treatment of Type 2 Diabetes: A Review of Randomized Controlled Trials. Diabetes Metab. Res. Rev. 2020, 36, e3213. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Stratigou, T.; Christodoulatos, G.S.; Tsigalou, C.; Dalamaga, M. Probiotics, Prebiotics, Synbiotics, Postbiotics, and Obesity: Current Evidence, Controversies, and Perspectives. Curr. Obes. Rep. 2020, 9, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Zikou, E.; Dovrolis, N.; Dimosthenopoulos, C.; Gazouli, M.; Makrilakis, K. The Effect of Probiotic Supplements on Metabolic Parameters of People with Type 2 Diabetes in Greece-A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 4663. [Google Scholar] [CrossRef] [PubMed]
- Bock, P.M.; Telo, G.H.; Ramalho, R.; Sbaraini, M.; Leivas, G.; Martins, A.F.; Schaan, B.D. The Effect of Probiotics, Prebiotics or Synbiotics on Metabolic Outcomes in Individuals with Diabetes: A Systematic Review and Meta-Analysis. Diabetologia 2021, 64, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Colantonio, A.G.; Werner, S.L.; Brown, M. The Effects of Prebiotics and Substances with Prebiotic Properties on Metabolic and Inflammatory Biomarkers in Individuals with Type 2 Diabetes Mellitus: A Systematic Review. J. Acad. Nutr. Diet. 2020, 120, 587–607.e2. [Google Scholar] [CrossRef] [PubMed]
- Carnahan, S.; Balzer, A.; Panchal, S.K.; Brown, L. Prebiotics in Obesity. Panminerva Med. 2014, 56, 165–175. [Google Scholar] [PubMed]
- Hijova, E. Probiotics and Prebiotics, Targeting Obesity with Functional Foods. Bratisl. Lek. Listy 2021, 122, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Naseri, K.; Saadati, S.; Ashtary-Larky, D.; Asbaghi, O.; Ghaemi, F.; Pashayee-Khamene, F.; Yari, Z.; de Courten, B. Probiotics and Synbiotics Supplementation Improve Glycemic Control Parameters in Subjects with Prediabetes and Type 2 Diabetes Mellitus: A GRADE-Assessed Systematic Review, Meta-Analysis, and Meta-Regression of Randomized Clinical Trials. Pharmacol. Res. 2022, 184, 106399. [Google Scholar] [CrossRef]
- Kanazawa, A.; Aida, M.; Yoshida, Y.; Kaga, H.; Katahira, T.; Suzuki, L.; Tamaki, S.; Sato, J.; Goto, H.; Azuma, K.; et al. Effects of Synbiotic Supplementation on Chronic Inflammation and the Gut Microbiota in Obese Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Study. Nutrients 2021, 13, 558. [Google Scholar] [CrossRef]
- Álvarez-Arraño, V.; Martín-Peláez, S. Effects of Probiotics and Synbiotics on Weight Loss in Subjects with Overweight or Obesity: A Systematic Review. Nutrients 2021, 13, 3627. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ji, Y.-Y.; Wen, X.-L.; Duan, S.-L. Fecal Microbiota Transplantation in the Metabolic Diseases: Current Status and Perspectives. World J. Gastroenterol. 2022, 28, 2546. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yu, X.; Li, Y.; Guo, Y.; Ge, L.; Pu, F.; Ma, X.; Cui, W.; Marrota, F.; He, F.; et al. Bifidobacterium Bifidum TMC3115 Can Characteristically Influence Glucose and Lipid Profile and Intestinal Microbiota in the Middle-Aged and Elderly. Probiotics Antimicrob. Proteins 2019, 11, 1182–1194. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal Microbiota Transplantation: In Perspective. Ther. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, N.M.J.; de Vos, W.M.; Nieuwdorp, M. Fecal Microbiota Transplantation in Human Metabolic Diseases: From a Murky Past to a Bright Future? Cell Metab. 2021, 33, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Gravina, A.G.; Pellegrino, R.; Palladino, G.; Coppola, A.; Brandimarte, G.; Tuccillo, C.; Ciardiello, F.; Romano, M.; Federico, A. Hericium Erinaceus, in Combination with Natural Flavonoid/Alkaloid and B3/B8 Vitamins, Can Improve Inflammatory Burden in Inflammatory Bowel Diseases Tissue: An Ex Vivo Study. Front. Immunol. 2023, 14, 1215329. [Google Scholar] [CrossRef] [PubMed]
- Auchtung, T.A.; Fofanova, T.Y.; Stewart, C.J.; Nash, A.K.; Wong, M.C.; Gesell, J.R.; Auchtung, J.M.; Ajami, N.J.; Petrosino, J.F. Investigating Colonization of the Healthy Adult Gastrointestinal Tract by Fungi. mSphere 2018, 3, e00092-18. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, Stability and Resilience of the Human Gut Microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Li, X.; Shen, J.; Feng, Q. Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease: An Update. Front. Pharmacol. 2020, 11, 574533. [Google Scholar] [CrossRef]
- Holster, S.; Lindqvist, C.M.; Repsilber, D.; Salonen, A.; de Vos, W.M.; König, J.; Brummer, R.J. The Effect of Allogenic Versus Autologous Fecal Microbiota Transfer on Symptoms, Visceral Perception and Fecal and Mucosal Microbiota in Irritable Bowel Syndrome: A Randomized Controlled Study. Clin. Transl. Gastroenterol. 2019, 10, e00034. [Google Scholar] [CrossRef]
- Taur, Y.; Coyte, K.; Schluter, J.; Robilotti, E.; Figueroa, C.; Gjonbalaj, M.; Littmann, E.R.; Ling, L.; Miller, L.; Gyaltshen, Y.; et al. Reconstitution of the Gut Microbiota of Antibiotic-Treated Patients by Autologous Fecal Microbiota Transplant. Sci. Transl. Med. 2018, 10, eaap9489. [Google Scholar] [CrossRef]
- Basson, A.R.; Zhou, Y.; Seo, B.; Rodriguez-Palacios, A.; Cominelli, F. Autologous Fecal Microbiota Transplantation for the Treatment of Inflammatory Bowel Disease. Transl. Res. 2020, 226, 1–11. [Google Scholar] [CrossRef]
- Pession, A.; Zama, D.; Muratore, E.; Leardini, D.; Gori, D.; Guaraldi, F.; Prete, A.; Turroni, S.; Brigidi, P.; Masetti, R. Fecal Microbiota Transplantation in Allogeneic Hematopoietic Stem Cell Transplantation Recipients: A Systematic Review. J. Pers. Med. 2021, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Tsuchiya, A.; Mizusawa, T.; Matsumoto, A.; Minemura, A.; Oka, K.; Takahashi, M.; Yoshida, T.; Kojima, Y.; Ogawa, K.; et al. Utility of Autologous Fecal Microbiota Transplantation and Elucidation of Microbiota in Diversion Colitis. DEN Open 2022, 2, e63. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.H.; Quraishi, M.N.; Segal, J.P.; McCune, V.L.; Baxter, M.; Marsden, G.L.; Moore, D.J.; Colville, A.; Bhala, N.; Iqbal, T.H.; et al. The Use of Faecal Microbiota Transplant as Treatment for Recurrent or Refractory Clostridium Difficile Infection and Other Potential Indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) Guidelines. Gut 2018, 67, 1920–1941. [Google Scholar] [CrossRef]
- Kim, K.O.; Schwartz, M.A.; Lin, O.S.T.; Chiorean, M.V.; Gluck, M. Reducing Cost and Complexity of Fecal Microbiota Transplantation Using Universal Donors for Recurrent Clostridium Difficile Infection. Adv. Ther. 2019, 36, 2052–2061. [Google Scholar] [CrossRef]
- Costello, S.P.; Tucker, E.C.; La Brooy, J.; Schoeman, M.N.; Andrews, J.M. Establishing a Fecal Microbiota Transplant Service for the Treatment of Clostridium Difficile Infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, 908–914. [Google Scholar] [CrossRef]
- Rode, A.A.; Bytzer, P.; Pedersen, O.B.; Engberg, J. Establishing a Donor Stool Bank for Faecal Microbiota Transplantation: Methods and Feasibility. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2019, 38, 1837–1847. [Google Scholar] [CrossRef]
- Commission Directive 2006/17/EC of 8 February 2006 Implementing Directive 2004/23/EC of the European Parliament and of the Council as Regards Certain Technical Requirements for the Donation, Procurement and Testing of Human Tissues and Cells (Text with EEA Relevance). 2006, Volume 038. Available online: http://data.europa.eu/eli/dir/2006/17/oj (accessed on 12 August 2024).
- Nicco, C.; Paule, A.; Konturek, P.; Edeas, M. From Donor to Patient: Collection, Preparation and Cryopreservation of Fecal Samples for Fecal Microbiota Transplantation. Diseases 2020, 8, 9. [Google Scholar] [CrossRef]
- Resolution of Severe Clostridium Difficile Infection Following Sequential Fecal Microbiota Transplantation—Abstract—Europe PMC. Available online: https://europepmc.org/article/pmc/3735621 (accessed on 25 June 2024).
