miRNA Profiles in Patients with Hematological Malignancy at Different Stages of the Disease: A Preliminary Study
Abstract
:1. Background
2. Methods
2.1. Subjects and Selection Criteria
- ND: A patient who is newly diagnosed with the disease (leukemia/lymphoma) but has not started any form of treatment. Samples were taken at diagnosis.
- Rem: A leukemia/lymphoma patient who received a course of treatment and their medical tests show no signs of the disease.
- Res: A leukemia/lymphoma patient who received treatment but failed to show any signs of improvement. Some patients who provided blood samples eventually passed away months later.
2.2. Sample Collection, Handling, and Transport
2.3. Molecular Studies
2.3.1. miRNA Extraction and cDNA Synthesis
2.3.2. miRNA Quantification by Real-Time qPCR
2.4. Statistical Analysis
2.4.1. Fold Change Calculation
2.4.2. Statistical Testing
2.5. Bioinformatics Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Overview of miRNA Expression across Disease Stages
3.3. miRNA Differential Expression in Newly Diagnosed Leukemia and Lymphoma Patients vs. Healthy Controls
3.4. Comparative Analysis for the Differential Expression of 5 miRNAs in Leukemia and Lymphoma
3.5. Functional Enrichment Analysis for Target Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Abbreviation | Description |
| miRNAs | MicroRNA |
| HM | Hematological malignancy |
| AML | Acute myeloid leukemia |
| T-ALL | T-lymphoblastic leukemia |
| cDNA | Complementary DNA |
| MDS | Myelodysplastic syndrome |
| KAUH | King Abdullah University Hospital |
| IRB | Institutional Review Board |
| rpm | Revolutions per minute |
| μL | Microliter |
| CT | Cycle threshold |
| ∆CT | Delta CT |
| GO | Gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| ND | Newly diagnosed |
| Rem | Remission |
| Res | Resistance |
| PI3K/AKT | Phosphatidylinositol 3-kinase/protein kinase B |
| ERK | Extracellular signal-regulated kinase |
| TORC2 | Target of rapamycin complex 2 |
| MAPKK | Mitogen-activated protein kinase kinase |
| cHL | Classical Hodgkin lymphoma |
| DLBCL | Diffuse large B-cell lymphoma |
| TGF-β | Transforming growth factor-β |
| Cat # | Catalogue number |
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [PubMed]
- Hematologic Malignancies|MEI Pharma [Internet]. Available online: https://meipharma.com/focus/hematologic-malignancies.html (accessed on 25 June 2024).
- Blood Cancers|Leukaemia|Lymphoma|Myeloma|Cancer Council NSW. Available online: https://www.cancercouncil.com.au/blood-cancers/ (accessed on 25 June 2024).
- Alhmoud, J.F.; Mustafa, A.G.; Malki, M.I. Molecular Sciences Targeting DNA Repair Pathways in Hematological Malignancies. Int. J. Mol. Sci. 2020, 21, 7365. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Chen, H.; Collins, A.R.; Connell, M.; Damia, G.; Dasgupta, S.; Malhotra, M.; Meeker, A.K.; Amedei, A.; Amin, A.; et al. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin. Cancer Biol. 2015, 35, S5–S24. [Google Scholar] [CrossRef] [PubMed]
- Boxer, L.M. The role of oncogenes in hematologic malignancies. Annu. Rev. Med. 1994, 45, 1–11. [Google Scholar] [CrossRef]
- Nicot, C. Tumor Suppressor Inactivation in the Pathogenesis of Adult T-Cell Leukemia. J. Oncol. 2015, 2015, 183590. [Google Scholar] [CrossRef] [PubMed]
- Grech, G.; Avellino, R.; Wismayer, P.S. Molecular mechanisms in haematological malignancies. Malta Med. J. 2009, 21, 6–11. [Google Scholar]
- Al-Saihati, H.A.; Al-Toub, M.; Sharaf, H.I.; Singh, M.; Ansari, M.N.; Bin Saeedan, A. Cell Signaling Pathways in Cancer. Mol. Targets Cancer Ther. (Part 1) 2023, 242–272. [Google Scholar] [CrossRef]
- The Ras Pathway and Cancer: Regulation, Challenges and Therapeutic Progress|Technology Networks. Available online: https://www.technologynetworks.com/cell-science/articles/the-ras-pathway-and-cancer-regulation-challenges-and-therapeutic-progress-347806 (accessed on 26 June 2024).
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Lang, W.; Mei, C.; Luo, Y.; Ye, L.; Wang, L.; Zhou, X.; Xu, G.; Ma, L.; Jin, J.; et al. Co-mutation landscape and clinical significance of RAS pathway related gene mutations in patients with myelodysplastic syndrome. Hematol. Oncol. 2022, 41, 159–166. [Google Scholar] [CrossRef]
- Wei, J.F.; Qiu, H.Y.; Chen, Z.; Miao, L.; Wang, Y.; Zhao, L.D.; Cai, Z.M. Clinical Significance of RAS Gene Mutations in Patients with Acute Myeloid Leukemia. J. Exp. Hematol. 2022, 30, 1391–1396. [Google Scholar]
- Dahl, L. Transforming Growth Factor-β1 and Tumor Development. Sci. Insights 2023, 42, 909–914. [Google Scholar] [CrossRef]
- Chan, M.K.K.; Chan, E.L.Y.; Ji, Z.Z.; Chan, A.S.W.; Li, C.; Leung, K.T.; To, K.F.; Tang, P.M.K. Transforming growth factor-β signaling: From tumor microenvironment to anticancer therapy. Explor. Target. Anti-Tumor Ther. 2023, 4, 316–343. [Google Scholar] [CrossRef] [PubMed]
- Steelman, L.S.; Abrams, S.L.; Whelan, J.; Bertrand, F.E.; Ludwig, D.E.; Bäsecke, J.; Libra, M.; Stivala, F.; Milella, M.; Tafuri, A.; et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia 2008, 22, 686–707. [Google Scholar] [CrossRef]
- Milella, M.; Kornblau, S.M.; Estrov, Z.; Carter, B.Z.; Lapillonne, H.; Harris, D.; Konopleva, M.; Zhao, S.; Estey, E.; Andreeff, M. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J. Clin. Investig. 2001, 108, 851. [Google Scholar] [CrossRef] [PubMed]
- What Is Noncoding DNA?: MedlinePlus Genetics. Available online: https://medlineplus.gov/genetics/understanding/basics/noncodingdna/ (accessed on 25 June 2024).
- Li, S.; Ma, Y.; Tan, Y.; Ma, X.; Zhao, M.; Chen, B.; Zhang, R.; Chen, Z.; Wang, K. Profiling and functional analysis of circular RNAs in acute promyelocytic leukemia and their dynamic regulation during all-trans retinoic acid treatment. Cell Death Dis. 2018, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Feng, T.; Xu, J.; Miao, M.; Ji, X.; Zhu, H.; Shao, X.J. Low expression of microRNA-340 confers adverse clinical outcome in patients with acute myeloid leukemia. J. Cell. Physiol. 2018, 234, 4200–4205. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2023, 25, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Sevcikova, A.; Fridrichova, I.; Nikolaieva, N.; Kalinkova, L.; Omelka, R.; Martiniakova, M.; Ciernikova, S. Clinical Significance of microRNAs in Hematologic Malignancies and Hematopoietic Stem Cell Transplantation. Cancers. 2023, 15, 2658. [Google Scholar] [CrossRef] [PubMed]
- Tchernitsa, O.; Kasajima, A.; Schäfer, R.; Kuban, R.J.; Ungethüm, U.; Györffy, B.; Neumann, U.; Simon, E.; Weichert, W.; Ebert, M.P.; et al. Systematic evaluation of the miRNA-ome and its downstream effects on mRNA expression identifies gastric cancer progression. J. Pathol. 2010, 222, 310–319. [Google Scholar] [CrossRef]
- Chakrabortty, A.; Patton, D.J.; Smith, B.F.; Agarwal, P. miRNAs: Potential as Biomarkers and Therapeutic Targets for Cancer. Genes 2023, 14, 1375. [Google Scholar] [CrossRef]
- Han, Y.; Li, H. miRNAs as biomarkers and for the early detection of non-small cell lung cancer (NSCLC). J. Thorac. Dis. 2018, 10, 3119–3131. [Google Scholar] [CrossRef]
- Cui, M.; Wang, H.; Yao, X.; Zhang, D.; Xie, Y.; Cui, R.; Zhang, X. Circulating MicroRNAs in Cancer: Potential and Challenge. Front. Genet. 2019, 10, 434306. [Google Scholar]
- Skipar, P.; Dey, M.; Piątkowski, J.; Sulejczak, D.; Rutkowski, P.; Czarnecka, A.M. MicroRNAs as Prognostic Biomarkers and Therapeutic Targets in Chondrosarcoma. Int. J. Mol. Sci. 2024, 25, 3176. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Cervellera, C.F.; Lanzillotti, C.; Touzé, A.; Gaboriaud, P.; Tognon, M.; Martini, F.; Rotondo, J.C. MicroRNA dysregulations in Merkel cell carcinoma: Molecular mechanisms and clinical applications. J. Med Virol. 2022, 95, e28375. [Google Scholar] [CrossRef] [PubMed]
- Zhi, F.; Cao, X.; Xie, X.; Wang, B.; Dong, W.; Gu, W.; Ling, Y.; Wang, R.; Yang, Y.; Liu, Y.; et al. Identification of Circulating MicroRNAs as Potential Biomarkers for Detecting Acute Myeloid Leukemia. PLoS ONE. 2013, 8, e56718. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, J.; Giefing, M. MicroRNA signature in classical Hodgkin lymphoma. J. Appl. Genet. 2021, 62, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, L.; Cogdell, D.E.; Zheng, H.; Schetter, A.J.; Nykter, M.; Harris, C.C.; Chen, K.; Hamilton, S.R.; Zhang, W. Circulating Plasma MiR-141 Is a Novel Biomarker for Metastatic Colon Cancer and Predicts Poor Prognosis. PLoS ONE 2011, 6, e17745. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Alonci, A.; Campo, S.; Penna, G.; Petrungaro, A.; Gerace, D.; Musolino, C. Circulating microRNAs: New biomarkers in diagnosis, prognosis and treatment of cancer (review). Int. J. Oncol. 2012, 41, 1897–1912. [Google Scholar] [CrossRef] [PubMed]
- Slaby, O.; Svoboda, M.; Fabian, P.; Smerdova, T.; Knoflickova, D.; Bednarikova, M.; Nenutil, R.; Vyzula, R. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology 2007, 72, 397–402. [Google Scholar] [CrossRef]
- Xiang, J.; Wu, J. Feud or Friend? The Role of the miR-17-92 Cluster in Tumorigenesis. Curr. Genom. 2010, 11, 129–135. [Google Scholar] [CrossRef]
- Shahid, S.; Shahid, W.; Shaheen, J.; Akhtar, M.W.; Sadaf, S. Circulating miR-146a expression as a non-invasive predictive biomarker for acute lymphoblastic leukemia. Sci. Rep. 2021, 11, 22783. [Google Scholar] [CrossRef]
- Wong, K.Y.; So, C.C.; Loong, F.; Chung, L.P.; Lam, W.W.L.; Liang, R.; Li, G.K.; Jin, D.Y.; Chim, C.S. Epigenetic inactivation of the miR-124-1 in haematological malignancies. PLoS ONE 2011, 6, e19027. [Google Scholar] [CrossRef] [PubMed]
- Machová Polaková, K.; Lopotová, T.; Klamová, H.; Burda, P.; Trněný, M.; Stopka, T.; Moravcová, J. Expression patterns of microRNAs associated with CML phases and their disease related targets. Mol. Cancer 2011, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Meerson, A.; Ploug, T. Assessment of six commercial plasma small RNA isolation kits using qRT-PCR and electrophoretic separation: Higher recovery of microRNA following ultracentrifugation. Biol. Methods Protoc. 2016, 1, bpw003. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W. [Real time quantitative PCR]. Exp. Mol. Med. 2001, 33 (Suppl. S1), 101–109. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Goni, R.; García, P.; Foissac, S. The qPCR Data Statistical Analysis. Control. 2009, 1, pp. 1–9. Available online: http://www.mendeley.com/research/qpcr-data-statistical-analysis (accessed on 25 June 2024).
- Moyé, L. What can we do about exploratory analyses in clinical trials? Contemp. Clin. Trials 2015, 45, 302–310. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Felekkis, K.; Papaneophytou, C. The Circulating Biomarkers League: Combining miRNAs with Cell-Free DNAs and Proteins. Int. J. Mol. Sci. 2024, 25, 3403. [Google Scholar] [CrossRef]
- Favero, A.; Segatto, I.; Perin, T.; Belletti, B. The many facets of miR-223 in cancer: Oncosuppressor, oncogenic driver, therapeutic target, and biomarker of response. Wiley Interdiscip. Rev. RNA 2021, 12, e1659. [Google Scholar] [CrossRef]
- Yuan, Y.; Kluiver, J.; Koerts, J.; de Jong, D.; Rutgers, B.; Abdul Razak, F.R.; Terpstra, M.; Plaat, B.E.; Nolte, I.M.; Diepstra, A.; et al. miR-24-3p Is Overexpressed in Hodgkin Lymphoma and Protects Hodgkin and Reed-Sternberg Cells from Apoptosis. Am. J. Pathol. 2017, 187, 1343–1355. [Google Scholar] [CrossRef]
- Tominaga, E.; Yuasa, K.; Shimazaki, S.; Hijikata, T. MicroRNA-1 targets Slug and endows lung cancer A549 cells with epithelial and anti-tumorigenic properties. Exp. Cell Res. 2013, 319, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Migliore, C.; Martin, V.; Leoni, V.P.; Restivo, A.; Atzori, L.; Petrelli, A.; Isella, C.; Zorcolo, L.; Sarotto, I.; Casula, G.; et al. MiR-1 downregulation cooperates with MACC1 in promoting MET overexpression in human colon cancer. Clin. Cancer Res. 2012, 18, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Hudson, R.S.; Yi, M.; Esposito, D.; Watkins, S.K.; Hurwitz, A.A.; Yfantis, H.G.; Lee, D.H.; Borin, J.F.; Naslund, M.J.; Alexander, R.B.; et al. MicroRNA-1 is a candidate tumor suppressor and prognostic marker in human prostate cancer. Nucleic Acids Res. 2011, 40, 3689–3703. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Enokida, H.; Chiyomaru, T.; Tatarano, S.; Hidaka, H.; Yamasaki, T.; Gotannda, T.; Tachiwada, T.; Nohata, N.; Yamane, T.; et al. Tumor suppressive microRNA-1 mediated novel apoptosis pathways through direct inhibition of splicing factor serine/arginine-rich 9 (SRSF9/SRp30c) in bladder cancer. Biochem. Biophys. Res. Commun. 2012, 417, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Tang, J.; Qiu, L.; Zhang, Z.; Shi, S.; Xue, L.; Kui, L.; Huang, T.; Nan, W.; Zhou, B.; et al. Semaphorin 4D is a potential biomarker in pediatric leukemia and promotes leukemogenesis by activating PI3K/AKT and ERK signaling pathways. Oncol. Rep. 2021, 45, 1. [Google Scholar] [CrossRef] [PubMed]
- Ghazaryan, A.; Wallace, J.A.; Tang, W.W.; Barba, C.; Lee, S.-H.; Bauer, K.M.; Nelson, M.C.; Kim, C.N.; Stubben, C.; Voth, W.P.; et al. miRNA-1 promotes acute myeloid leukemia cell pathogenesis through metabolic regulation. Front. Genet. 2023, 14, 1192799. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Han, X.; Roy, M.; Liu, W.; Zhao, X.; Liu, J. Differential expression profiles and functional analysis of plasma miRNAs associated with chronic myeloid leukemia phases. Future Oncol. 2018, 15, 763–776. [Google Scholar] [CrossRef]
- Panico, A.; Tumolo, M.R.; Leo, C.G.; De Donno, A.; Grassi, T.; Bagordo, F.; Serio, F.; Idolo, A.; Masi, R.; Mincarone, P.; et al. The influence of lifestyle factors on miRNA expression and signal pathways: A review. Epigenomics 2021, 13, 145–164. [Google Scholar] [CrossRef]
- Xiong, M.; Wang, P.; Pan, B.; Nie, J.; Wang, S.; He, B. The diagnostic and prognostic values of microRNA-196a in cancer. Biosci. Rep. 2021, 41, BSR20203559. [Google Scholar] [CrossRef] [PubMed]
- Coskun, E.; von der Heide, E.K.; Schlee, C.; Goekbuget, N.; Hoelzer, D.; Hofmann, W.-K.; Thiel, E.; