Nesfatin-1: A Novel Diagnostic and Prognostic Biomarker in Digestive Diseases
Abstract
1. Introduction
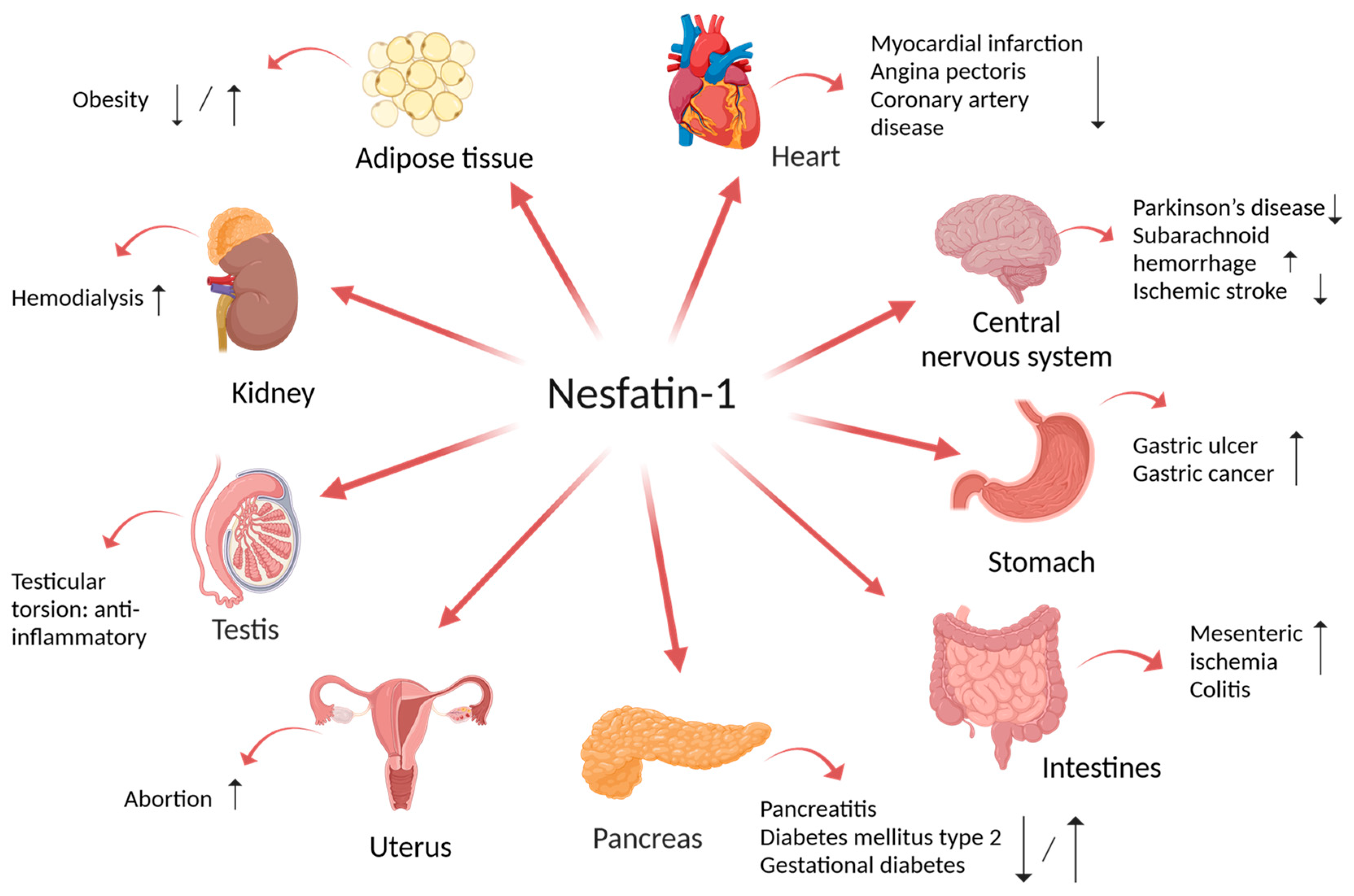
2. Implication of Nesfatin-1 in Multiple Disorders
3. Implication of Nesfatin-1 in Digestive Disorders
3.1. Gastric Ulcer
3.2. Gastric Cancer
3.3. Necrotizing Enterocolitis
3.4. Mesenteric Ischemia
3.5. Colitis
3.6. Pancreatitis
3.7. Cholecystitis
3.8. Obstructive Jaundice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oh-I, S.; Shimizu, H.; Satoh, T.; Okada, S.; Adachi, S.; Inoue, K.; Eguchi, H.; Yamamoto, M.; Imaki, T.; Hashimoto, K.; et al. Identification of Nesfatin-1 as a Satiety Molecule in the Hypothalamus. Nature 2006, 443, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Oh-I, S.; Okada, S.; Mori, M. Nesfatin-1: An Overview and Future Clinical Application. Endocr. J. 2009, 56, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.S.; Brismar, H.; Broberger, C. Distribution and Neuropeptide Coexistence of Nucleobindin-2 MRNA/Nesfatin-like Immunoreactivity in the Rat CNS. Neuroscience 2008, 156, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Goebel, M.; Taché, Y. Nesfatin-1: A Novel Inhibitory Regulator of Food Intake and Body Weight. Obes. Rev. 2011, 12, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Goebel-Stengel, M.; Wang, L.; Stengel, A.; Taché, Y. Localization of Nesfatin-1 Neurons in the Mouse Brain and Functional Implication. Brain Res. 2011, 1396, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Hsuchou, H.; Kastin, A.J. Nesfatin-1 Crosses the Blood–Brain Barrier without Saturation. Peptides 2007, 28, 2223–2228. [Google Scholar] [CrossRef] [PubMed]
- Kras, K.; Muszyński, M.; Muszyński, S.; Tomaszewska, E.; Arciszewski, M.B.; Klećkowskaklećkowska-Nawrot, J.; Goździewskagoździewska-Harłajczuk, K.; Kras, K.; Muszyński, S.; Tomaszewska, E.; et al. Minireview: Peripheral Nesfatin-1 in Regulation of the Gut Activity—15 Years since the Discovery. Animals 2022, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, F. Antioxidant, Anti-Inflammatory and Anti-Apoptotic Activities of Nesfatin-1: A Review. J. Inflamm. Res. 2020, 13, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.-Q.; Li, X.-L.; Jiang, C.-Y.; Lin, L.; Shi, R.-H.; Chen, J.-D.; Oomura, Y. Expression of Nesfatin-1/NUCB2 in Rodent Digestive System. World J. Gastroenterol. 2010, 16, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Goebel, M.; Yakubov, I.; Wang, L.; Witcher, D.; Coskun, T.; Taché, Y.; Sachs, G.; Lambrecht, N.W.G. Identification and Characterization of Nesfatin-1 Immunoreactivity in Endocrine Cell Types of the Rat Gastric Oxyntic Mucosa. Endocrinology 2009, 150, 232. [Google Scholar] [CrossRef] [PubMed]
- Puzio, I.; Muszyński, S.; Dobrowolski, P.; Kapica, M.; Pawłowska-Olszewska, M.; Donaldson, J.; Tomaszewska, E. Alterations in Small Intestine and Liver Morphology, Immunolocalization of Leptin, Ghrelin and Nesfatin-1 as Well as Immunoexpression of Tight Junction Proteins in Intestinal Mucosa after Gastrectomy in Rat Model. J. Clin. Med. 2021, 10, 272. [Google Scholar] [CrossRef]
- Mohan, H.; Unniappan, S. Ontogenic Pattern of Nucleobindin-2/Nesfatin-1 Expression in the Gastroenteropancreatic Tissues and Serum of Sprague Dawley Rats. Regul. Pept. 2012, 175, 61–69. [Google Scholar] [CrossRef]
- Jiang, S.; Zhou, W.; Zhang, X.; Wang, D.; Zhu, H.; Hong, M.; Gong, Y.; Ye, J.; Fang, F. Developmental Expression and Distribution of Nesfatin-1/NUCB2 in the Canine Digestive System. Acta Histochem. 2016, 118, 90–96. [Google Scholar] [CrossRef]
- Gonkowski, S.; Rychlik, A.; Nowicki, M.; Nieradka, R.; Bulc, M.; Całka, J. A Population of Nesfatin 1-like Immunoreactive (LI) Cells in the Mucosal Layer of the Canine Digestive Tract. Res. Vet. Sci. 2012, 93, 1119–1121. [Google Scholar] [CrossRef]
- Morton, K.A.; Hargreaves, L.; Mortazavi, S.; Weber, L.P.; Blanco, A.M.; Unniappan, S. Tissue-Specific Expression and Circulating Concentrations of Nesfatin-1 in Domestic Animals. Domest. Anim. Endocrinol. 2018, 65, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Feijóo-Bandín, S.; Rodríguez-Penas, D.; García-Rúa, V.; Mosquera-Leal, A.; Otero, M.F.; Pereira, E.; Rubio, J.; Martínez, I.; Seoane, L.M.; Gualillo, O.; et al. Nesfatin-1 in Human and Murine Cardiomyocytes: Synthesis, Secretion, and Mobilization of GLUT-4. Endocrinology 2013, 154, 4757–4767. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Chen, J.; Brown, J.E.; Tripathi, G.; Hallschmid, M.; Patel, S.; Kern, W.; Hillhouse, E.W.; Lehnert, H.; Tan, B.K.; et al. Identification of Nesfatin-1 in Human and Murine Adipose Tissue: A Novel Depot-Specific Adipokine with Increased Levels in Obesity. Endocrinology 2010, 151, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Shepperd, E.; Thiruppugazh, V.; Lohan, S.; Grey, C.L.; Chang, J.P.; Unniappan, S. Nesfatin-1 Regulates the Hypothalamo-Pituitary-Ovarian Axis of Fish. Biol. Reprod. 2012, 87, 84. [Google Scholar] [CrossRef]
- García-Galiano, D.; Pineda, R.; Ilhan, T.; Castellano, J.M.; Ruiz-Pino, F.; Sánchez-Garrido, M.A.; Vazquez, M.J.; Sangiao-Alvarellos, S.; Romero-Ruiz, A.; Pinilla, L.; et al. Cellular Distribution, Regulated Expression, and Functional Role of the Anorexigenic Peptide, NUCB2/Nesfatin-1, in the Testis. Endocrinology 2012, 153, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Author, C.; Yang, H.; Kim, J.; Chung, Y.; Kim, H.; Im, E.; Lee, H. The Tissue Distribution of Nesfatin-1/NUCB2 in Mouse. Dev. Reprod. 2014, 18, 301–309. [Google Scholar] [CrossRef]
- Prinz, P.; Goebel-Stengel, M.; Teuffel, P.; Rose, M.; Klapp, B.F.; Stengel, A. Peripheral and Central Localization of the Nesfatin-1 Receptor Using Autoradiography in Rats. Biochem. Biophys. Res. Commun. 2016, 470, 521–527. [Google Scholar] [CrossRef]
- Brailoiu, G.C.; Dun, S.L.; Brailoiu, E.; Inan, S.; Yang, J.; Jaw, K.C.; Dun, N.J. Nesfatin-1: Distribution and Interaction with a G Protein-Coupled Receptor in the Rat Brain. Endocrinology 2007, 148, 5088–5094. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, Z.; Gao, L.; Li, Y.; Zhao, J.; Zhang, W. AMPK-Dependent Modulation of Hepatic Lipid Metabolism by Nesfatin-1. Mol. Cell Endocrinol. 2015, 417, 20–26. [Google Scholar] [CrossRef]
- Dong, J.; Xu, H.; Wang, P.F.; Cai, G.J.; Song, H.F.; Wang, C.C.; Dong, Z.T.; Ju, Y.J.; Jiang, Z.Y. Nesfatin-1 Stimulates Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase in STZ-Induced Type 2 Diabetic Mice. PLoS ONE 2013, 8, e83397. [Google Scholar] [CrossRef]
- Kan, J.-Y.; Yen, M.-C.; Wang, J.-Y.; Wu, D.-C.; Chiu, Y.-J.; Ho, Y.-W.; Kuo, P.-L.; Kan, J.-Y.; Yen, M.-C.; Wang, J.-Y.; et al. Nesfatin-1/Nucleobindin-2 Enhances Cell Migration, Invasion, and Epithelial-Mesenchymal Transition via LKB1/AMPK/TORC1/ZEB1 Pathways in Colon Cancer. Oncotarget 2016, 7, 31336–31349. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.; Wang, C.; Li, K.; Li, S.; Boden, G.; Li, N.; Yang, G. Nesfatin-1 Action in the Brain Increases Insulin Sensitivity through Akt/AMPK/TORC2 Pathway in Diet-Induced Insulin Resistance. Diabetes 2012, 61, 1959–1968. [Google Scholar] [CrossRef]
- Tan, J.; Jin, L.; Wang, Y.; Cao, B.; Wang, S.; Zhang, F.; Wei, L. Nesfatin-1 Acts as an Inhibitory Factor in Human Gastrointestinal Smooth Muscle Cells in Diabetes Mellitus-Induced Delayed Gastric Emptying. Int. J. Clin. Exp. Pathol. 2016, 9, 11214–11221. [Google Scholar]
- Ge, J.F.; Xu, Y.Y.; Qin, G.; Pan, X.Y.; Cheng, J.Q.; Chen, F.H. Nesfatin-1, a Potent Anorexic Agent, Decreases Exploration and Induces Anxiety-like Behavior in Rats without Altering Learning or Memory. Brain Res. 2015, 1629, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Tanida, M.; Gotoh, H.; Yamamoto, N.; Wang, M.; Kuda, Y.; Kurata, Y.; Mori, M.; Shibamoto, T. Hypothalamic Nesfatin-1 Stimulates Sympathetic Nerve Activity via Hypothalamic ERK Signaling. Diabetes 2015, 64, 3725–3736. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Y.Y.; Ge, J.F.; Chen, F.H. CRHR1 Mediates the Up-Regulation of Synapsin I Induced by Nesfatin-1 Through ERK 1/2 Signaling in SH-SY5Y Cells. Cell Mol. Neurobiol. 2018, 38, 627–633. [Google Scholar] [CrossRef]
- Yuan, J.H.; Chen, X.; Dong, J.; Zhang, D.; Song, K.; Zhang, Y.; Wu, G.B.; Hu, X.H.; Jiang, Z.Y.; Chen, P. Nesfatin-1 in the Lateral Parabrachial Nucleus Inhibits Food Intake, Modulates Excitability of Glucosensing Neurons, and Enhances UCP1 Expression in Brown Adipose Tissue. Front. Physiol. 2017, 8, 239809. [Google Scholar] [CrossRef] [PubMed]
- Yosten, G.L.C.; Samson, W.K. Nesfatin-1 Exerts Cardiovascular Actions in Brain: Possible Interaction with the Central Melanocortin System. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 330–336. [Google Scholar] [CrossRef]
- Ying, J.; Zhang, Y.; Gong, S.; Chang, Z.; Zhou, X.; Li, H.; Tao, J.; Zhang, G. Nesfatin-1 Suppresses Cardiac L-Type Ca2+ Channels Through Melanocortin Type 4 Receptor and the Novel Protein Kinase C Theta Isoform Pathway. Cell. Physiol. Biochem. 2015, 36, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Tasatargil, A.; Kuscu, N.; Dalaklioglu, S.; Adiguzel, D.; Celik-Ozenci, C.; Ozdem, S.; Barutcigil, A.; Ozdem, S. Cardioprotective Effect of Nesfatin-1 against Isoproterenol-Induced Myocardial Infarction in Rats: Role of the Akt/GSK-3β Pathway. Peptides 2017, 95, 1–9. [Google Scholar] [CrossRef]
- Lu, Q.B.; Wang, H.P.; Tang, Z.H.; Cheng, H.; Du, Q.; Wang, Y.B.; Feng, W.B.; Li, K.X.; Cai, W.W.; Qiu, L.Y.; et al. Nesfatin-1 Functions as a Switch for Phenotype Transformation and Proliferation of VSMCs in Hypertensive Vascular Remodeling. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2018, 1864, 2154–2168. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, L.; Tang, H.; Yin, Y.; Xiang, X.; Li, Y.; Zhao, J.; Mulholland, M.; Zhang, W. Peripheral Effects of Nesfatin-1 on Glucose Homeostasis. PLoS ONE 2013, 8, e71513. [Google Scholar] [CrossRef]
- Feng, H.; Wang, Q.; Guo, F.; Han, X.; Pang, M.; Sun, X.; Gong, Y.; Xu, L.; Xu, L. Nesfatin-1 Influences the Excitability of Gastric Distension-Responsive Neurons in the Ventromedial Hypothalamic Nucleus of Rats. Physiol. Res. 2017, 66, 335–344. [Google Scholar] [CrossRef]
- Stengel, A.; Goebel, M.; Wang, L.; Rivier, J.; Kobelt, P.; Mönnikes, H.; Lambrecht, N.W.G.; Taché, Y. Central Nesfatin-1 Reduces Dark-Phase Food Intake and Gastric Emptying in Rats: Differential Role of Corticotropin-Releasing Factor2 Receptor. Endocrinology 2009, 150, 4911. [Google Scholar] [CrossRef]
- Nakata, M.; Manaka, K.; Yamamoto, S.; Mori, M.; Yada, T. Nesfatin-1 Enhances Glucose-Induced Insulin Secretion by Promoting Ca2+ Influx through L-Type Channels in Mouse Islet β-Cells. Endocr. J. 2011, 58, 305–313. [Google Scholar] [CrossRef]
- Maejima, Y.; Horita, S.; Kobayashi, D.; Aoki, M.; O’hashi, R.; Imai, R.; Sakamoto, K.; Mori, M.; Takasu, K.; Ogawa, K.; et al. Nesfatin-1 Inhibits Voltage Gated K+ Channels in Pancreatic Beta Cells. Peptides 2017, 95, 10–15. [Google Scholar] [CrossRef]
- Ayada, C.; Turgut, G.; Turgut, S. The Effect of Nesfatin-1 on Heart L-Type Ca2+ Channel Aα1c Subunit in Rats Subjected to Chronic Restraint Stress. Bratisl. Med. J. 2015, 116, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Takahashi, M.; Mukohda, M.; Morita, T.; Okada, M.; Hara, Y. A Novel Adipocytokine, Nesfatin-1 Modulates Peripheral Arterial Contractility and Blood Pressure in Rats. Biochem. Biophys. Res. Commun. 2012, 418, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Angelone, T.; Filice, E.; Pasqua, T.; Amodio, N.; Galluccio, M.; Montesanti, G.; Quintieri, A.M.; Cerra, M.C. Nesfatin-1 as a Novel Cardiac Peptide: Identification, Functional Characterization, and Protection against Ischemia/Reperfusion Injury. Cell. Mol. Life Sci. 2013, 70, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Rupp, S.K.; Stengel, A. Interactions between Nesfatin-1 and the Autonomic Nervous System—An Overview. Peptides 2022, 149, 170719. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Q.; Bai, J.; Li, G.; Tao, K.; Wang, G.; Xia, Z. RYGB Increases Postprandial Gastric Nesfatin-1 and Rapid Relieves NAFLD via Gastric Nerve Detachment. PLoS ONE 2020, 15, e0243640. [Google Scholar] [CrossRef] [PubMed]
- Pena-Leon, V.; Perez-Lois, R.; Seoane, L.M. MTOR Pathway Is Involved in Energy Homeostasis Regulation as a Part of the Gut–Brain Axis. Int. J. Mol. Sci. 2020, 21, 5715. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wen, F.; Qiu, D.; Wang, S. Nesfatin-1 in Lipid Metabolism and Lipid-Related Diseases. Clin. Chim. Acta 2021, 522, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nasri, A.; Kowaluk, M.; Widenmaier, S.B.; Unniappan, S. Nesfatin-1 and Nesfatin-1-like Peptide Attenuate Hepatocyte Lipid Accumulation and Nucleobindin-1 Disruption Modulates Lipid Metabolic Pathways. Commun. Biol. 2024, 7, 623. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Qu, Y.; Song, L.; Lin, Q.; Li, M.; Su, K.; Li, Y.; Dong, J. Central Nesfatin-1 Activates Lipid Mobilization in Adipose Tissue and Fatty Acid Oxidation in Muscle via the Sympathetic Nervous System. BioFactors 2020, 46, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Özdemir-Kumral, Z.N.; Koyuncuoğlu, T.; Arabacı-Tamer, S.; Çilingir-Kaya, Ö.T.; Köroğlu, A.K.; Yüksel, M.; Yeğen, B.Ç. High-Fat Diet Enhances Gastric Contractility, but Abolishes Nesfatin-1-Induced Inhibition of Gastric Emptying. J. Neurogastroenterol. Motil. 2021, 27, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Dore, R.; Krotenko, R.; Reising, J.P.; Murru, L.; Sundaram, S.M.; Di Spiezio, A.; Müller-Fielitz, H.; Schwaninger, M.; Jöhren, O.; Mittag, J.; et al. Nesfatin-1 Decreases the Motivational and Rewarding Value of Food. Neuropsychopharmacology 2020, 45, 1645–1655. [Google Scholar] [CrossRef] [PubMed]
- Dokumacioglu, E.; Iskender, H.; Sahin, A.; Erturk, E.Y.; Kaynar, O. Serum Levels of Nesfatin-1 and Irisin in Obese Children. Eur. Cytokine Netw. 2020, 31, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Pałasz, A.; Rojczyk, E.; Siwiec, A.; Janas-Kozik, M. Nesfatin-1 in the Neurochemistry of Eating Disorders. Psychiatr. Pol. 2020, 54, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Li, S.-Z.; Fan, X.-T.; Tian, Z.; Lu, X.-Q.; Dong, J. Circulating Nesfatin-1 Levels and Type 2 Diabetes: A Systematic Review and Meta-Analysis. J Diabetes Res 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Öztürk Özkan, G. Effects of Nesfatin-1 on Food Intake and Hyperglycemia. J. Am. Coll. Nutr. 2020, 39, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liang, Y.; Wang, K.; Wu, J.; Luo, H.; Yi, B. Influence of Circulating Nesfatin-1, GSH and SOD on Insulin Secretion in the Development of T2DM. Front. Public. Health 2022, 10, 882686. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Choubey, M.; Yada, T.; Krishna, A. Nesfatin-1 Ameliorates Type-2 Diabetes-Associated Reproductive Dysfunction in Male Mice. J. Endocrinol. Investig. 2020, 43, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Matta, R.A.; El-Hini, S.H.; Salama, A.M.S.E.; Moaness, H.M. Serum Nesfatin-1 Is a Biomarker of Pre-Diabetes and Interplays with Cardiovascular Risk Factors. Egypt. J. Intern. Med. 2022, 34, 15. [Google Scholar] [CrossRef]
- Mohammad, N.; Gallaly, D. Serum Nesfatin-1 in Patients with Type 2 Diabetes Mellitus: A Cross Sectional Study. Zanco J. Med. Sci. 2020, 24, 1–7. [Google Scholar] [CrossRef]
- Kadim, B.M.; Hassan, E.A. Nesfatin-1—As a Diagnosis Regulatory Peptide in Type 2 Diabetes Mellitus. J. Diabetes Metab. Disord. 2022, 21, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Baek, S.-E.; Yoon, J.-W.; Kim, H.-S.; Kwon, Y.; Yeom, E. Nesfatin-1 Ameliorates Pathological Abnormalities in Drosophila HTau Model of Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2024, 727, 150311. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, X.; Ma, H.; Zheng, W.; Shen, X. Reduction in Nesfatin-1 Levels in the Cerebrospinal Fluid and Increased Nigrostriatal Degeneration Following Ventricular Administration of Anti-Nesfatin-1 Antibody in Mice. Front. Neurosci. 2021, 15, 621173. [Google Scholar] [CrossRef]
- Acik, V.; Matyar, S.; Arslan, A.; İstemen, İ.; Olguner, S.K.; Arslan, B.; Gezercan, Y.; Ökten, A.İ. Relationshıp of Spontaneous Subarachnoid Haemorrhage and Cerebral Aneurysm to Serum Visfatin and Nesfatin-1 Levels. Clin. Neurol. Neurosurg. 2020, 194, 105837. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak-Kabzińska, A.; Marek, B.; Borgiel-Marek, H.; Kajdaniuk, D.; Kos-Kudła, B. Assessing the Blood Concentration of New Adipocytokines in Patients with Ischaemic Stroke. Endokrynol. Pol. 2020, 71, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A.; Epelbaum, J.; Gourcerol, G.; Stengel, A.; Prinz, P.; Teuffel, P.; Lembke, V.; Kobelt, P.; Goebel-Stengel, M.; Hofmann, T.; et al. Nesfatin-1 30–59 Injected Intracerebroventricularly Differentially Affects Food Intake Microstructure in Rats Under Normal Weight and Diet-Induced Obese Conditions. Front. Neurosci. 2015, 9, 422. [Google Scholar] [CrossRef]
- Elthakaby, A.H.M.; Esso, H.Y.; Elsayed, H.M.; Youssef Ahmed, K.; Abd El-Ghaffar Mohamed, N.; Elthakaby, M.; Youssef, K.; Mohamed, E.-G.; Authors Ahmed M Elthakaby, A.H.; Mohamed, R.R. Effect of Nesfatin-1 on the Nutritional Status of Hemodialysis Patients. J. Med. Sci. Res. 2022, 5, 30. [Google Scholar] [CrossRef]
- Yin, C.; Liu, W.; Xu, E.; Zhang, M.; Lv, W.; Lu, Q.; Xiao, Y. Copeptin and Nesfatin-1 Are Interrelated Biomarkers with Roles in the Pathogenesis of Insulin Resistance in Chinese Children with Obesity. Ann. Nutr. Metab. 2020, 76, 223–232. [Google Scholar] [CrossRef]
- Afifi, M.A.; Khalil, F.M.; Assal, M.A.; El Matueny, R.M.; Rizk, M. Serum Nesfatin-1 in Patients with Metabolic Associated Fatty Liver Disease. Egypt. J. Hosp. Med. 2024, 94, 1056. [Google Scholar]
- Samani, S.M.; Ghasemi, H.; Bookani, K.R.; Shokouhi, B. Serum nesfatin-1 level in healthy subjects with weight-related abnormalities and newly diagnosed patients with type 2 diabetes mellitus—A case-control study. Acta Endocrinol. 2019, XV, 69–73. [Google Scholar] [CrossRef]
- Caroleo, M.; Carbone, E.A.; Arcidiacono, B.; Greco, M.; Primerano, A.; Mirabelli, M.; Fazia, G.; Rania, M.; Hribal, M.L.; Gallelli, L.; et al. Does NUCB2/Nesfatin-1 Influence Eating Behaviors in Obese Patients with Binge Eating Disorder? Toward a Neurobiological Pathway. Nutrients 2023, 15, 348. [Google Scholar] [CrossRef]
- Jiang, X.-H.; Song, H.-H.; Wang, R. Expression and Clinical Significance of Serum SHBG, Nesfatin-1 and SFRP4 in Patients with Gestational Diabetes Mellitus. J. Trop. Med. 2020, 20, 815–819. [Google Scholar]
- Çaltekin, M.D.; Caniklioğlu, A. Maternal Serum Delta-Like 1 and Nesfatin-1 Levels in Gestational Diabetes Mellitus: A Prospective Case-Control Study. Cureus 2021, 13, e17001. [Google Scholar] [CrossRef]
- Mahmood, N.; Al-Sammarai, R.; Al-Fanar, R. Evaluation of the Role of Nesfatin-1 and Myonectin as A Diagnostic Marker for Polycystic Ovary Syndrome and Also for Treatment Response with Metformin. Egypt. Acad. J. Biol. Sci. C Physiol. Mol. Biol. 2023, 15, 577–584. [Google Scholar] [CrossRef]
- Obstet, T.J.; Demir Çaltekin, M.; Caniklioğlu, A.; Yalçın, S.E.; Kırmızı, D.A.; Baser, E.; Yalvaç, E.S. Clinical Investigation/Araştırma PRECIS: DLK1 and Nesfatin-1 Protein Levels Are Lower in Women with PCOS. Polikistik over Sendromunda DLK1 ve Nesfatin-1 Düzeyleri ve Metabolik Parametrelerle Ilişkisi: Prospektif Kontrollü Çalışma. J. Turk. Soc. Obstet. Gynecol. 2021, 18, 124–130. [Google Scholar] [CrossRef]
- Su, R.-Y.; Geng, X.-Y.; Yang, Y.; Yin, H.-S.; Rui-Ying Su, C. Nesfatin-1 Inhibits Myocardial Ischaemia/Reperfusion Injury through Activating Akt/ERK Pathway-Dependent Attenuation of Endoplasmic Reticulum Stress. J. Cell. Mol. Med. 2021, 25, 5050–5059. [Google Scholar] [CrossRef] [PubMed]
- Naseroleslami, M.; Sharifi, M.; Rakhshan, K.; Mokhtari, B.; Aboutaleb, N. Nesfatin-1 Attenuates Injury in a Rat Model of Myocardial Infarction by Targeting Autophagy, Inflammation, and Apoptosis. Arch. Physiol. Biochem. 2020, 129, 122–130. [Google Scholar] [CrossRef]
- Nejati, A.; Doustkami, H.; Babapour, B.; Ebrahimoghlou, V.; Aslani, M.R. Serum Correlation of Nesfatin-1 with Angiographic, Echocardiographic, and Biochemical Findings in Patients with Coronary Artery Disease. Iran. Red. Crescent Med. J. 2021, 23, 245. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Korakas, E.; Lampropoulos, S.; Maratou, E.; Kassimis, G.; Patsourakos, N.; Plotas, P.; Moutsatsou, P.; Lambadiari, V. Plasma Nesfatin-1 and DDP-4 Levels in Patients with Coronary Artery Disease: Kozani Study. Cardiovasc. Diabetol. 2021, 20, 166. [Google Scholar] [CrossRef]
- Yilmaz, M.S.; Altinbas, B.; Guvenc, G.; Erkan, L.G.; Avsar, O.; Savci, V.; Kucuksen, D.U.; Arican, I.; Yalcin, M. The Role of Centrally Injected Nesfatin-1 on Cardiovascular Regulation in Normotensive and Hypotensive Rats. Auton. Neurosci. 2015, 193, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Rong, G.; Xu, Y.; Jing, J. Elevated Nesfatin-1 Level in Synovium and Synovial Fluid Is Associated with pro-Inflammatory Cytokines in Patients with Rheumatoid Arthritis. Int. J. Gen. Med. 2021, 14, 5269–5278. [Google Scholar] [CrossRef]
- Seewordova, L.; Polyakova, J.; Papichev, E.; Akhverdyan, Y.; Zavodovsky, В. Ab0078 Nesfatin-1 Expression Associated with A Marker for Bone Matrix Formation P1np in Rheumatoid Arthritis. Ann. Rheum. Dis. 2022, 81, 1171. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, T.; Xu, Y.; Rong, G.; Jing, J. Inhibition of NUCB2 Suppresses the Proliferation, Migration, and Invasion of Rheumatoid Arthritis Synovial Fibroblasts from Patients with Rheumatoid Arthritis in Vitro. J. Orthop. Surg. Res. 2022, 17, 574. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Choubey, M.; Yada, T.; Krishna, A. Direct Effects of Neuropeptide Nesfatin-1 on Testicular Spermatogenesis and Steroidogenesis of the Adult Mice. Gen. Comp. Endocrinol. 2019, 271, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Choubey, M.; Yada, T.; Krishna, A. Immunohistochemical Localization and Possible Functions of Nesfatin-1 in the Testis of Mice during Pubertal Development and Sexual Maturation. J. Mol. Histol. 2019, 50, 533–549. [Google Scholar] [CrossRef]
- Arabaci Tamer, S.; Yildirim, A.; Köroğlu, M.K.; Çevik, Ö.; Ercan, F.; Yeğen, B. Nesfatin-1 Ameliorates Testicular Injury and Supports Gonadal Function in Rats Induced with Testis Torsion. Peptides 2018, 107, 1–9. [Google Scholar] [CrossRef]
- Chung, Y.; Kim, H.; Seon, S.; Yang, H. Serum Cytokine Levels Are Related to Nesfatin-1/NUCB2 Expression in the Implantation Sites of Spontaneous Abortion Model of CBA/j × DBA/2. Dev. Reprod. 2017, 21, 35. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.Z.; Chen, S.C.; Zou, X.B.; Tian, L.L.; Sui, S.H.; Liu, N.Z. Nesfatin-1 Alleviates Acute Lung Injury through Reducing Inflammation and Oxidative Stress via the Regulation of HMGB1. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5071–5081. [Google Scholar] [CrossRef]
- Jiang, L.; Xu, K.; Li, J.; Zhou, X.; Xu, L.; Wu, Z.; Ma, C.; Ran, J.; Hu, P.; Bao, J.; et al. Nesfatin-1 Suppresses Interleukin-1β-Induced Inflammation, Apoptosis, and Cartilage Matrix Destruction in Chondrocytes and Ameliorates Osteoarthritis in Rats. Aging 2020, 12, 1760–1777. [Google Scholar] [CrossRef]
- Sun, J.; Gao, N.; Wu, Q.; Li, Y.; Zhang, L.; Jiang, Z.; Wang, Z.; Liu, J. High Plasma Nesfatin-1 Level in Chinese Adolescents with Depression. Sci. Rep. 2023, 13, 15288. [Google Scholar] [CrossRef]
- Kolgazi, M.; Cantali-Ozturk, C.; Deniz, R.; Ozdemir-Kumral, Z.N.; Yuksel, M.; Sirvanci, S.; Yeğen, B.C. Nesfatin-1 Alleviates Gastric Damage via Direct Antioxidant Mechanisms. J. Surg. Res. 2015, 193, 111–118. [Google Scholar] [CrossRef]
- Szlachcic, A.; Sliwowski, Z.; Krzysiek-Maczka, G.; Majka, J.; Surmiak, M.; Pajdo, R.; Drozdowicz, D.; Konturek, S.J.; Brzozowski, T. New Satiety Hormone Nesfatin-1 Protects Gastric Mucosa against Stress-Induced Injury: Mechanistic Roles of Prostaglandins, Nitric Oxide, Sensory Nerves and Vanilloid Receptors. Peptides 2013, 49, 9–20. [Google Scholar] [CrossRef]
- Szlachcic, A.; Majka, J.; Strzalka, M.; Szmyd, J.; Pajdo, R.; Ptak-Belowska, A.; Kwiecien, S.; Brzozowski, T. Experimental Healing of Preexisting Gastric Ulcers Induced by Hormones Controlling Food Intake Ghrelin, Orexin-A and Nesfatin-1 Is Impaired under Diabetic Conditions. A Key to Understanding the Diabetic Gastropathy? J. Physiol. Pharmacol. 2013, 64, 625–637. [Google Scholar] [PubMed]
- Kolgazi, M.; Mehmet, A.; Üniversitesi, A.A.; Özdemir, Z.N.; Demirci, E.K. Anti-Inflammatory Effects of Nesfatin-1 on Acetic Acid-Induced Gastric Ulcer in Rats: Involvement of Cyclo-Oxygenase Pathway. J. Physiol. Pharmacol. 2017, 68, 765–777. [Google Scholar] [PubMed]
- Wang, X.Q.; Zheng, Y.; Fang, P.F.; Song, X.B. Nesfatin-1 Is a Potential Diagnostic Biomarker for Gastric Cancer. Oncol. Lett. 2020, 19, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.H.; Go, S.I.; Lee, W.S.; Lee, J.H.; Jeong, S.H.; Lee, Y.J.; Hong, S.C.; Ha, W.S.; Yang, F. Prognostic Impact of Ki-67 in Patients with Gastric Cancer—The Importance of Depth of Invasion and Histologic Differentiation. Medicine 2017, 96, e7181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, J.; Wang, H.; Xiao, L.; Wei, Y.; He, J.; Wang, G. The Level of Nesfatin-1 in a Mouse Gastric Cancer Model and Its Role in Gastric Cancer Comorbid with Depression. Shanghai Arch. Psychiatry 2018, 30, 119–126. [Google Scholar] [PubMed]
- Yoshida, N.; Maejima, Y.; Sedbazar, U.; Ando, A.; Kurita, H.; Damdindorj, B.; Takano, E.; Gantulga, D.; Iwasaki, Y.; Kurashina, T.; et al. Stressor-Responsive Central Nesfatin-1 Activates Corticotropin-Releasing Hormone, Noradrenaline and Serotonin Neurons and Evokes Hypothalamic-Pituitary-Adrenal Axis. Aging 2010, 2, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Sampah, M.E.S.; Salazar, A.J.G.; Hackam, D.J. Necrotizing Enterocolitis. In Principles of Neonatology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 707–714. [Google Scholar] [CrossRef]
- Karadeniz Cerit, K.; Koyuncuoğlu, T.; Yağmur, D.; Peker Eyüboğlu, İ.; Şirvancı, S.; Akkiprik, M.; Aksu, B.; Dağlı, E.T.; Yeğen, B. Nesfatin-1 Ameliorates Oxidative Bowel Injury in Rats with Necrotizing Enterocolitis: The Role of the Microbiota Composition and Claudin-3 Expression. J. Pediatr. Surg. 2020, 55, 2797–2810. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, S.; Fan, Z. Oxidative Stress in Intestinal Ischemia-Reperfusion. Front. Med. 2022, 8, 750731. [Google Scholar] [CrossRef] [PubMed]
- Tatar, C.; Ahlatci, F.A.; Idiz, U.O.; Nayci, A.E.; Incir, S.; Agcaoglu, O.; Idiz, C.; Balik, E. May Nesfatin-1 Be a Biomarker in Acute Mesenteric Ischemia? J. Coll. Physicians Surg. Pak. 2019, 29, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Sánchez, G.; García-Alonso, I.; María, J.G.S.d.S.; Alonso-Varona, A.; de la Parte, B.H. Antioxidant-Based Therapy Reduces Early-Stage Intestinal Ischemia-Reperfusion Injury in Rats. Antioxidants 2021, 10, 853. [Google Scholar] [CrossRef]
- Ozturk, C.; Oktay, S.; Yuksel, M.; Akakin, D.; Yarat, A.; Kasimay, C. Anti-Inflammatory Effects of Nesfatin-1 in Rats with Acetic Acid—Induced Colitis and Underlying Mechanisms. J. Physiol. Pharmacol. 2017, 66, 741–750. [Google Scholar]
- Beyaz, A.E.; Akbal, E. Increased Serum Nesfatin-1 Levels in Patients with Inflammatory Bowel Diseases. Postgrad. Med. J. 2022, 98, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.S.; Pecchi, E.; Trouslard, J.; Jean, A.; Dallaporta, M.; Troadec, J.D. Central Nesfatin-1-Expressing Neurons Are Sensitive to Peripheral Inflammatory Stimulus. J. Neuroinflam. 2009, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Buzcu, H.; Ozbeyli, D.; Yuksel, M.; Cilingir Kaya, O.T.; Kasimay Cakir, O. Nesfatin-1 Protects from Acute Pancreatitis: Role of Melanocortin Receptors. J. Physiol. Pharmacol. 2019, 70, 1–10. [Google Scholar] [CrossRef]
- Ferlengez, A.G.; Tatar, C.; Degerli, M.S.; Koyuncu, A.; Ahlatci, F.A.; Nayci, A.E.; Ari, A.; Idiz, U.O. The Role of Serum Nesfatin-1 in a Rat Model of Acute Pancreatitis. Adv. Clin. Exp. Med. 2023, 32, 545–549. [Google Scholar] [CrossRef]
- Ulger, B.V.; Gül, M.; Uslukaya, O.; Oguz, A.; Bozdag, Z.; Yüksel, H.; Böyük, A. New Hormones to Predict the Severity of Gallstone-Induced Acute Pancreatitis. Turk. J. Gastroenterol. 2014, 25, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Tekin, O.; Sevinç, M.M.; Albayrak, Ö.; Batıkan, O.; İdiz, U.O. Prognostic Effect of Nesfatin-1 on the Diagnosis and Staging of Acute Cholecystitis. Ulus. Travma Acil Cerrahi Derg. 2022, 28, 1583–1589. [Google Scholar] [CrossRef]
- Solmaz, A.; Gülçiçek, O.B.; Erçetin, C.; Yiğitbaş, H.; Yavuz, E.; Arıcı, S.; Erzik, C.; Zengi, O.; Demirtürk, P.; Çelik, A.; et al. Nesfatin-1 Alleviates Extrahepatic Cholestatic Damage of Liver in Rats. Bosn. J. Basic. Med. Sci. 2016, 16, 247. [Google Scholar] [CrossRef]
| Pathway | Study Type | Description of the Pathway/Effect | Conclusion | Citation |
|---|---|---|---|---|
| AMPK | Animal models: C57BL/6J mice with induced hepatic steatosis Hepatocyte cultures | Decreased lipogenesis transcription factors PPARγ and SREBP1 In cultured hepatocytes, it stimulates AMPK phosphorylation | Reduces the accumulation of lipids in the liver | Yin et al., 2015 [23] |
| Animal model: Male Kunming SPF mice with high-calorie diet + 2 Streptozotocin doses to induce type 2 Diabetes Mellitus | Lowered food intake, insulin resistance coefficient, and blood glucose Nesfatin-1 in low doses stimulates the AMPK-ACC pathway and activates free fatty acid utilization | Inhibits free fatty acid production and stimulates their oxidation | Dong et al., 2013 [24] | |
| Human colon cancer samples SW480 and SW620 cancer cell lines | In colon cancer tissue samples and SW480 and SW620 cancer cell lines: increased nesfatin-1 expression Nesfatin-1 activated LKB1/AMPK/TORC1/ZEB1 pathway in vivo and in vitro | Nesfatin-1/NUCB2 promotes migration and invasion in colon cancer | Kan et al., 2016 [25] | |
| Animal model: male Sprague-Dawley rats on normal or a high-fat diet | Activation of AKT/AMPK/TORC2, insulin receptor, and insulin substrate 1 phosphorylation Inhibition of hepatic gluconeogenesis and increased glucose uptake in the muscle | Nesfatin-1 increases insulin peripheral uptake and decreases production of glucose in the liver | Yang et al., 2012 [26] | |
| ERK/MAPK | Human gastrointestinal smooth muscle cells (HGSMC) | Up-regulates eNOS activity by involving the ERK/MAPK/mTOR cascade Increases some pro-apoptosis factors (p53 and Fas) | Nesfatin-1 accelerates the apoptosis rate in HGSMC | Tan et al., 2016 [27] |
| Rats | Decreases the expression of brain-derived neurotrophic factor (BDNF) and of phosphorylated ERK in the prefrontal cortex and the hippocampus | Nesfatin-1 induces anxiety-like behavior in rats | Ge et al., 2015 [28] | |
| Male Wistar rats and Zucker fatty rats | Increase in p-ERK1/2 positive neurons in the paraventricular hypothalamic nucleus Increased co-expression in the corticotropin-releasing hormone cells Activation of sympathetic activity in the kidney, liver, and white adipose tissue Hypertensive effects | Nesfatin-1 regulates the autonomic nervous system | Tanida et al., 2015 [29] | |
| Human SH-SY5Y neuroblastoma cells | Stimulates the protein expression of synapsin-1, p-ERK1/2, and CRH | Nesfatin-1 could be involved in the hypothalamic–pituitary–adrenal axis and in synaptic plasticity | Chen et al., 2018 [30] | |
| Melanocortin pathway | Adult male Wistar rats | Enhances the excitability of glucose-sensitive neurons in the lateral parabrachial nucleus Weight loss and increased the expression of uncoupling protein 1 (UCP1) in brown adipose tissue in chronic administration | Nesfatin-1 inhibits food intake and regulates body weight | Yuan et al., 2017 [31] |
| Adult male Harlan Sprague-Dawley rats | Nesfatin-1 increases mean arterial pressure | Nesfatin-1 stimulates sympathetic activity | Yosten et al., 2009 [32] | |
| Ventricular myocytes from adult male Wistar rats | Treatment with nesfatin-1 inhibited L-type Ca2+ channels in cardiomyocytes | Nesfatin-1 modulates cardiac performance | Ying et al., 2015 [33] | |
| AKT | Wistar rats with isoproterenol-induced myocardial infarction | Nesfatin-1 increases p-GSK-3β/GSK-3β and p-Akt/Akt expression in the myocardium Nesfatin-1 decreases the levels of troponin-T and proinflammatory cytokines Reduces the apoptotic and necrotic cells | Nesfatin-1 has cardioprotective activity against myocardial infarction | Tasatargil et al., 2017 [34] |
| Wistar–Kyoto rats Human aortic vascular smooth muscle cells (VSMCs) | Nesfatin-1 activates PI3K/Akt/mTOR and JAK2/STAT pathways induces VSMCs proliferation and phenotypic transformation Down-regulation of the nesfatin-1 gene inhibited cardiovascular remodeling | Nesfatin-1 is involved in vascular remodeling and hypertension by regulating VSMC proliferation | Lu et al., 2018 [35] | |
| Sprague-Dawley rats, C57BL/6J mice, and high-fat-diet-induced obese mice Myocytes, hepatocytes, and adipose cells isolated from C57BL/6J mice | Nesfatin-1 increased insulin secretion in vivo and in min6 cells via AKT phosphorylation Nesfatin-1 up-regulated the phosphorylation of AKT in adipose tissue, skeletal muscle, and liver in the mice that received a high-fat diet Nesfatin-1 promotes GLUT4 translocation in the adipose tissue and in the skeletal muscle irrespective of the diet (high-fat or normal diet) | Nesfatin-1 increases the secretion of insulin and the sensitivity to insulin in peripheral tissues | Li et al., 2013 [36] | |
| CRF pathway | Adult male Wistar rats | Nesfatin-1 inhibits excitatory neurons involved in gastric distention Nesfatin-1 stimulates the inhibitory gastric distention neurons in the ventromedial hypothalamic nucleus | Nesfatin-1 has implications for digestive disorders and obesity | Feng et al., 2017 [37] |
| Adult male Sprague-Dawley rats | Nesfatin-1 decreased dark-phase food intake in case of intracerebral administration, with no effect after ip administration | Nesfatin-1 is involved in the central regulation of food intake | Stengel et al., 2009 [38] | |
| Ion currents | Hypothalamic neuronal cultures from Sprague-Dawley rats | Nesfatin-1 increased intracellular Ca2+ concentrations in hypothalamic neurons | Nesfatin-1 increases calcium influx in hypothalamic neurons via a G protein-coupled receptor | Brailoiu et al., 2007 [22] |
| Beta cells from ICR mice | Nesfatin-1 increases the Ca2+ influx in pancreatic beta cells through the L-type Ca2+ channels after treatment with glucose | Nesfatin-1 stimulates the release of insulin from beta islet cells in a glucose-dependent manner | Nakata et al., 2011 [39] | |
| Islet cells from male C57BL/6J mice | Nesfatin-1 inhibits the activity of the Kv channels that stimulate insulin secretion | Nesfatin-1 increases the release of insulin from beta islet cells via the inhibition of Kv channels | Maejima et al., 2017 [40] | |
| Heart samples from Wistar albino rats | The expression level of the α1c subunit protein of the L-type Ca2+ channels in cardiac extracts was elevated in the rats subjected to chronic restraint stress | Nesfatin-1 may be involved in cardiac failure | Ayada et al., 2015 [41] | |
| NO-cGMP system | Mesenteric artery isolated from Wistar rats | Nesfatin-1 impairs the production of SNP-induced cGMP, which leads to reduced smooth muscle relaxation | Nesfatin-1 regulates blood pressure by modulating peripheral arterial resistance | Yamawaki et al., 2012 [42] |
| Heart samples from Wistar rats | Nesfatin-1 recruits specific GC-receptors (NPR-A—natriuretic peptide receptor A) activating the ERK1/2 and protein kinase G pathways Pretreatment decreases the size of the infarct and lactate dehydrogenase levels | Nesfatin-1 has negative inotropic and lusitropic effects and modulates heart activity Nesfatin-1 protects against ischemia/reperfusion injury | Angelone et al., 2013 [43] |
| Group of Disorder | Disease/Process | Type of Study | Observations | Conclusion | First Author and Year |
|---|---|---|---|---|---|
| Neurological disorders | Alzheimer’s disease (AD) | Animal model: Drosophila melanogaster | Nesfatin-1 reduces the neurodegenerative process in the eye and bristle of AD-induced models Nesfatin-1 regulates neuromuscular activity Nesfatin-1 reduces the levels of human Tau protein, particularly phospho-tau | Nesfatin-1 impedes neurodegeneration | Yang et al., 2024 [61] |
| Parkinson’s disease | C57BL/6 mice | Anti-nesfatin-1 antibody in the lateral ventricle led to: Reduction in the nesfatin-1 level of the cerebrospinal fluid ROS production and depletion of intraneuronal mitochondria Increased membrane permeability, cytochrome c leakage, caspase-3 induced apoptosis | Dopaminergic neuronal depletion is closely correlated with a reduced level of nesfatin-1 in the cerebrospinal fluid | Chen et al., 2021 [62] | |
| Subarachnoid hemorrhage | Case-control study | Nesfatin-1 levels were increased in the subarachnoid hemorrhage group compared to healthy controls Nesfatin-1 levels positively correlate with the presence, number, and size of the aneurysm | Potential role of nesfatin-1 as a predictive biomarker for acute subarachnoid hemorrhage and the existence of aneurysms | Acik et al., 2020 [63] | |
| Endocrine and metabolic disorders | Ischemic stroke | Case-control study | Nesfatin-1 levels were lower in the ischemic stroke group in comparison to controls No significant association between nesfatin-1 levels and the severity of ischemic stroke | Nesfatin-1 may play a role in the pathogenesis of ischemic stroke | Kazimierczak-Kabzińska et al., 2020 [64] |
| Feeding Behavior/Obesity | Male Sprague-Dawley rats | Nesfatin-1 decreases food ingestion by reducing the meal size not frequency or the intervals between meals Increase in satiation (size of a meal) under normal weight conditions Increase in satiety (frequency of meals) under diet-induced obesity conditions | Nesfatin-1 has anorexigenic effects | Carr et al., 2015 [65] | |
| Case-control study | High nesfatin-1 levels in the patients under hemodialysis vs. healthy subjects Significant positive correlation between nesfatin-1 levels and malnutrition inflammation score and the increased interleukin-6 levels Significant negative correlation with body mass index (BMI) | Nesfatin-1 is associated with the nutritional status in end-stage renal disease | Elthakaby et al., 2022 [66] | ||
| Case-control study | Higher serum nesfatin-1 levels in the obese group Nesfatin-1 positively correlates with copeptin, serum insulin level, and homeostasis model assessment of insulin resistance | Nesfatin-1 might be involved in the appearance of insulin resistance Nesfatin-1 regulates food intake | Yin et al., 2020 [67] | ||
| Case-control study | Lower nesfatin-1 level in the metabolic-associated fatty liver disease (MAFLD) group Nesfatin-1 correlated negatively with high-sensitivity-C reactive protein (hs-CRP), alanine aminotransferase, and fasting glucose | Nesfatin-1 could be a new diagnostic biomarker for MAFLD | Afifi et al., 2024 [68] | ||
| Diabetes Mellitus | Case-control study | Nesfatin-1 levels are significantly lower in the healthy obese and diabetic group in comparison to healthy individuals, being the highest in the underweight group | Nesfatin-1 may be part of the common pathogenetic pathway between diabetes and obesity | Samani et al., 2019 [69] | |
| 3T3-L1 mouse adipocytes Human adipocytes (omental visceral adipose tissue) | Nesfatin-1 expression was increased in hypoxic murine adipocytes Nesfatin-1 was highly detectable in the visceral adipose tissue of obese subjects vs. controls Nesfatin-1 levels correlated with binge eating, sweets craving, and hyperphagic behavior | Nesfatin-1 may be an indicator of eating disorders in obese patients | Caroleo et al., 2023 [70] | ||
| Case-control study | Serum nesfatin-1 level was the highest in the patients with diabetes mellitus type 2 (DMT2) Higher nesfatin-1 level DMT2 and pre-diabetic patients vs. controls Nesfatin-1 positively correlated with glucose, insulin resistance, and lipid profile parameters | Nesfatin-1 could be a predictor biomarker for pre-diabetes and DMT2 | Matta et al., 2022 [58] | ||
| Case-control study | Higher serum nesfatin-1 levels in gestational diabetes mellitus patients Nesfatin-1 is associated with blood glucose, HOMA-IR. and high-density lipoprotein cholesterol (HDL-C) | Nesfatin-1 has diagnostic value for gestational diabetes | Jiang et al., 2020 [71] | ||
| Case-control study | Lower nesfatin-1 levels in the gestational diabetes mellitus group | Nesfatin-1 is an alternative screening method to the conventional oral glucose tolerance test (OGTT) | Çaltekin et al., 2021 [72] | ||
| Polycystic ovary syndrome (PCOS) | Case-control study | Higher nesfatin-1 levels in PCOS patients Normal nesfatin-1 levels after treatment with Metformin | Nesfatin-1 can be a diagnostic biomarker for PCOS Nesfatin-1 levels can indicate the response to treatment | Mahmood et al., 2023 [73] | |
| Case-control study | Lower nesfatin-1 levels in PCOS patients Negative correlations between nesfatin-1 levels, visceral adiposity index, BMI, HOMA-IR, and triglyceride levels | Low nesfatin-1 levels may play an important role in the pathogenesis of PCOS | Çaltekin et al., 2021 [74] | ||
| Cardiovascular disorders | Ischemia/reperfusion injury | C57BL/6 mice H9c2 cardiomyocytes | Ischemia led to down-regulation of NUCB2 transcription with low nesfatin-1 levels Wortmannin (inhibitor of Akt/ERK pathway) abolished the beneficial effects of nesfatin-1 Activation of the Akt/ERK pathway reduces endoplasmic reticulum stress | Nesfatin-1 attenuates ischemia-reperfusion stress | Su et al., 2021 [75] |
| Myocardial infarction, angina pectoris | Adult male Wistar rats | Restoration of SOD and the GSH content by nesfatin-1 Nesfatin-1 inhibited autophagy and apoptosis (reduction of caspase 3 and Bax) | Nesfatin-1 could be used to treat myocardial infarction | Naseroleslami et al., 2020 [76] | |
| Case-control study | Significantly lower nesfatin-1 levels in stable-angina pectoris and acute-myocardial infarction patients No significant difference between the stable-angina pectoris group and the acute-myocardial infarction group Negative correlation between nesfatin-1 low-density lipoproteins, coronary atherosclerosis status (Gensini score), troponin T, and creatine kinase-MB | Nesfatin-1 is involved in the pathogenesis of atherosclerosis and myocardial infarction | Nejati et al., 2021 [77] | ||
| Coronary artery disease (CAD) | Case-control study | Low nesfatin-1 levels in the CAD groups (stable chronic CAD and unstable angina) vs. controls Nesfatin-1 correlated negatively with the Gensini score used to quantify the severity of CAD Nesfatin-1 is closely associated with high-sensitivity-C reactive protein (hsCRP) levels | Nesfatin-1 is negatively associated with the presence and severity of CAD | Kadoglou et al., 2021 [78] | |
| Normotension, Hypotension | Male Sprague-Dawley rats | Nesfatin-1 increases catecholamine, vasopressin, and renin concentrations Significant increase in the mean arterial pressure in both normotensive and hypotensive rats | Nesfatin-1 has pressor effects and regulates sympathetic activity | Yilmaz et al., 2015 [79] | |
| Rheumatologic disorders | Rheumatoid arthritis (RA) | Case-control study | Positive relationship between nesfatin-1, IL-1β, and TNF-alfa levels in synovium and synovial fluid samples Nesfatin-1 synovial levels correlated positively with rheumatoid factor Nesfatin-1 has a sensibility of 77.5% and a specificity of 60% in differentiating patients with RA from healthy individuals | Synovial nesfatin-1 is involved in the inflammatory process in RA and correlates with the severity of the disease | Zhang et al., 2021 [80] |
| Case-control study | Nesfatin-1 serum level was higher in the RA group High nesfatin-1 level correlates with CRP and N-terminal propeptide of type I procollagen (P1NP), a biomarker of the formation of bone matrix | Nesfatin-1 might regulate the function of osteoblasts in RA patients | Seewordova et al., 2022 [81] | ||
| Synovial fibroblasts from patients with RA | Higher expression of NUCB2 mRNA in the synovium in the patients with RA The high expression of NUCB2 correlated with other genes controlling DNA replication, intercellular adhesion, and the interaction with the surrounding extracellular matrix | Nesfatin-1 is involved in the pathogenesis and progression of RA | Zhang et al., 2022 [82] | ||
| Reproductive disorders | Testicular function | Mice testis | Nesfatin-1 had an increased stimulatory role in testosterone production Nesfatin-1 increased intratesticular glucose transport It accelerated cell proliferation and decreased apoptosis; higher sperm production | Nesfatin-1 regulates testicular function | Ranjan et al., 2019 [83] |
| Puberty | Male parks strain mice Testes from pre-pubertal mice | Nesfatin-1 facilitated GnRH-R expression, the production of steroid hormones, spermatogenic biomarkers (Bcl2, PCNA), glucose-metabolism-related proteins (GLUT8, insulin receptor) | Nesfatin-1 facilitates spermatogenesis and steroidogenesis Nesfatin-1 promotes pubertal transition | Ranjan et al., 2019 [84] | |
| Testicular torsion | Male Sprague-Dawley rats | Nesfatin-1 induced a lower percentage of spermatozoa with head defects and lower levels of pro-inflammatory cytokines: TNF-α, IL-6, caspase-3 | Nesfatin-1 prevents the degeneration of spermatogenic cells induced by torsion | Arabaci et al., 2018 [85] | |
| Pregnancy/Abortion | CBA⁄j female mice, BALB⁄c male mice, and DBA⁄2 male mice | The level of nesfatin-1 was higher in the uteri of the abortion animals The expression in the uterus might be down-regulated by cytokines and chemokines in pregnancy | Nesfatin-1 expression might be involved in the maintenance of pregnancy | Chung et al., 2017 [86] | |
| Other diseases | Acute lung injury (ALI) | Mice BEAS-2B human alveolar epithelial cell line | Administration of nesfatin-1 reduced oxidative stress and inflammation both in mouse lung tissue and BEAS-2B cells Nesfatin-1 inhibits inflammatory pathways downstream of HMGB-1 | Nesfatin-1 ameliorates inflammation in ALI | Wang et al., 2020 [87] |
| Osteoarthritis (OA) | Sprague-Dawley rats | Nesfatin-1 inhibits MAPK, the IL-1β activation of NF-kB and Bax/Bcl-2 signaling pathways in chondrocytes Nesfatin-1 has anti-inflammatory functions and reduces the levels of MMPs, caspase-3, COX-2, and IL-6 | Nesfatin-1 has a protective role in OA | Jiang et al., 2020 [88] | |
| Depression | Case-control study | Nesfatin-1 serum levels significantly higher in adolescents with major depressive disorders Positive correlation between nesfatin-1 levels and Hamilton Depression Rating Scale (HAMD-17) used to assess the severity of the disorder | Nesfatin-1 may be a diagnostic and disease-severity biomarker for depression | Sun et al., 2023 [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damian-Buda, A.-C.; Matei, D.M.; Ciobanu, L.; Damian-Buda, D.-Z.; Pop, R.M.; Buzoianu, A.D.; Bocsan, I.C. Nesfatin-1: A Novel Diagnostic and Prognostic Biomarker in Digestive Diseases. Biomedicines 2024, 12, 1913. https://doi.org/10.3390/biomedicines12081913
Damian-Buda A-C, Matei DM, Ciobanu L, Damian-Buda D-Z, Pop RM, Buzoianu AD, Bocsan IC. Nesfatin-1: A Novel Diagnostic and Prognostic Biomarker in Digestive Diseases. Biomedicines. 2024; 12(8):1913. https://doi.org/10.3390/biomedicines12081913
Chicago/Turabian StyleDamian-Buda, Adriana-Cezara, Daniela Maria Matei, Lidia Ciobanu, Dana-Zamfira Damian-Buda, Raluca Maria Pop, Anca Dana Buzoianu, and Ioana Corina Bocsan. 2024. "Nesfatin-1: A Novel Diagnostic and Prognostic Biomarker in Digestive Diseases" Biomedicines 12, no. 8: 1913. https://doi.org/10.3390/biomedicines12081913
APA StyleDamian-Buda, A.-C., Matei, D. M., Ciobanu, L., Damian-Buda, D.-Z., Pop, R. M., Buzoianu, A. D., & Bocsan, I. C. (2024). Nesfatin-1: A Novel Diagnostic and Prognostic Biomarker in Digestive Diseases. Biomedicines, 12(8), 1913. https://doi.org/10.3390/biomedicines12081913









