The Relationship between Circulating Kidney Injury Molecule-1 and Cardiovascular Morbidity and Mortality in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Clinical and Biochemical Evaluation
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Plasma KIM-1
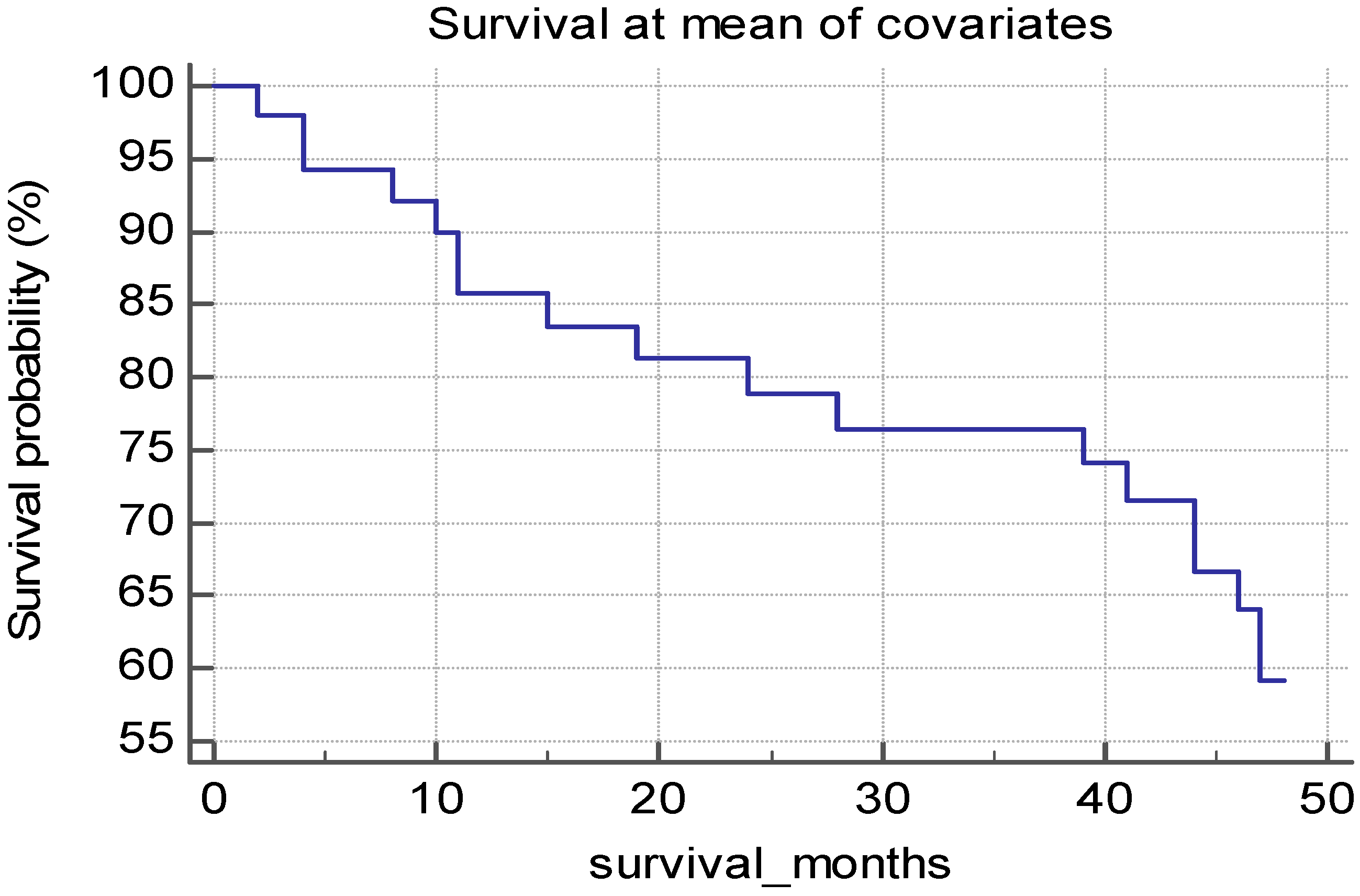
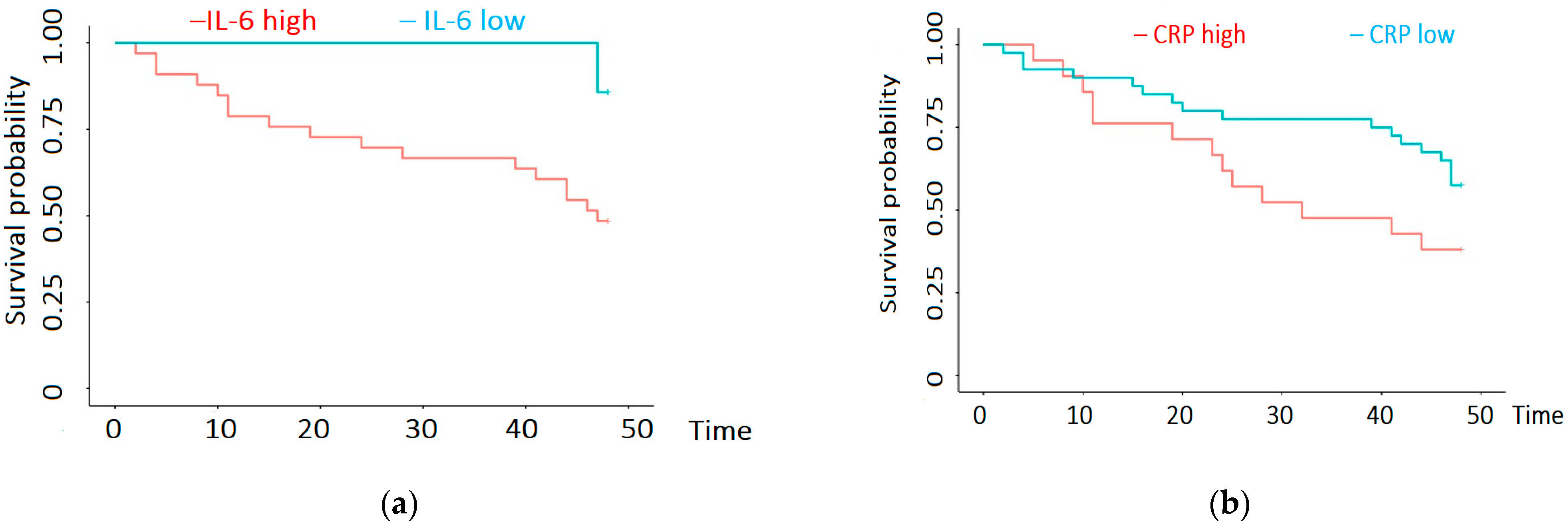
3.2. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication—Worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-Cause and cause-Specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Pecoits-Filho, R.; Okpechi, I.G.; Donner, J.-A.; Harris, D.C.; Aljubori, H.M.; Bello, A.K.; Bellorin-Font, E.; Caskey, F.J.; Collins, A.; Cueto-Manzano, A.M.; et al. Capturing and monitoring global differences in untreated and treated end-stage kidney disease, kidney replacement therapy modality, and outcomes. Kidney Int. Suppl. 2020, 10, e3–e9. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.; Albertus, P.; Ayanian, J.; Balkrishnan, R.; Bragg-Gresham, J.; Cao, J.; Chen, J.L.T.; et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2017, 69, A7–A8, Erratum in Am. J. Kidney Dis. 2017, 69, 712. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M. Cause of Death in Patients with Reduced Kidney Function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G.; Nadkarni, G.N.; Huang, Y.; Moledina, D.G.; Rao, V.; Zhang, J.; Ferket, B.; Crowley, S.T.; Fried, L.F.; Parikh, C.R. Plasma Biomarkers and Kidney Function Decline in Early and Established Diabetic Kidney Disease. J. Am. Soc. Nephrol. 2017, 28, 2786–2793. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.-A.; Engström, G.; Nilsson, J.; Almgren, P.; Petkovic, M.; Christensson, A.; Nilsson, P.M.; Melander, O.; Orho-Melander, M. Plasma kidney injury molecule-1 (p-KIM-1) levels and deterioration of kidney function over 16 years. Nephrol. Dial. Transplant. 2019, 35, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Braden, G.L.; Landry, D.L. The Next Frontier: Biomarkers and Artificial Intelligence Predicting Cardiorenal Outcomes in Diabetic Kidney Disease. Kidney360 2022, 3, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Karmakova, T.; Sergeeva, N.; Kanukoev, K.; Alekseev, B.; Kaprin, A. Kidney