- Youngster, I.; Sauk, J.; Pindar, C.; Wilson, R.G.; Kaplan, J.L.; Smith, M.B.; Alm, E.J.; Gevers, D.; Russell, G.H.; Hohmann, E.L. Fecal Microbiota Transplant for Relapsing Clostridium Difficile Infection Using a Frozen Inoculum from Unrelated Donors: A Randomized, Open-Label, Controlled Pilot Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2014, 58, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Steiner, T.; Petrof, E.O.; Smieja, M.; Roscoe, D.; Nematallah, A.; Weese, J.S.; Collins, S.; Moayyedi, P.; Crowther, M.; et al. Frozen vs Fresh Fecal Microbiota Transplantation and Clinical Resolution of Diarrhea in Patients with Recurrent Clostridium Difficile Infection: A Randomized Clinical Trial. JAMA 2016, 315, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Preidis, G.A.; Versalovic, J. Targeting the Human Microbiome with Antibiotics, Probiotics, and Prebiotics: Gastroenterology Enters the Metagenomics Era. Gastroenterology 2009, 136, 2015–2031. [Google Scholar] [CrossRef] [PubMed]
- Bilinski, J.; Dziurzynski, M.; Grzesiowski, P.; Podsiadly, E.; Stelmaszczyk-Emmel, A.; Dzieciatkowski, T.; Dziewit, L.; Basak, G.W. Multimodal Approach to Assessment of Fecal Microbiota Donors Based on Three Complementary Methods. J. Clin. Med. 2020, 9, 2036. [Google Scholar] [CrossRef]
- Satokari, R.; Mattila, E.; Kainulainen, V.; Arkkila, P.E.T. Simple Faecal Preparation and Efficacy of Frozen Inoculum in Faecal Microbiota Transplantation for Recurrent Clostridium Difficile Infection--an Observational Cohort Study. Aliment. Pharmacol. Ther. 2015, 41, 46–53. [Google Scholar] [CrossRef]
- Gough, E.; Shaikh, H.; Manges, A.R. Systematic Review of Intestinal Microbiota Transplantation (Fecal Bacteriotherapy) for Recurrent Clostridium Difficile Infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2011, 53, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, Y.K.; Chen, Z.; Hui, M.; Wong, M.C.S.; Ho, W.C.S.; Chin, M.L.; Ng, S.C.; Chan, F.K.L.; Chan, P.K.S. Impact of Inter- and Intra-Individual Variation, Sample Storage and Sampling Fraction on Human Stool Microbial Community Profiles. PeerJ 2019, 7, e6172. [Google Scholar] [CrossRef]
- Costello, S.P.; Conlon, M.A.; Vuaran, M.S.; Roberts-Thomson, I.C.; Andrews, J.M. Faecal Microbiota Transplant for Recurrent Clostridium Difficile Infection Using Long-Term Frozen Stool Is Effective: Clinical Efficacy and Bacterial Viability Data. Aliment. Pharmacol. Ther. 2015, 42, 1011–1018. [Google Scholar] [CrossRef]
- Burz, S.D.; Abraham, A.-L.; Fonseca, F.; David, O.; Chapron, A.; Béguet-Crespel, F.; Cénard, S.; Le Roux, K.; Patrascu, O.; Levenez, F.; et al. A Guide for Ex Vivo Handling and Storage of Stool Samples Intended for Fecal Microbiota Transplantation. Sci. Rep. 2019, 9, 8897. [Google Scholar] [CrossRef]
- Hamilton, M.J.; Weingarden, A.R.; Sadowsky, M.J.; Khoruts, A. Standardized Frozen Preparation for Transplantation of Fecal Microbiota for Recurrent Clostridium Difficile Infection. Am. J. Gastroenterol. 2012, 107, 761–767. [Google Scholar] [CrossRef]
- Smits, L.P.; Bouter, K.E.C.; de Vos, W.M.; Borody, T.J.; Nieuwdorp, M. Therapeutic Potential of Fecal Microbiota Transplantation. Gastroenterology 2013, 145, 946–953. [Google Scholar] [CrossRef]
- Grehan, M.J.; Borody, T.J.; Leis, S.M.; Campbell, J.; Mitchell, H.; Wettstein, A. Durable Alteration of the Colonic Microbiota by the Administration of Donor Fecal Flora. J. Clin. Gastroenterol. 2010, 44, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Covasa, M. Microbiota Transplant in the Treatment of Obesity and Diabetes: Current and Future Perspectives. Front. Microbiol. 2020, 11, 590370. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Buch, H.; Wen, Q.; Long, C.; Cui, B.; Zhang, F. Colonic Transendoscopic Enteral Tubing: Route for a Novel, Safe, and Convenient Delivery of Washed Microbiota Transplantation in Children. Gastroenterol. Res. Pract. 2021, 2021, 6676962. [Google Scholar] [CrossRef] [PubMed]
- Verdier, C.; Denis, S.; Gasc, C.; Boucinha, L.; Uriot, O.; Delmas, D.; Dore, J.; Le Camus, C.; Schwintner, C.; Blanquet-Diot, S. An Oral FMT Capsule as Efficient as an Enema for Microbiota Reconstruction Following Disruption by Antibiotics, as Assessed in an In Vitro Human Gut Model. Microorganisms 2021, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cui, B.; He, X.; Nie, Y.; Wu, K.; Fan, D. Microbiota Transplantation: Concept, Methodology and Strategy for Its Modernization. Protein Cell 2018, 9, 462–473. [Google Scholar] [CrossRef]
- Long, C.; Yu, Y.; Cui, B.; Jagessar, S.A.R.; Zhang, J.; Ji, G.; Huang, G.; Zhang, F. A Novel Quick Transendoscopic Enteral Tubing in Mid-Gut: Technique and Training with Video. BMC Gastroenterol. 2018, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Kao, D.; Roach, B.; Silva, M.; Beck, P.; Rioux, K.; Kaplan, G.G.; Chang, H.-J.; Coward, S.; Goodman, K.J.; Xu, H.; et al. Effect of Oral Capsule– vs Colonoscopy-Delivered Fecal Microbiota Transplantation on Recurrent Clostridium Difficile Infection. JAMA 2017, 318, 1985–1993. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European Consensus Conference on Faecal Microbiota Transplantation in Clinical Practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Wang, H.; Fang, H.; Gao, Q.; Zhang, J.; Song, M.; Zhou, Y.; Wang, Y.; Wang, W. Association between IGF2BP2 Polymorphisms and Type 2 Diabetes Mellitus: A Case–Control Study and Meta-Analysis. Int. J. Environ. Res. Public Health 2016, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Azimirad, M.; Yadegar, A.; Gholami, F.; Shahrokh, S.; Asadzadeh Aghdaei, H.; Ianiro, G.; Suzuki, H.; Cammarota, G.; Zali, M.R. Treatment of Recurrent Clostridioides Difficile Infection Using Fecal Microbiota Transplantation in Iranian Patients with Underlying Inflammatory Bowel Disease. J. Inflamm. Res. 2020, 13, 563–570. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Widlak, M.; Bhala, N.; Moore, D.; Price, M.; Sharma, N.; Iqbal, T.H. Systematic Review with Meta-Analysis: The Efficacy of Faecal Microbiota Transplantation for the Treatment of Recurrent and Refractory Clostridium Difficile Infection. Aliment. Pharmacol. Ther. 2017, 46, 479–493. [Google Scholar] [CrossRef]
- Hui, W.; Li, T.; Liu, W.; Zhou, C.; Gao, F. Fecal Microbiota Transplantation for Treatment of Recurrent C. Difficile Infection: An Updated Randomized Controlled Trial Meta-Analysis. PLoS ONE 2019, 14, e0210016. [Google Scholar] [CrossRef]
- Nowak, A.; Hedenstierna, M.; Ursing, J.; Lidman, C.; Nowak, P. Efficacy of Routine Fecal Microbiota Transplantation for Treatment of Recurrent Clostridium Difficile Infection: A Retrospective Cohort Study. Int. J. Microbiol. 2019, 2019, 7395127. [Google Scholar] [CrossRef]
- Ashraf, M.F.; Tageldin, O.; Nassar, Y.; Batool, A. Fecal Microbiota Transplantation in Patients with Recurrent Clostridium Difficile Infection: A Four-Year Single-Center Retrospective Review. Gastroenterol. Res. 2021, 14, 237–243. [Google Scholar] [CrossRef]
- Tian, H.; Ge, X.; Nie, Y.; Yang, L.; Ding, C.; McFarland, L.V.; Zhang, X.; Chen, Q.; Gong, J.; Li, N. Fecal Microbiota Transplantation in Patients with Slow-Transit Constipation: A Randomized, Clinical Trial. PLoS ONE 2017, 12, e0171308. [Google Scholar] [CrossRef] [PubMed]
- Moayyedi, P.; Surette, M.G.; Kim, P.T.; Libertucci, J.; Wolfe, M.; Onischi, C.; Armstrong, D.; Marshall, J.K.; Kassam, Z.; Reinisch, W.; et al. Fecal Microbiota Transplantation Induces Remission in Patients with Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology 2015, 149, 102–109.e6. [Google Scholar] [CrossRef] [PubMed]
- Costello, S.P.; Hughes, P.A.; Waters, O.; Bryant, R.V.; Vincent, A.D.; Blatchford, P.; Katsikeros, R.; Makanyanga, J.; Campaniello, M.A.; Mavrangelos, C.; et al. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients with Ulcerative Colitis: A Randomized Clinical Trial. JAMA 2019, 321, 156–164. [Google Scholar] [CrossRef]
- Borody, T.J.; Clancy, A. Fecal Microbiota Transplantation for Ulcerative Colitis—Where to from Here? Transl. Gastroenterol. Hepatol. 2019, 4, 48. [Google Scholar] [CrossRef]
- Fehily, S.R.; Basnayake, C.; Wright, E.K.; Kamm, M.A. Fecal Microbiota Transplantation Therapy in Crohn’s Disease: Systematic Review. J. Gastroenterol. Hepatol. 2021, 36, 2672–2686. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Huang, Z.; Wei, W.; Li, Z. Fecal Microbiota Transplantation for Crohn’s Disease: A Systematic Review and Meta-Analysis. Tech. Coloproctol. 2021, 25, 495–504. [Google Scholar] [CrossRef]
- Gutin, L.; Piceno, Y.; Fadrosh, D.; Lynch, K.; Zydek, M.; Kassam, Z.; LaMere, B.; Terdiman, J.; Ma, A.; Somsouk, M.; et al. Fecal Microbiota Transplant for Crohn Disease: A Study Evaluating Safety, Efficacy, and Microbiome Profile. United Eur. Gastroenterol. J. 2019, 7, 807–814. [Google Scholar] [CrossRef]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal Microbiota Transplantation to Maintain Remission in Crohn’s Disease: A Pilot Randomized Controlled Study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, P.H.; Hilpüsch, F.; Cavanagh, J.P.; Leikanger, I.S.; Kolstad, C.; Valle, P.C.; Goll, R. Faecal Microbiota Transplantation versus Placebo for Moderate-to-Severe Irritable Bowel Syndrome: A Double-Blind, Randomised, Placebo-Controlled, Parallel-Group, Single-Centre Trial. Lancet Gastroenterol. Hepatol. 2018, 3, 17–24. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of Faecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome in a Randomised, Double-Blind, Placebo-Controlled Study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef]
- Holvoet, T.; Joossens, M.; Vázquez-Castellanos, J.F.; Christiaens, E.; Heyerick, L.; Boelens, J.; Verhasselt, B.; van Vlierberghe, H.; De Vos, M.; Raes, J.; et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients with Irritable Bowel Syndrome with Predominant Abdominal Bloating: Short- and Long-Term Results from a Placebo-Controlled Randomized Trial. Gastroenterology 2021, 160, 145–157.e8. [Google Scholar] [CrossRef]
- El-Salhy, M.; Patcharatrakul, T.; Gonlachanvit, S. Fecal Microbiota Transplantation for Irritable Bowel Syndrome: An Intervention for the 21st Century. World J. Gastroenterol. 2021, 27, 2921–2943. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Bouter, K.E.; van Raalte, D.H.; Groen, A.K.; Nieuwdorp, M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology 2017, 152, 1671–1678. [Google Scholar] [CrossRef]
- Fuhri Snethlage, C.M.; Nieuwdorp, M.; Hanssen, N.M.J. Faecal Microbiota Transplantation in Endocrine Diseases and Obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101483. [Google Scholar] [CrossRef]
- Davis, C.D. The Gut Microbiome and Its Role in Obesity. Nutr. Today 2016, 51, 167–174. [Google Scholar] [CrossRef]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Cantone, E.; Cassarano, S.; Tuccinardi, D.; Barrea, L.; Savastano, S.; Colao, A.; on behalf of the Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group. Gut Microbiota: A New Path to Treat Obesity. Int. J. Obes. Suppl. 2019, 9, 10–19. [Google Scholar] [CrossRef]
- Marotz, C.A.; Zarrinpar, A. Treating Obesity and Metabolic Syndrome with Fecal Microbiota Transplantation. Yale J. Biol. Med. 2016, 89, 383–388. [Google Scholar]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Liu, B.-N.; Liu, X.-T.; Liang, Z.-H.; Wang, J.-H. Gut Microbiota in Obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Xu, X.; Ming, J.; Wang, Z.; Gao, B.; Xing, Y.; Zhou, J.; Fu, J.; Liu, T.; et al. The Fecal Microbiota Is Already Altered in Normoglycemic Individuals Who Go on to Have Type 2 Diabetes. Front. Cell. Infect. Microbiol. 2021, 11, 598672. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Matute, P.; Íñiguez, M.; de Toro, M.; Recio-Fernández, E.; Oteo, J.A. Autologous Fecal Transplantation from a Lean State Potentiates Caloric Restriction Effects on Body Weight and Adiposity in Obese Mice. Sci. Rep. 2020, 10, 9388. [Google Scholar] [CrossRef] [PubMed]
- Guirro, M.; Costa, A.; Gual-Grau, A.; Herrero, P.; Torrell, H.; Canela, N.; Arola, L. Effects from Diet-Induced Gut Microbiota Dysbiosis and Obesity Can Be Ameliorated by Fecal Microbiota Transplantation: A Multiomics Approach. PLoS ONE 2019, 14, e0218143. [Google Scholar] [CrossRef]
- Yu, E.W.; Gao, L.; Stastka, P.; Cheney, M.C.; Mahabamunuge, J.; Torres Soto, M.; Ford, C.B.; Bryant, J.A.; Henn, M.R.; Hohmann, E.L. Fecal Microbiota Transplantation for the Improvement of Metabolism in Obesity: The FMT-TRIM Double-Blind Placebo-Controlled Pilot Trial. PLoS Med. 2020, 17, e1003051. [Google Scholar] [CrossRef]
- Allegretti, J.R.; Kassam, Z.; Mullish, B.H.; Chiang, A.; Carrellas, M.; Hurtado, J.; Marchesi, J.R.; McDonald, J.A.K.; Pechlivanis, A.; Barker, G.F.; et al. Effects of Fecal Microbiota Transplantation with Oral Capsules in Obese Patients. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 855–863.e2. [Google Scholar] [CrossRef]
- Mocanu, V.; Zhang, Z.; Deehan, E.C.; Kao, D.H.; Hotte, N.; Karmali, S.; Birch, D.W.; Samarasinghe, K.K.; Walter, J.; Madsen, K.L. Fecal Microbial Transplantation and Fiber Supplementation in Patients with Severe Obesity and Metabolic Syndrome: A Randomized Double-Blind, Placebo-Controlled Phase 2 Trial. Nat. Med. 2021, 27, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, P.; Juuti, A.; Luostarinen, M.; Niskanen, L.; Liukkonen, T.; Tillonen, J.; Kössi, J.; Ilvesmäki, V.; Viljakka, M.; Satokari, R.; et al. Effectiveness of Fecal Microbiota Transplantation for Weight Loss in Patients with Obesity Undergoing Bariatric Surgery: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2247226. [Google Scholar] [CrossRef]
- Leong, K.S.W.; Jayasinghe, T.N.; Wilson, B.C.; Derraik, J.G.B.; Albert, B.B.; Chiavaroli, V.; Svirskis, D.M.; Beck, K.L.; Conlon, C.A.; Jiang, Y.; et al. Effects of Fecal Microbiome Transfer in Adolescents with Obesity: The Gut Bugs Randomized Controlled Trial. JAMA Netw. Open 2020, 3, e2030415. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, M.; Yang, J.; Xu, Q.; Liang, C.; Chen, B.; Zhang, J.; Yang, Y.; Wang, H.; Shang, Y.; et al. Response of Gut Microbiota in Type 2 Diabetes to Hypoglycemic Agents. Endocrine 2019, 66, 485–493. [Google Scholar] [CrossRef]
- Proença, I.M.; Allegretti, J.R.; Bernardo, W.M.; de Moura, D.T.H.; Ponte Neto, A.M.; Matsubayashi, C.O.; Flor, M.M.; Kotinda, A.P.S.T.; de Moura, E.G.H. Fecal Microbiota Transplantation Improves Metabolic Syndrome Parameters: Systematic Review with Meta-Analysis Based on Randomized Clinical Trials. Nutr. Res. 2020, 83, 1–14. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Clément, K.; Nieuwdorp, M. Fecal Microbiota Transplantation: A Future Therapeutic Option for Obesity/Diabetes? Curr. Diab. Rep. 2019, 19, 51. [Google Scholar] [CrossRef]
- Rinott, E.; Youngster, I.; Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain. Gastroenterology 2021, 160, 158–173.e10. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wei, J.; Liu, P.; Zhang, Q.; Tian, Y.; Hou, G.; Meng, L.; Xin, Y.; Jiang, X. Role of the Gut Microbiota in Type 2 Diabetes and Related Diseases. Metabolism 2021, 117, 154712. [Google Scholar] [CrossRef]
- Zhang, P.; Li, L.; Han, X.; Li, Q.; Zhang, X.; Liu, J.J.; Wang, Y. Fecal Microbiota Transplantation Improves Metabolism and Gut Microbiome Composition in Db/Db Mice. Acta Pharmacol. Sin. 2020, 41, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Y.; Yan, Y.; Tian, S.; Zheng, D.; Leng, D.; Wang, C.; Jiao, J.; Wang, Z.; Bai, Y. Promising Treatment for Type 2 Diabetes: Fecal Microbiota Transplantation Reverses Insulin Resistance and Impaired Islets. Front. Cell. Infect. Microbiol. 2020, 9, 455. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, B.; Chen, F.; Xia, R.; Zhu, D.; Chen, B.; Lin, A.; Zheng, C.; Hou, D.; Li, X.; et al. Fecal Microbiota Transplantation Reverses Insulin Resistance in Type 2 Diabetes: A Randomized, Controlled, Prospective Study. Front. Cell. Infect. Microbiol. 2022, 12, 1089991. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Xu, Z.; Mak, J.W.Y.; Yang, K.; Liu, Q.; Zuo, T.; Tang, W.; Lau, L.; Lui, R.N.; Wong, S.H.; et al. Microbiota Engraftment after Faecal Microbiota Transplantation in Obese Subjects with Type 2 Diabetes: A 24-Week, Double-Blind, Randomised Controlled Trial. Gut 2022, 71, 716–723. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bhardwaj, P.; Singh, R. Administration of Lactobacillus Casei and Bifidobacterium Bifidum Ameliorated Hyperglycemia, Dyslipidemia, and Oxidative Stress in Diabetic Rats. Int. J. Prev. Med. 2016, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Yong, H.; You, N.; Lu, W.; Yang, X.; Ye, X.; Wang, Y.; Cai, T.; Zheng, X.; Chen, H.; et al. Prospective Study Reveals Host Microbial Determinants of Clinical Response to Fecal Microbiota Transplant Therapy in Type 2 Diabetes Patients. Front. Cell. Infect. Microbiol. 2022, 12, 820367. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M.; SPRING Trial Group. Connections between the Gut Microbiome and Metabolic Hormones in Early Pregnancy in Overweight and Obese Women. Diabetes 2016, 65, 2214–2223. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Su, L.; Hong, Z.; Zhou, T.; Jian, Y.; Xu, M.; Zhang, X.; Zhu, X.; Wang, J. Health Improvements of Type 2 Diabetic Patients through Diet and Diet plus Fecal Microbiota Transplantation. Sci. Rep. 2022, 12, 1152. [Google Scholar] [CrossRef]
- Verbrugghe, P.; Brynjólfsson, J.; Jing, X.; Björck, I.; Hållenius, F.; Nilsson, A. Evaluation of Hypoglycemic Effect, Safety and Immunomodulation of Prevotella Copri in Mice. Sci. Rep. 2021, 11, 21279. [Google Scholar] [CrossRef]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of Insulin Sensitivity after Lean Donor Feces in Metabolic Syndrome Is Driven by Baseline Intestinal Microbiota Composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota from Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Stratigou, T.; Tsagarakis, S. Microbiome and Diabetes: Where Are We Now? Diabetes Res. Clin. Pract. 2018, 146, 111–118. [Google Scholar] [CrossRef]
- Witjes, J.J.; Smits, L.P.; Pekmez, C.T.; Prodan, A.; Meijnikman, A.S.; Troelstra, M.A.; Bouter, K.E.C.; Herrema, H.; Levin, E.; Holleboom, A.G.; et al. Donor Fecal Microbiota Transplantation Alters Gut Microbiota and Metabolites in Obese Individuals with Steatohepatitis. Hepatol. Commun. 2020, 4, 1578–1590. [Google Scholar] [CrossRef]
- Craven, L.; Rahman, A.; Nair Parvathy, S.; Beaton, M.; Silverman, J.; Qumosani, K.; Hramiak, I.; Hegele, R.; Joy, T.; Meddings, J.; et al. Allogenic Fecal Microbiota Transplantation in Patients with Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am. J. Gastroenterol. 2020, 115, 1055–1065. [Google Scholar] [CrossRef]
- Rapoport, E.A.; Baig, M.; Puli, S.R. Adverse Events in Fecal Microbiota Transplantation: A Systematic Review and Meta-Analysis. Ann. Gastroenterol. 2022, 35, 150–163. [Google Scholar] [CrossRef]
- Wang, S.; Xu, M.; Wang, W.; Cao, X.; Piao, M.; Khan, S.; Yan, F.; Cao, H.; Wang, B. Systematic Review: Adverse Events of Fecal Microbiota Transplantation. PLoS ONE 2016, 11, e0161174. [Google Scholar] [CrossRef] [PubMed]
- Kim, S. The Safety and Effectiveness of Fecal Microbiota Transplantation: Systematic Review and Meta-Anylysis. Value Health 2018, 21, S41–S42. [Google Scholar] [CrossRef]
- Michailidis, L.; Currier, A.C.; Le, M.; Flomenhoft, D.R. Adverse Events of Fecal Microbiota Transplantation: A Meta-Analysis of High-Quality Studies. Ann. Gastroenterol. 2021, 34, 802–814. [Google Scholar] [CrossRef]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.-B.; Hohmann, E.L. Drug-Resistant E. Coli Bacteremia Transmitted by Fecal Microbiota Transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Brandt, L.J.; Aroniadis, O.C.; Mellow, M.; Kanatzar, A.; Kelly, C.; Park, T.; Stollman, N.; Rohlke, F.; Surawicz, C. Long-Term Follow-up of Colonoscopic Fecal Microbiota Transplant for Recurrent Clostridium Difficile Infection. Am. J. Gastroenterol. 2012, 107, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.; Amaratunga, T.; Barnes, E.L.; Fischer, M.; Kassam, Z.; Allegretti, J.R. The Risk of Inflammatory Bowel Disease Flares after Fecal Microbiota Transplantation: Systematic Review and Meta-Analysis. Gut Microbes 2017, 8, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Perler, B.K.; Chen, B.; Phelps, E.; Allegretti, J.R.; Fischer, M.; Ganapini, V.; Krajiceck, E.; Kumar, V.; Marcus, J.; Nativ, L.; et al. Long-Term Efficacy and Safety of Fecal Microbiota Transplantation for Treatment of Recurrent Clostridioides Difficile Infection. J. Clin. Gastroenterol. 2020, 54, 701–706. [Google Scholar] [CrossRef]
- Keller, J.J.; Ooijevaar, R.E.; Hvas, C.L.; Terveer, E.M.; Lieberknecht, S.C.; Högenauer, C.; Arkkila, P.; Sokol, H.; Gridnyev, O.; Mégraud, F.; et al. A Standardised Model for Stool Banking for Faecal Microbiota Transplantation: A Consensus Report from a Multidisciplinary UEG Working Group. United Eur. Gastroenterol. J. 2021, 9, 229–247. [Google Scholar] [CrossRef] [PubMed]
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Study Design | Study Population | FMT Intervention and Duration | Major Findings | Reference |
|---|---|---|---|---|
| Randomized, double-blinded, placebo-controlled, pilot study | n = 22 metabolically healthy obese patients | Oral capsules derived from a single lean donor (induction dose of 30 capsules at week 4 and maintenance dose of 12 capsules at week 8), follow-up of 26 weeks | No effects on BMI and GLP1 postprandial response, sustained changes in gut microbiota and bile acid profiles resembling the lean donor profile, safe procedure, well-tolerated, no adverse events | [113] |
| Randomized, double-blinded, placebo-controlled trial (Gut Bugs Trial) | n = 87 obese adolescents aged 14–18 years old, New Zealand | Single course of oral FMT capsules derived from 4 healthy lean donors, follow-up of 26 weeks | No effects on weight loss, insulin sensitivity, liver function, lipid profile, inflammatory markers, blood pressure, total body fat %, gut health and health-related quality of life, reduction of abdominal adiposity, resolution of metabolic syndrome in post hoc analyses, maintained shift in gut microbiota profiling for up to 12 weeks, no serious adverse events | [116] |
| Randomized, double-blinded, placebo-controlled pilot trial | n = 24 obese patients with mild to moderate insulin resistance, treated at a single academic medical center of US | Weekly oral FMT capsules derived from healthy lean donors Treatment for 6 weeks Follow-up of 12 weeks | No effects on insulin sensitivity, body composition and other secondary metabolic outcomes, variable engraftment of donor gut microbiota maintained for at least 12 weeks, no serious adverse events | [112] |
| Randomized, double-blinded, placebo-controlled, parallel four-arm, phase 2 clinical trial | n = 70 patients with severe obesity (BMI > 40) and metabolic syndrome 4 groups compared: FMT + HF (n = 17) FMT + LF (n = 17) HF (n = 17) LF (n = 19) | Single-dose oral FMT capsules derived from healthy lean donors combined with daily fiber supplementation of high or low fermentability Treatment for 6 weeks | Improved insulin sensitivity only in FMT + LF group, effects independent of diet and medications, but associated with altered microbial ecology, improved enteroendocrine responses after OGTT and increased engraftment of donor microbes, safe and well-tolerated intervention | [114] |
| Randomized, placebo-controlled clinical trial DIRECT PLUS weight loss trial (Dietary Intervention Randomized Controlled Trial Polyphenols-Unprocessed) | n = 90 abdominally obese or dyslipidemic Israel patients undergoing weight loss with a 6-month Mediterranean diet, follow-up over the weight regain phase (6–14 months) | Diet-modulated Auto-FMT in the form of 100 frozen capsules performed over the weight regain phase, after the 6-month weight loss phase | Attenuated weight regain, waist circumference gain, and insulin rebound only in the group having lost weight with a polyphenol-enriched, green plant-based version of Mediterranean diet No adverse events reported | [120] |
| Randomized, double-blinded, placebo-controlled, multi-center trial | n = 41 severely obese adults undergoing bariatric surgery at two centers in Finland | Allo-FMT derived from a lean donor vs. Auto-placebo performed by gastroscopy into duodenum, bariatric surgery (LRYGB or LSG) performed 6 months after baseline FMT intervention, follow-up of 18 months | No effects on total weight loss % at 6 months (prior to surgery) or at 18 months (post bariatric surgery) No effect of FMT in potentiating the bariatric surgery weight loss effect | [115] |
| Non-blinded, single-arm, FMT intervention trial (prospective cohort study) | n = 17 patients with T2DM | FMT derived from healthy lean donors, follow-up of 12 weeks | Reduced HbA1c, fasting plasma glucose and uric acid levels after FMT, increased postprandial c-peptide levels, pretreated fecal abundance of Rikenellaceae and Anaerotruncus genera may predict an enhanced metabolic response to FMT | [128] |
| Randomized, double-blinded, parallel 3-arm, placebo-controlled trial | n = 61 obese patients with T2DM 3 groups: FMT + lifestyle intervention FMT alone Sham transplantation | FMT derived from healthy lean donors ± lifestyle intervention Repeated sessions of FMT Follow-up of 24 weeks | Sustained engraftment of lean donor microbiota after repeated FMTs, increased fecal abundance of Lactobacillus and Bifidobacterium species, improved lipid profile and reduced liver stiffness 24 weeks after combined FMT and lifestyle intervention | [125] |
| Randomized, open-label, controlled clinical trial | n = 16 patients with T2DM (n = 13 completed the study) | Oral FMT (capsules) derived from young healthy donors administered on a weekly basis for 3 weeks, concomitant dietary intervention with dietary formulations rich in prebiotics, probiotics, and whole-grain products, follow-up of 3 months | Accelerated changes in gut microbiota with diet + FMT vs. diet alone, improved blood glucose and blood pressure control, increased fecal abundance of Bifidobacterium and decreased abundance of sulfate-reducing bacteria | [131] |
| Randomized, controlled, prospective study | n = 31 Chinese patients with newly diagnosed T2DM | FMT derived from healthy donors delivered via nasojejunal feeding tubes ± metformin, follow-up of 4 weeks | Improved insulin resistance, BMI, fasting, and postprandial glucose levels and HbA1c, enhanced engraftment of donor-associated microbiota | [124] |
| Randomized, double-blinded, placebo-controlled trial | n = 18 male obese Caucasian subjects with metabolic syndrome | Allo-FMT derived from healthy lean, age-matched, male donors (small intestinal infusion via gastroduodenoscopy) vs. Auto-FMT (serving as placebo), follow-up of 6 weeks | Increased peripheral insulin sensitivity assessed by clamps and elevated levels of butyrate-producing fecal bacteria at 6 weeks | [134] |
| Randomized, double-blinded, controlled trial | n = 38 male obese Caucasian subjects with metabolic syndrome | Allo-FMT derived from healthy lean donors (infusion via nasoduodenal tube) vs. Auto-FMT (serving as placebo), Allo-FMT repeated at 6 weeks, follow-up of 18 weeks | Transient short-term beneficial effect of Allo-FMT on peripheral insulin sensitivity assessed by clamps at 6 weeks, metabolic response associated with plasma metabolite and fecal microbial changes and predicted based on baseline gut microbiota diversity and composition | [133] |
| Randomized, double-blinded, controlled trial | n = 21 patients with NAFLD | Allo-FMT derived from a healthy lean donor vs. Auto-FMT delivered endoscopically into distal duodenum, follow-up of up to 6 months post-FMT | No effects on insulin resistance and MRI-assessed hepatic fat content, reduced small intestinal permeability in those with elevated gut permeability at baseline at 6 weeks after Allo-FMT | [137] |
| Randomized, double-blinded, controlled, proof-of-concept trial | n = 21 obese subjects with ultrasound-confirmed hepatic steatosis | Allo-FMT derived from lean vegan donor vs. Auto-FMT (serving as placebo) performed 3 times at 8-week intervals, liver biopsy performed at baseline and at 24 weeks | Trend towards reduced necro-inflammatory burden in liver histology, reduced hepatic gene expression involved in inflammation and lipid metabolism, beneficial changes in gut microbiota composition and plasma metabolites | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zikou, E.; Koliaki, C.; Makrilakis, K. The Role of Fecal Microbiota Transplantation (FMT) in the Management of Metabolic Diseases in Humans: A Narrative Review. Biomedicines 2024, 12, 1871. https://doi.org/10.3390/biomedicines12081871
Zikou E, Koliaki C, Makrilakis K. The Role of Fecal Microbiota Transplantation (FMT) in the Management of Metabolic Diseases in Humans: A Narrative Review. Biomedicines. 2024; 12(8):1871. https://doi.org/10.3390/biomedicines12081871
Chicago/Turabian StyleZikou, Eva, Chrysi Koliaki, and Konstantinos Makrilakis. 2024. "The Role of Fecal Microbiota Transplantation (FMT) in the Management of Metabolic Diseases in Humans: A Narrative Review" Biomedicines 12, no. 8: 1871. https://doi.org/10.3390/biomedicines12081871





