Icaritin Exerts Anti-Cancer Effects through Modulating Pyroptosis and Immune Activities in Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds and Antibodies
2.2. Colony Formation Assay
2.3. Cytotoxicity Assays
2.4. LDH Release Assay
2.5. Annexin V-FITC/PI Staining
2.6. Pyroptosis-Related Inhibitors
2.7. Western Blotting
2.8. Co-Culture of THP-1 Cells with HepG2 Cells
2.9. Quantitative PCR Analyses
2.10. ELISA
2.11. Cell Transfection and siRNA Knockdown
2.12. Orthotopic Liver Cancer Mice Model
2.13. H&E Staining
2.14. Immunohistochemistry
2.15. Multiplex Immunohistochemistry
2.16. Bio-Plex Multiplex Mouse Cytokine 23-Plex Assay
2.17. Statistical Analysis
3. Results
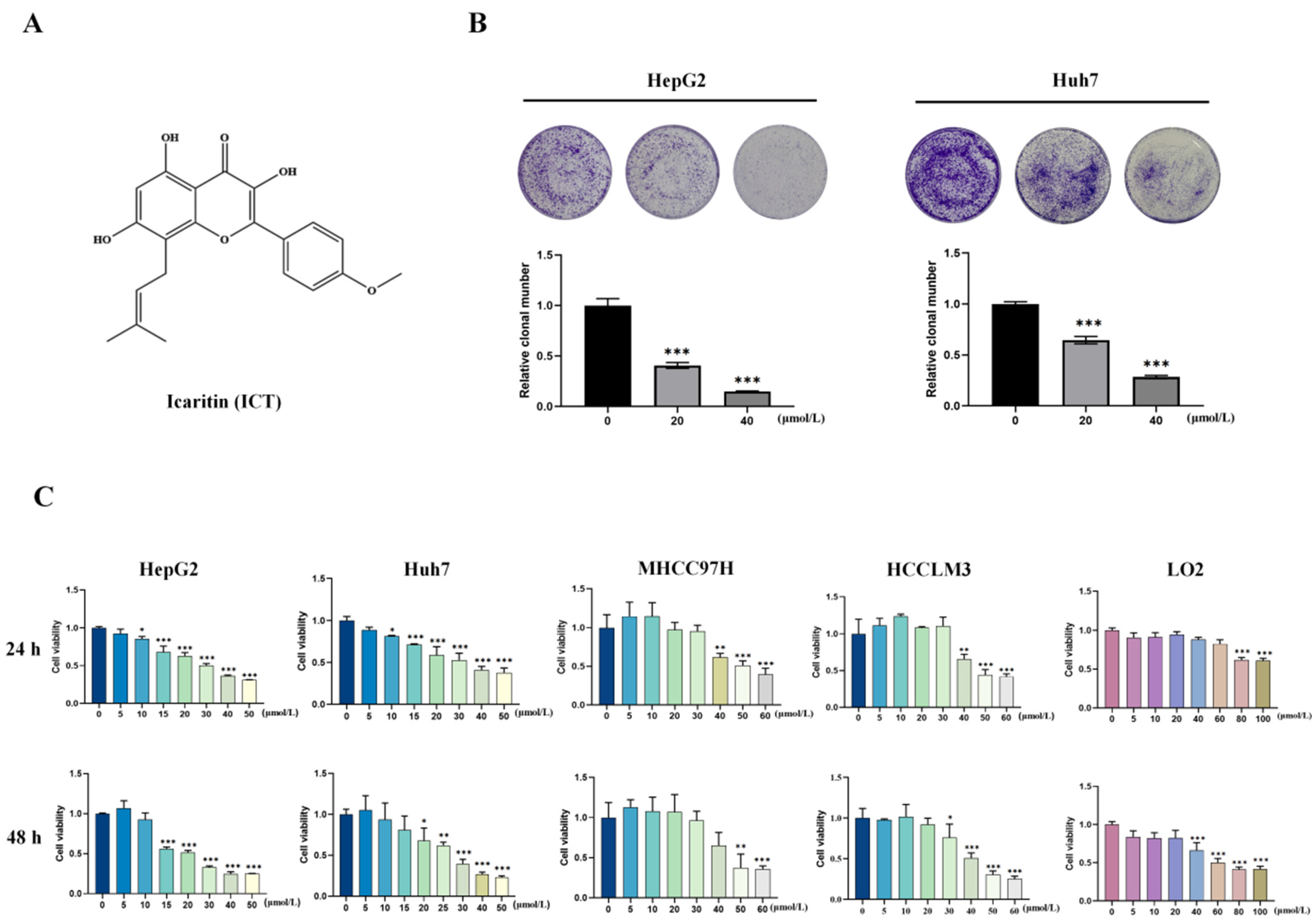
3.1. ICT Inhibited Survival and Proliferation of HCC Cells
3.2. ICT Induced Pyroptosis in HCC Cells
3.3. The Caspase1-GSDMD and Caspase3-GSDME Pathways Are Involved in ICT-Triggered Pyroptosis
3.4. ICT-Induced Polarization of Tumor-Associated Macrophages In Vitro
3.5. ICT Inhibits Tumor Growth by Regulating Pyroptosis and Immune Response In Vivo
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, X.; Wang, Z.; Huang, L.; Xie, Y.; Jiang, S.; Feng, B. eALT-F: A New Non-Invasive Staging Method to Identify Medium to High-Risk Patients with HCC from Ultra-High HBV Viral Load Population—China, 2010–2023. China CDC Wkly. 2023, 5, 1107–1114. [Google Scholar] [PubMed]
- Zhou, Z.; He, H.; Wang, K.; Shi, X.; Wang, Y.; Su, Y.; Wang, Y.; Li, D.; Liu, W.; Zhang, Y.; et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 2020, 368, eaaz7548. [Google Scholar] [CrossRef]
- Liu, J.; Hong, M.; Li, Y.; Chen, D.; Wu, Y.; Hu, Y. Programmed Cell Death Tunes Tumor Immunity. Front. Immunol. 2022, 13, 847345. [Google Scholar] [CrossRef]
- Guo, Z.; Su, Z.; Wei, Y.; Zhang, X.; Hong, X. Pyroptosis in glioma: Current management and future application. Immunol. Rev. 2023, 321, 152–168. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Z.; Zhu, J.; Liu, Z.; Zhang, Y.; Qin, G.; Ren, J.; Qu, X. Bioorthogonal Disruption of Pyroptosis Checkpoint for High-Efficiency Pyroptosis Cancer Therapy. J. Am. Chem. Soc. 2023, 145, 16658–16668. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, H.; Yuan, Z.; Zundell, J.; Towers, M.; Lin, J.; Lombardi, S.; Nie, H.; Murphy, B.; Yang, T.; et al. Targeting the mevalonate pathway suppresses ARID1A-inactivated cancers by promoting pyroptosis. Cancer Cell 2023, 41, 740–756.e10. [Google Scholar] [CrossRef] [PubMed]
- Khanova, E.; Wu, R.; Wang, W.; Yan, R.; Chen, Y.; French, S.W.; Llorente, C.; Pan, S.Q.; Yang, Q.; Li, Y.; et al. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology 2018, 67, 1737–1753. [Google Scholar]
- Hou, J.; Zhao, R.; Xia, W.; Chang, C.W.; You, Y.; Hsu, J.M.; Nie, L.; Chen, Y.; Wang, Y.C.; Liu, C.; et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020, 22, 1264–1275. [Google Scholar]
- Liu, J.; Du, S.; Kong, Q.; Zhang, X.; Jiang, S.; Cao, X.; Li, Y.; Li, C.; Chen, H.; Ding, Z.; et al. HSPA12A attenuates lipopolysaccharide-induced liver injury through inhibiting caspase-11-mediated hepatocyte pyroptosis via PGC-1α-dependent acyloxyacyl hydrolase expression. Cell Death Differ. 2020, 27, 2651–2667. [Google Scholar] [CrossRef] [PubMed]
- Gaul, S.; Alegre, A.; Kaufmann, F. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. J. Eur. Assoc. Study Liver 2021, 74, 156–167. [Google Scholar]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, Z. Cancer-associated pyroptosis: A new license to kill tumor. Front. Immunol. 2023, 14, 1082165. [Google Scholar] [CrossRef]
- Magnani, L.; Colantuoni, M.; Mortellaro, A. Gasdermins: New Therapeutic Targets in Host Defense, Inflammatory Diseases, and Cancer. Front. Immunol. 2022, 13, 898298. [Google Scholar] [CrossRef]
- Li, M.; Jiang, P.; Yang, Y.; Xiong, L.; Wei, S.; Wang, J.; Li, C. The role of pyroptosis and gasdermin family in tumor progression and immune microenvironment. Exp. Hematol. Oncol. 2023, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zhao, M.; Yang, Y.; Xie, Y.; Li, Z.; Zhou, L.; Shang, R.; Zhou, P. The role of pyroptosis in hepatocellular carcinoma. Cell. Oncol. 2023, 46, 811–823. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Xia, S.; Kong, Q.; Li, S.; Liu, X.; Junqueira, C.; Meza-Sosa, K.F.; Mok, T.M.Y.; Ansara, J.; et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020, 579, 415–420. [Google Scholar] [CrossRef]
- Li, X.; Zhang, T.; Kang, L.; Xin, R.; Sun, M.; Chen, Q.; Pei, J.; Chen, Q.; Gao, X.; Lin, Z. Apoptotic caspase-7 activation inhibits non-canonical pyroptosis by GSDMB cleavage. Cell Death Differ. 2023, 30, 2120–2134. [Google Scholar] [CrossRef]
- Atabaki, R.; Khaleghzadeh-Ahangar, H.; Esmaeili, N.; Mohseni-Moghaddam, P. Role of Pyroptosis, a Pro-inflammatory Programmed Cell Death, in Epilepsy. Cell. Mol. Neurobiol. 2022, 43, 1049–1059. [Google Scholar] [CrossRef]
- Hu, M.; Deng, F.; Song, X.; Zhao, H.; Yan, F. The crosstalk between immune cells and tumor pyroptosis: Advancing cancer immunotherapy strategies. J. Exp. Clin. Cancer Res. 2024, 43, 190. [Google Scholar] [CrossRef]
- Chai, R.; Li, Y.; Shui, L.; Ni, L.; Zhang, A. The role of pyroptosis in inflammatory diseases. Front. Cell Dev. Biol. 2023, 11, 1173235. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Shi, X.; Ding, J.; Liu, W.; He, H.; Wang, K.; Shao, F. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 2017, 547, 99–103. [Google Scholar] [CrossRef]
- Shao, F. Gasdermins: Making pores for pyroptosis. Nat. Rev. Immunol. 2021, 21, 620–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H. Chemotherapy drugs induce pyroptosis through caspase-3-dependent cleavage of GSDME. Sci. China Life Sci. 2018, 61, 129–130. [Google Scholar]
- Liu, X.; Lieberman, J. A Mechanistic Understanding of Pyroptosis: The Fiery Death Triggered by Invasive Infection. Adv. Immunol. 2017, 135, 81–117. [Google Scholar]
- Chen, D.; Guo, S.; Tang, X.; Rong, Y.; Bo, H.; Shen, H.; Zhao, Z.; Qiao, A.; Shen, J.; Wang, J. Combination of ruthenium (II) polypyridyl complex Δ-Ru1 and Taxol enhances the anti-cancer effect on Taxol-resistant cancer cells through Caspase-1/GSDMD-mediated pyroptosis. J. Inorg. Biochem. 2022, 230, 111749. [Google Scholar]
- Defourny, J.; Aghaie, A.; Perfettini, I.; Avan, P.; Delmaghani, S.; Petit, C. Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proc. Natl. Acad. Sci. USA 2019, 116, 8010–8017. [Google Scholar] [CrossRef]
- Yang, X.; Tang, Z. Role of gasdermin family proteins in cancers (Review). Int. J. Oncol. 2023, 63, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, J.; Cui, J.; Wang, Y.; Jin, Y.; Zhang, D.; Lin, D.; Lin, J. Tumor-associated macrophages in tumor progression and the role of traditional Chinese medicine in regulating TAMs to enhance antitumor effects. Front. Immunol. 2022, 13, 1026898. [Google Scholar] [CrossRef]
- Jia, W.; Yuan, J.; Cheng, B.; Ling, C. Targeting tumor-derived exosome-mediated premetastatic niche formation: The metastasis-preventive value of traditional Chinese medicine. Cancer Lett. 2023, 567, 216261. [Google Scholar] [CrossRef]
- Fan, Y.; Li, S.; Ding, X.; Yue, J.; Jiang, J.; Zhao, H.; Hao, R.; Qiu, W.; Liu, K.; Li, Y.; et al. First-in-class immune-modulating small molecule Icaritin in advanced hepatocellular carcinoma: Preliminary results of safety, durable survival and immune biomarkers. BMC Cancer 2019, 19, 279. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, Y.; Yang, H.; Hu, Y.; Li, X. Nanomedicine-boosting icaritin-based immunotherapy of advanced hepatocellular carcinoma. Mil. Med. Res. 2022, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Zhang, W.; Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed. Pharmacother. 2022, 151, 113180. [Google Scholar] [CrossRef]
- Huang, C.; Li, Z.; Zhu, J.; Chen, X.; Hao, Y.; Yang, R.; Huang, R.; Zhou, J.; Wang, Z.; Xiao, W.; et al. Systems pharmacology dissection of Epimediumtargeting tumor microenvironment to enhance cytotoxic T lymphocyte responses in lung cancer. Aging 2021, 13, 2912–2940. [Google Scholar]
- Zhou, J.; Wu, J.; Chen, X.; Fortenbery, N.; Eksioglu, E.; Kodumudi, K.N.; Pk, E.-B.; Dong, J.; Djeu, J.Y.; Wei, S. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int. Immunopharmacol. 2011, 11, 890–898. [Google Scholar] [CrossRef]
- Bailly, C. Molecular and cellular basis of the anticancer activity of the prenylated flavonoid icaritin in hepatocellular carcinoma. Chem. Biol. Interact. 2020, 325, 109124. [Google Scholar] [CrossRef]
- Hu, J.; Yang, T.; Xu, H.; Hu, M.; Wen, H.; Jiang, H. A novel anticancer agent icaritin inhibited proinflammatory cytokines in TRAMP mice. Int. Urol. Nephrol. 2016, 48, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Jiang, W.; Xue, J.; Cheng, Y.; Wang, J.; Yang, R.; Zhang, X. Icaritin promotes apoptosis and inhibits proliferation by down-regulating AFP gene expression in hepatocellular carcinoma. BMC Cancer 2021, 21, 318. [Google Scholar] [CrossRef]
- Tao, H.; Liu, M.; Wang, Y.; Luo, S.; Xu, Y.; Ye, B.; Zheng, L.; Meng, K.; Li, L. Icaritin Induces Anti-tumor Immune Responses in Hepatocellular Carcinoma by Inhibiting Splenic Myeloid-Derived Suppressor Cell Generation. Front. Immunol. 2021, 12, 609295. [Google Scholar] [CrossRef]
- Wei, Z.; Gao, R.; Sun, Z.; Yang, W.; He, Q.; Wang, C.; Zhang, J.; Zhang, X.; Guo, L.; Wang, S. Baicalin inhibits influenza A (H1N1)-induced pyroptosis of lung alveolar epithelial cells via caspase-3/GSDME pathway. J. Med. Virol. 2023, 95, e28790. [Google Scholar] [PubMed]
- Xue, C.; Gu, X.; Zheng, Q.; Shi, Q.; Yuan, X.; Chu, Q.; Jia, J.; Su, Y.; Bao, Z.; Lu, J.; et al. Effects of 3-HAA on HCC by Regulating the Heterogeneous Macrophages-A scRNA-Seq Analysis. Adv. Sci. 2023, 10, e2207074. [Google Scholar]
- Kong, X.; Gao, M.; Liu, Y.; Zhang, P.; Li, M.; Ma, P.; Shang, P.; Wang, W.; Liu, H.; Zhang, Q.; et al. GSDMD-miR-223-NLRP3 axis involved in B(a)P-induced inflammatory injury of alveolar epithelial cells. Ecotoxicol. Environ. Saf. 2022, 232, 113286. [Google Scholar]
- Xiang, J.; Zhang, N.; Sun, H.; Su, L.; Zhang, C.; Xu, H.; Feng, J.; Wang, M.; Chen, J.; Liu, L.; et al. Disruption of SIRT7 Increases the Efficacy of Checkpoint Inhibitor via MEF2D Regulation of Programmed Cell Death 1 Ligand 1 in Hepatocellular Carcinoma Cells. Gastroenterology 2020, 158, 664–678.e24. [Google Scholar] [CrossRef]
- Zhou, B.; Yan, J.; Guo, L.; Zhang, B.; Liu, S.; Yu, M.; Chen, Z.; Zhang, K.; Zhang, W.; Li, X.; et al. Hepatoma cell-intrinsic TLR9 activation induces immune escape through PD-L1 upregulation in hepatocellular carcinoma. Theranostics 2020, 10, 6530–6543. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Jiang, M.; Chu, Y.; Wang, W.; Chen, D.; Li, X.; Zhang, Z.; Zhang, D.; Fan, D.; Nie, Y.; et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J. Hepatol. 2017, 68, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Pitt, J.M.; Li, Q.; Yang, H. The renaissance of anti-neoplastic immunity from tumor cell demise. Immunol. Rev. 2017, 280, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Xu, J.; Zhang, B.; Liu, J.; Liang, C.; Hua, J.; Meng, Q.; Yu, X.; Shi, S. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 2020, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, J.; Zhang, C. What role does pyroptosis play in cancer? Mol. Metab. 2022, 65, 101587. [Google Scholar] [PubMed]
- Khan, M.; Ai, M.; Du, K.; Song, J.; Wang, B.; Lin, J.; Ren, A.; Chen, C.; Huang, Z.; Qiu, W.; et al. Pyroptosis relates to tumor microenvironment remodeling and prognosis: A pan-cancer perspective. Front. Immunol. 2022, 13, 1062225. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [PubMed]
- Kang, R.; Xie, Y.; Zhang, Q.; Hou, W.; Jiang, Q.; Zhu, S.; Liu, J.; Zeng, D.; Wang, H.; Bartlett, D.L.; et al. Intracellular HMGB1 as a novel tumor suppressor of pancreatic cancer. Cell Res. 2017, 27, 916–932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Ding, J.; Wang, C.; Zhou, X.; Gao, W.; Huang, H.; Shao, F.; Liu, Z. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 2020, 579, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Naugler, W.E.; Sakurai, T.; Kim, S.; Maeda, S.; Kim, K.; Elsharkawy, A.M.; Karin, M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007, 317, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Zhao, E.; Kryczek, I.; Vatan, L.; Sadovskaya, A.; Ludema, G.; Simeone, D.M.; Zou, W.; Welling, T.H. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 2014, 147, 1393–1404. [Google Scholar] [CrossRef]






| Primers | Sequences (5′-3′) |
|---|---|
| IL-6 | F: CGGGAACGAAAGAGAAGCTCTA |
| IL-6 | R: CGCTTGTGGAGAAGGAGTTCA |
| IL-10 | F: TCAAGGCGCATGTGAACTCC |
| IL-10 | R: GATGTCAAACTCACTCATGGCT |
| CD163 | F: CAGCGGCTTGCAGTTTCCTC |
| CD163 | R: TGGCCTCCTTTTCCATTCCAGA |
| COX-2 | F: CCACCCGCAGTACAGAAAGT |
| COX-2 | R: CAGGATACAGCTCCACAGCA |
| TNF-α | F: GAGGCCAAGCCCTGGTATG |
| TNF-α | R: CGGGCCGATTGATCTCAGC |
| β-actin | F: CTCTTCCAGCCTTCCTTCCT |
| β-actin | R: CAGGGCAGTGATCTCCTTCT |
| Primers | Sequences (5′-3′) |
|---|---|
| SiGSDMD-1 | F: GAGCUUCCACUUCUACGAUTT |
| SiGSDMD-1 | R: AUCGUAGAAGUGGAAGCUCTT |
| SiGSDMD-2 | F: GACACAGAAGGAGGUGGAATT |
| SiGSDMD-2 | R: UUCCACCUCCUUCUGUGUCTT |
| SiGSDMD-3 | F: GCCAUCUGAGCCAGAAGAATT |
| SiGSDMD-3 | R: UUCUUCUGGCUCAGAUGGCTT |
| SiGSDMD-4 | F: CCACAACUUCCUGACAGAUTT |
| SiGSDMD-4 | R: AUCUGUCAGGAAGUUGUGGTT |
| SiGSDME-1 | F: GGUGACCUGAUUGCAGUAUTT |
| SiGSDME-1 | R: AUACUGCAAUCAGGUCACCTT |
| SiGSDME-2 | F: GCAGCAAGCAGCUGUUUAUTT |
| SiGSDME-2 | R: AUAAACAGCUGCUUGCUGCTT |
| SiGSDME-3 | F: GGAUUGUGCAGCGCUUGUUTT |
| SiGSDME-3 | R: AACAAGCGCUGCACAAUCCTT |
| SiGSDME-4 | F: GCUGCGCAUGGGAUAUCUUTT |
| SiGSDME-4 | R: AAGAUAUCCCAUGCGCAGCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Li, W.; Yang, W.; Wang, M.; Xing, Y.; Wang, S. Icaritin Exerts Anti-Cancer Effects through Modulating Pyroptosis and Immune Activities in Hepatocellular Carcinoma. Biomedicines 2024, 12, 1917. https://doi.org/10.3390/biomedicines12081917
Jiao Y, Li W, Yang W, Wang M, Xing Y, Wang S. Icaritin Exerts Anti-Cancer Effects through Modulating Pyroptosis and Immune Activities in Hepatocellular Carcinoma. Biomedicines. 2024; 12(8):1917. https://doi.org/10.3390/biomedicines12081917
Chicago/Turabian StyleJiao, Yuanyuan, Wenqian Li, Wen Yang, Mingyu Wang, Yaling Xing, and Shengqi Wang. 2024. "Icaritin Exerts Anti-Cancer Effects through Modulating Pyroptosis and Immune Activities in Hepatocellular Carcinoma" Biomedicines 12, no. 8: 1917. https://doi.org/10.3390/biomedicines12081917
APA StyleJiao, Y., Li, W., Yang, W., Wang, M., Xing, Y., & Wang, S. (2024). Icaritin Exerts Anti-Cancer Effects through Modulating Pyroptosis and Immune Activities in Hepatocellular Carcinoma. Biomedicines, 12(8), 1917. https://doi.org/10.3390/biomedicines12081917