Injury Molecule 1 (KIM-1): A Multifunctional Glycoprotein and Biological Marker (Review). Sovrem. Teh. Med. 2021, 13, 64–78. [Google Scholar] [CrossRef]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood Kidney Injury Molecule-1 Is a Biomarker of Acute and Chronic Kidney Injury and Predicts Progression to ESRD in Type I Diabetes. J. Am. Soc. Nephrol. 2014, 25, 2177–2186. [Google Scholar] [CrossRef]
- Grodin, J.L.; Perez, A.L.; Wu, Y.; Hernandez, A.F.; Butler, J.; Metra, M.; Felker, G.M.; Voors, A.A.; McMurray, J.J.; Armstrong, P.W.; et al. Circulating Kidney Injury Molecule-1 Levels in Acute Heart Failure. JACC Heart Fail. 2015, 3, 777–785. [Google Scholar] [CrossRef]
- Nowak, N.; Skupien, J.; Niewczas, M.A.; Yamanouchi, M.; Major, M.; Croall, S.; Smiles, A.; Warram, J.H.; Bonventre, J.V.; Krolewski, A.S. Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int. 2016, 89, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, A.; Wen, J.; Zhen, J.; Hao, Q.; Zhang, Y.; Hu, Z.; Xiao, X. Kidney Injury Molecule-1 Level is Associated with the Severity of Renal Interstitial Injury and Prognosis in Adult Henoch–Schönlein Purpura Nephritis. Arch. Med Res. 2017, 48, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, J.H.; Abraham, A.G.; Xu, Y.; Schelling, J.R.; Feldman, H.I.; Sabbisetti, V.S.; Gonzalez, M.C.; Coca, S.; Schrauben, S.J.; Waikar, S.S.; et al. Plasma Biomarkers of Tubular Injury and Inflammation Are Associated with CKD Progression in Children. J. Am. Soc. Nephrol. 2020, 31, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Brilland, B.; Boud’Hors, C.; Wacrenier, S.; Blanchard, S.; Cayon, J.; Blanchet, O.; Piccoli, G.B.; Henry, N.; Djema, A.; Coindre, J.-P.; et al. Kidney injury molecule 1 (KIM-1): A potential biomarker of acute kidney injury and tubulointerstitial injury in patients with ANCA-glomerulonephritis. Clin. Kidney J. 2023, 16, 1521–1533. [Google Scholar] [CrossRef]
- Ichimura, T.; Asseldonk, E.J.; Humphreys, B.D.; Gunaratnam, L.; Duffield, J.S.; Bonventre, J.V. Kidney injury molecule–1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Investig. 2008, 118, 1657–1668. [Google Scholar] [CrossRef]
- Liu, C.; Debnath, N.; Mosoyan, G.; Chauhan, K.; Vasquez-Rios, G.; Soudant, C.; Menez, S.; Parikh, C.R.; Coca, S.G. Systematic Review and Meta-Analysis of Plasma and Urine Biomarkers for CKD Outcomes. J. Am. Soc. Nephrol. 2022, 33, 1657–1672. [Google Scholar] [CrossRef]
- Nowak, N.; Skupien, J.; Smiles, A.M.; Yamanouchi, M.; Niewczas, M.A.; Galecki, A.T.; Duffin, K.L.; Breyer, M.D.; Pullen, N.; Bonventre, J.V.; et al. Markers of early progressive renal decline in type 2 diabetes suggest different implications for etiological studies and prognostic tests development. Kidney Int. 2018, 93, 1198–1206. [Google Scholar] [CrossRef]
- Liu, H.; Sridhar, V.S.; Lovblom, L.E.; Lytvyn, Y.; Burger, D.; Burns, K.; Brinc, D.; Lawler, P.R.; Cherney, D.Z. Markers of Kidney Injury, Inflammation, and Fibrosis Associated With Ertugliflozin in Patients with CKD and Diabetes. Kidney Int. Rep. 2021, 6, 2095–2104. [Google Scholar] [CrossRef]
- Schmidt, I.M.; Srivastava, A.; Sabbisetti, V.; McMahon, G.M.; He, J.; Chen, J.; Kusek, J.W.; Taliercio, J.; Ricardo, A.C.; Hsu, C.-Y.; et al. Plasma Kidney Injury Molecule 1 in CKD: Findings from the Boston Kidney Biopsy Cohort and CRIC Studies. Am. J. Kidney Dis. 2022, 79, 231–243.e1. [Google Scholar] [CrossRef]
- Emmens, J.E.; ter Maaten, J.M.; Matsue, Y.; Metra, M.; O’Connor, C.M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; Davison, B.; Cleland, J.G.; et al. Plasma kidney injury molecule-1 in heart failure: Renal mechanisms and clinical outcome. Eur. J. Heart Fail. 2015, 18, 641–649. [Google Scholar] [CrossRef]
- McMillan, R.; Skiadopoulos, L.; Hoppensteadt, D.; Guler, N.; Bansal, V.; Parasuraman, R.; Fareed, J. Biomarkers of Endothelial, Renal, and Platelet Dysfunction in Stage 5 Chronic Kidney Disease Hemodialysis Patients with Heart Failure. Clin. Appl. Thromb. 2017, 24, 235–240. [Google Scholar] [CrossRef]
- Feldreich, T.; Nowak, C.; Fall, T.; Carlsson, A.C.; Carrero, J.-J.; Ripsweden, J.; Qureshi, A.R.; Heimbürger, O.; Barany, P.; Stenvinkel, P.; et al. Circulating proteins as predictors of cardiovascular mortality in end-stage renal disease. J. Nephrol. 2018, 32, 111–119. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, Z.; Cao, X.; Ding, X.; Zou, J.; Jin, H. Predictive role of cardiac valvular calcification in all-cause mortality of Chinese initial haemodialysis patients: A follow-up study of 4 years. BMC Nephrol. 2023, 24, 37. [Google Scholar] [CrossRef]
- Raggi, P.; Bellasi, A.; Gamboa, C.; Ferramosca, E.; Ratti, C.; Block, G.A.; Muntner, P. All-cause Mortality in Hemodialysis Patients with Heart Valve Calcification. Clin. J. Am. Soc. Nephrol. 2011, 6, 1990–1995. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, C. Effect of the Geometry and Severity of Left Ventricular Hypertrophy on Cardiovascular Mortality in Patients on Chronic Hemodialysis, PREPRINT (Version 1) available at Research Square. 2023; Volume 3, 2. [Google Scholar] [CrossRef]
- Kramer, A.; Pippias, M.; Noordzij, M.; Stel, V.S.; Afentakis, N.; Ambühl, P.M.; Andrusev, A.M.; Fuster, E.A.; Monzón, F.E.A.; Åsberg, A.; et al. The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2015: A summary. Clin. Kidney J. 2018, 11, 108–122. [Google Scholar] [CrossRef]



| Parameter | Patients Treated with HD (Mean Values ± Standard Deviation) |
|---|---|
| Age (years) | 60.1 ± 11.8 |
| Female:Male | 21:42 |
| Hemoglobin (g/dL) | 10.9 ± 1.0 |
| Hematocrit (%) | 33.3 ± 4.3 |
| Serum ferritin (ng/mL) | 995.9 ± 358.0 |
| TSAT (%) | 30.6 ± 10.5 |
| Serum creatinine (predialysis) mg/dL | 8.4 ± 1.9 |
| Serum urea (predialysis) mg/dL | 123.1 ± 28.7 |
| Plasma KIM-1 (pg/mL) | 403.8 ± 546.8 |
| IL-6 (pg/mL) | 10.8 ± 0.9 |
| CRP (mg/dL) | 1.0 ± 0.9 |
| Parameter | HD Patients (Mean Values ± Standard Deviation) | Control Group (Mean Values ± Standard Deviation) | Comparison between Groups (p-Value) |
|---|---|---|---|
| Age (years) | 60.1 ± 11.8 | 59.03 ± 14 | 0.65 |
| Female: Male | 21:42 | 32:29 | N.A. |
| Plasma KIM-1 (pg/mL) | 403.8 ± 546.8 | 217.48 ± 267.10 | 0.02 |
| IL-6 (pg/mL) | 10.8 ± 0.9 | 9.5 ± 7.6 | 0.18 |
| CRP (mg/dL) | 1.0 ± 0.9 | 1.28 ± 2.97 | 0.47 |
| Parameter | Correlation Coefficient |
|---|---|
| Age | NS |
| Hemoglobin | R = −0.5; p = 0.01 |
| Hematocrit | R = −0.5; p = 0.01 |
| Serum ferritin | NS |
| TSAT | NS |
| Serum creatinine (predialysis) | NS |
| Serum urea (predialysis) | NS |
| CRP | R = 0.28; p = 0.02 |
| IL-6 | R = 0.35; p = 0.005 |
| kT/V | NS |
| Ejection fraction | NS |
| Parameter | Number of Patients (Out of 63) | Plasma KIM-1: Mean Value ± Standard Deviation (Comparison between Patients with and without the Parameter) | Correlation Coefficient |
|---|---|---|---|
| Valvular calcifications LVH | 48 | 210.0 ± 414.1 vs. 462.5 ± 648.8 | 0.04 |
| 37 | 155.5 ± 214.0 vs. 432.1 ± 690.2 | 0.02 | |
| Signs of ischemia on ECG Coronary heart disease | 25 | 268.09 ± 515.06 vs. 266.34 ± 467.17 | 0.4 |
| 32 | 291.33 ± 516.62 vs. 244.286 ± 456.13 | 0.7 | |
| Cerebrovascular disease Diabetes mellitus | 8 | 109.9 ± 244.47 vs. 290.33 ± 506.17 | 0.97 |
| 27 | 219.16 ± 436.13 vs. 329.11 ± 539.68 | 0.37 | |
| Male gender Glomerular disease | 41 | 305.29 ± 562.06 vs. 192.37 ± 265.54 | 0.38 |
| 42 | 528.08 ± 661.38 vs. 209.69 ± 177.41 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sircuța, A.F.; Grosu, I.D.; Schiller, A.; Petrica, L.; Ivan, V.; Schiller, O.; Bodea, M.; Mircea, M.-N.; Goleț, I.; Bob, F. The Relationship between Circulating Kidney Injury Molecule-1 and Cardiovascular Morbidity and Mortality in Hemodialysis Patients. Biomedicines 2024, 12, 1903. https://doi.org/10.3390/biomedicines12081903
Sircuța AF, Grosu ID, Schiller A, Petrica L, Ivan V, Schiller O, Bodea M, Mircea M-N, Goleț I, Bob F. The Relationship between Circulating Kidney Injury Molecule-1 and Cardiovascular Morbidity and Mortality in Hemodialysis Patients. Biomedicines. 2024; 12(8):1903. https://doi.org/10.3390/biomedicines12081903
Chicago/Turabian StyleSircuța, Alexandru Florin, Iulia Dana Grosu, Adalbert Schiller, Ligia Petrica, Viviana Ivan, Oana Schiller, Madalina Bodea, Monica-Nicoleta Mircea, Ionuţ Goleț, and Flaviu Bob. 2024. "The Relationship between Circulating Kidney Injury Molecule-1 and Cardiovascular Morbidity and Mortality in Hemodialysis Patients" Biomedicines 12, no. 8: 1903. https://doi.org/10.3390/biomedicines12081903
APA StyleSircuța, A. F., Grosu, I. D., Schiller, A., Petrica, L., Ivan, V., Schiller, O., Bodea, M., Mircea, M.-N., Goleț, I., & Bob, F. (2024). The Relationship between Circulating Kidney Injury Molecule-1 and Cardiovascular Morbidity and Mortality in Hemodialysis Patients. Biomedicines, 12(8), 1903. https://doi.org/10.3390/biomedicines12081903







