The Interplay between Mitochondrial Metabolism and Nasal Mucociliary Function as a Surrogate Method to Diagnose Thyroid Dysfunction: Insights from a Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Participants, and Endpoints
2.2. Sample Size Calculation
2.3. Assessments
2.4. Ethical Issues
2.5. Statistical Analysis
3. Results
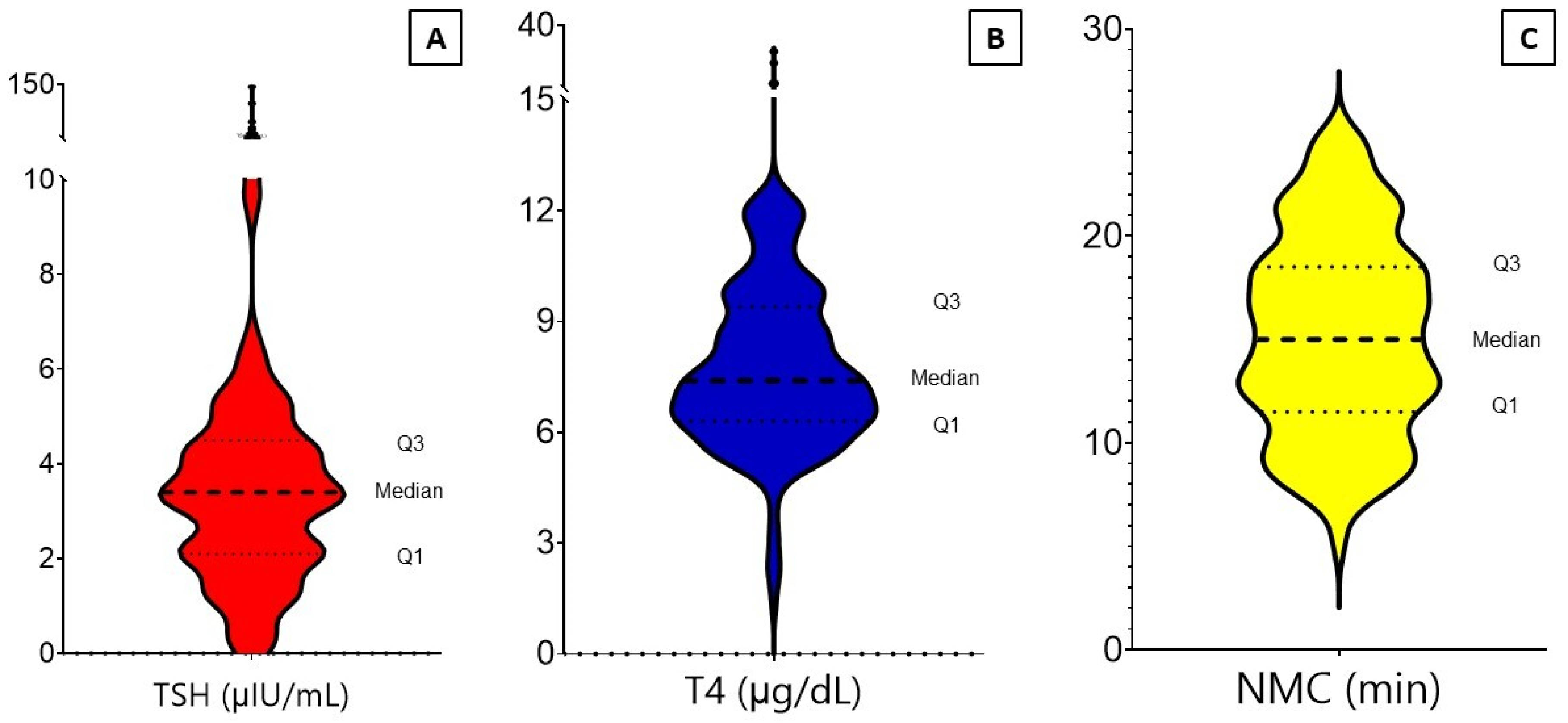
3.1. Descriptive Analysis
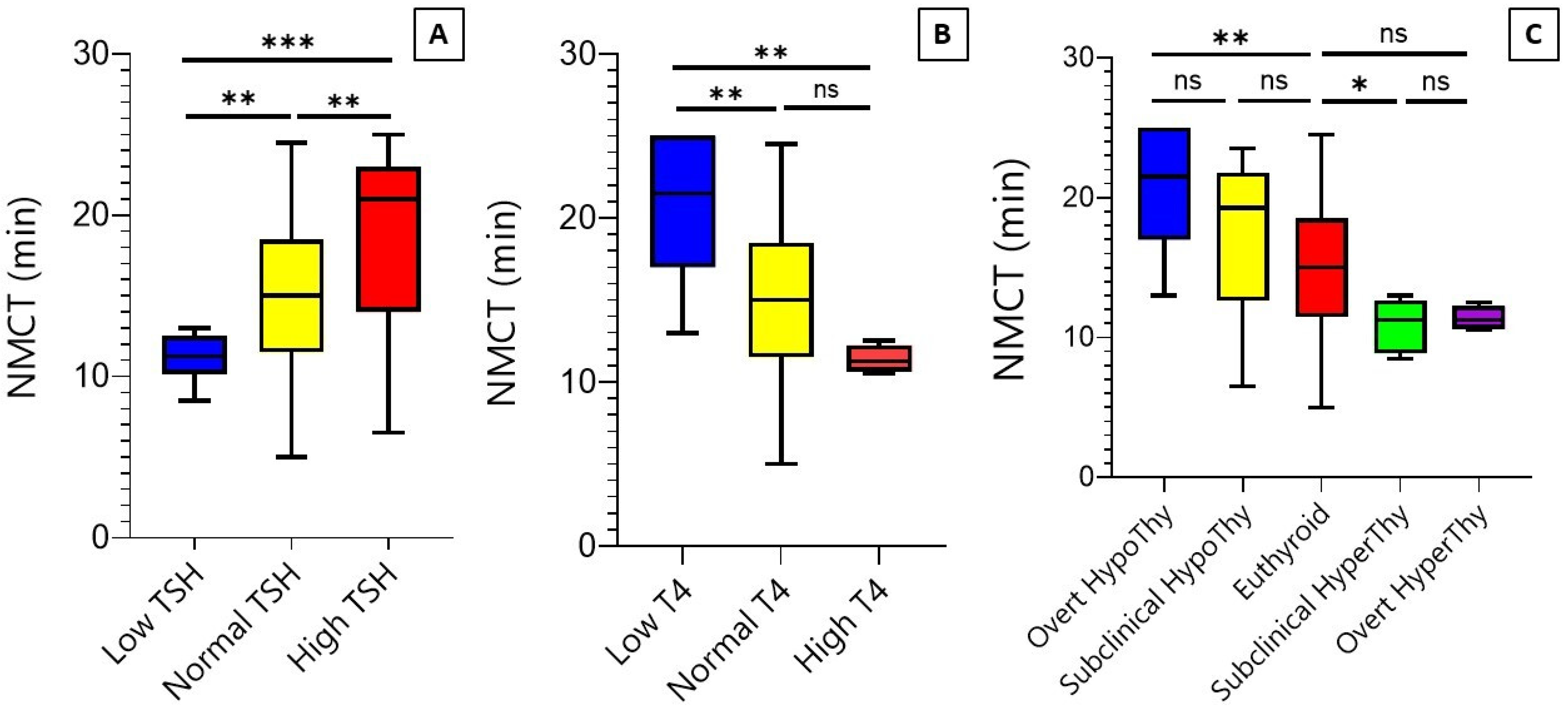
3.2. Correlation between Nasal Mucociliary Clearance Time and Thyroid Function Tests
3.3. Mucociliary Clearance over the TSH, T4, and Thyroid Function Groups
3.4. Diagnostic Value of Mucociliary Clearance Test in Differentiating Thyroid Function Disorders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Thyroid Association (ATA). Prevalence and Impact of Thyroid Disease 2023. Available online: https://www.thyroid.org/media-main/press-room/ (accessed on 5 July 2023).
- Humphreys, J. Considerations in evaluating the thyroid gland in a primary care setting. J. Fam. Med. Prim. Care 2020, 9, 5833–5836. [Google Scholar] [CrossRef] [PubMed]
- Razvi, S.; Bhana, S.; Mrabeti, S. Challenges in Interpreting Thyroid Stimulating Hormone Results in the Diagnosis of Thyroid Dysfunction. J. Thyroid. Res. 2019, 2019, 4106816. [Google Scholar] [CrossRef] [PubMed]
- Noli, L.; Khorsandi, S.E.; Pyle, A.; Giritharan, G.; Fogarty, N.; Capalbo, A.; Devito, L.; Jovanovic, V.M.; Khurana, P.; Rosa, H.; et al. Effects of thyroid hormone on mitochondria and metabolism of human preimplantation embryos. Stem Cells 2020, 38, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh-Hesary, F.; Houshyari, M.; Farhadi, M. Mitochondrial metabolism: A predictive biomarker of radiotherapy efficacy and toxicity. J. Cancer Res. Clin. Oncol. 2023, 149, 6719–6741. [Google Scholar] [CrossRef] [PubMed]
- Houshyari, M.; Taghizadeh-Hesary, F. Is Mitochondrial Metabolism a New Predictive Biomarker for Antiprogrammed Cell Death Protein-1 Immunotherapy? JCO Oncol. Pract. 2023, 19, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tian, P.; Zhong, H. WDPCP Modulates Cilia Beating Through the MAPK/ERK Pathway in Chronic Rhinosinusitis with Nasal Polyps. Front. Cell Dev. Biol. 2021, 8, 630340. [Google Scholar] [CrossRef]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef]
- Yücel, H.; Eşmen, S.E. Evaluation of nasal mucociliary clearance by saccharine test in rheumatoid arthritis. Braz. J. Otorhinolaryngol. 2022, 88 (Suppl. S5), S42–S46. [Google Scholar] [CrossRef]
- Priscilla, J.; Padmavathi, R.; Ghosh, S.; Paul, P.; Ramadoss, S.; Balakrishnan, K.; Thanasekaraan, V.; Subhashini, A. Evaluation of mucociliary clearance among women using biomass and clean fuel in a periurban area of Chennai: A preliminary study. Lung India 2011, 28, 30–33. [Google Scholar] [CrossRef]
- Pandya, V.; Tiwari, R. Nasal mucociliary clearance in health and disease. Indian J. Otolaryngol. Head Neck Surg. 2006, 58, 332–334. [Google Scholar] [CrossRef]
- Uysal, I.; Gökakın, A.K.; Karakuş, C.F.; Deveci, K.; Hasbek, Z.; Sancakdar, E. Evaluation of nasal mucociliary activity in iatrogenic hypothyroidism. Eur. Arch. Oto-Rhino-Laryngol. 2013, 270, 3075–3078. [Google Scholar] [CrossRef]
- Feeney, E.L.; Hayes, J.E. Regional differences in suprathreshold intensity for bitter and umami stimuli. Chemosens. Percept. 2014, 7, 147–157. [Google Scholar] [CrossRef]
- Spence, C. The tongue map and the spatial modulation of taste perception. Curr. Res. Food Sci. 2022, 5, 598–610. [Google Scholar] [CrossRef]
- Sample Size Calculator. 18 June 2023. Available online: https://www.calculator.net/sample-size-calculator.html?type=1&cl=95&ci=5&pp=15&ps=&x=121&y=9 (accessed on 18 June 2023).
- Aminorroaya, A.; Meamar, R.; Amini, M.; Feizi, A.; Tabatabae, A.; Imani, E.F. Incidence of thyroid dysfunction in an Iranian adult population: The predictor role of thyroid autoantibodies: Results from a prospective population-based cohort study. Eur. J. Med. Res. 2017, 22, 21. [Google Scholar] [CrossRef]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singap. Med. J. 2003, 44, 614–619. [Google Scholar]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Choi, H.G.; Kim, T.J.; Hong, S.K.; Min, C.; Yoo, D.M.; Kim, H.; Lee, J.S. Thyroid Diseases and Chronic Rhinosinusitis: A Nested Case–Control Study Using a National Health Screening Cohort. Int. J. Environ. Res. Public Health 2022, 19, 8372. [Google Scholar] [CrossRef]
- Niwa, S.; Nakajima, K.; Miki, H.; Minato, Y.; Wang, D.; Hirokawa, N. KIF19A Is a Microtubule-Depolymerizing Kinesin for Ciliary Length Control. Dev. Cell 2012, 23, 1167–1175. [Google Scholar] [CrossRef]
- Jonckheere, A.I.; Smeitink, J.A.M.; Rodenburg, R.J.T. Mitochondrial ATP synthase: Architecture, function and pathology. J. Inherit. Metab. Dis. 2012, 35, 211–225. [Google Scholar] [CrossRef]
- Droguett, K.; Rios, M.; Carreño, D.V.; Navarrete, C.; Fuentes, C.; Villalón, M.; Barrera, N.P. An autocrine ATP release mechanism regulates basal ciliary activity in airway epithelium. J. Physiol. 2017, 595, 4755–4767. [Google Scholar] [CrossRef]
- Tarasiuk, A.; Bar-Shimon, M.; Gheber, L.; Korngreen, A.; Grossman, Y.; Priel, Z. Extracellular ATP induces hyperpolarization and motility stimulation of ciliary cells. Biophys. J. 1995, 68, 1163–1169. [Google Scholar] [CrossRef]
- Schwiebert, E.M.; Zsembery, A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim. Biophys. Acta Biomembr. 2003, 1615, 7–32. [Google Scholar] [CrossRef]
- Behnam, B.; Taghizadeh-Hesary, F. Mitochondrial Metabolism: A New Dimension of Personalized Oncology. Cancers 2023, 15, 4058. [Google Scholar] [CrossRef]
- Moruzzi, N.; Valladolid-Acebes, I.; A Kannabiran, S.; Bulgaro, S.; Burtscher, I.; Leibiger, B.; Leibiger, I.B.; Berggren, P.-O.; Brismar, K. Mitochondrial impairment and intracellular reactive oxygen species alter primary cilia morphology. Life Sci. Alliance 2022, 5, e202201505. [Google Scholar] [CrossRef]
- Wang, X.; Mao, J.; Zhou, X.; Li, Q.; Gao, L.; Zhao, J. Thyroid Stimulating Hormone Triggers Hepatic Mitochondrial Stress through Cyclophilin D Acetylation. Oxidative Med. Cell. Longev. 2020, 2020, 1249630. [Google Scholar] [CrossRef]
- Ma, X.; Wang, F.; Zhen, X.; Zhao, L.; Fang, L.; Dong, Z.; Chen, W.; Zhou, X. gp91phox, a Novel Biomarker Evaluating Oxidative Stress, Is Elevated in Subclinical Hypothyroidism. Int. J. Endocrinol. 2020, 2020, 3161730. [Google Scholar] [CrossRef]
- Hu, H.; Liang, L.; Zheng, X. Fibulin-1: A novel biomarker for predicting disease activity of the thyroid-associated oph-thalmopathy. Eye 2022, 37, 2216–2219. [Google Scholar] [CrossRef]
- Kadkhodazadeh, H.; Amouzegar, A.; Mehran, L.; Gharibzadeh, S.; Azizi, F.; Tohidi, M. Smoking status and changes in thyroid-stimulating hormone and free thyroxine levels during a decade of follow-up: The Tehran thyroid study. Casp. J. Intern. Med. 2020, 11, 47–52. [Google Scholar]


| Characteristics | Patients (Total = 232) |
|---|---|
| Gender | |
| Female, n (%) | 165 (71.1) |
| Male, n (%) | 67 (28.9) |
| Patient age at diagnosis (years) | |
| 10–30, n (%) | 59 (25.4) |
| 31–50, n (%) | 91 (39.2) |
| 51–70, n (%) | 66 (28.4) |
| >70, n (%) | 16 (6.8) |
| TSH (μIU/mL) | |
| Mean/range/SD | 5.3/0.01–144.3/3.7 |
| Low (<0.4) | 10 (4.3) |
| Normal (0.4–6.1 or up to 10 in >50 years) | 201 (86.6) |
| High (>6.1 or 10 in >50 years) | 21 (9.1) |
| T4 (μg/dL) | |
| Mean/range/SD | 7.9/1.4–29.5/2.8 |
| Low (<4.5) | 7 (3.0) |
| Normal (4.5–12.5) | 221 (95.3) |
| High (>12.5) | 4 (1.7) |
| Condition | |
| Overt hypothyroidism | 7 (3.0) |
| Subclinical hypothyroidism | 14 (6.0) |
| Euthyroid | 201 (86.6) |
| Subclinical hyperthyroidism | 6 (2.6) |
| Overt hyperthyroidism | 4 (1.7) |
| NMCT (min) | |
| Right side | |
| Mean | 15.3 |
| Range/IQR | 4–28/11–19 |
| SD | 5.10 |
| Left side | |
| Mean | 15.1 |
| Range/IQR | 4–31/11–19 |
| SD | 4.96 |
| Average | |
| Mean | 15.2 |
| Range/IQR | 5–25/11.5–18.5 |
| SD | 4.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhadi, M.; Ghanbari, H.; Salehi, A.; Ashique, S.; Taghizadeh-Hesary, F. The Interplay between Mitochondrial Metabolism and Nasal Mucociliary Function as a Surrogate Method to Diagnose Thyroid Dysfunction: Insights from a Population-Based Study. Biomedicines 2024, 12, 1897. https://doi.org/10.3390/biomedicines12081897
Farhadi M, Ghanbari H, Salehi A, Ashique S, Taghizadeh-Hesary F. The Interplay between Mitochondrial Metabolism and Nasal Mucociliary Function as a Surrogate Method to Diagnose Thyroid Dysfunction: Insights from a Population-Based Study. Biomedicines. 2024; 12(8):1897. https://doi.org/10.3390/biomedicines12081897
Chicago/Turabian StyleFarhadi, Mohammad, Hadi Ghanbari, Ali Salehi, Sumel Ashique, and Farzad Taghizadeh-Hesary. 2024. "The Interplay between Mitochondrial Metabolism and Nasal Mucociliary Function as a Surrogate Method to Diagnose Thyroid Dysfunction: Insights from a Population-Based Study" Biomedicines 12, no. 8: 1897. https://doi.org/10.3390/biomedicines12081897
APA StyleFarhadi, M., Ghanbari, H., Salehi, A., Ashique, S., & Taghizadeh-Hesary, F. (2024). The Interplay between Mitochondrial Metabolism and Nasal Mucociliary Function as a Surrogate Method to Diagnose Thyroid Dysfunction: Insights from a Population-Based Study. Biomedicines, 12(8), 1897. https://doi.org/10.3390/biomedicines12081897






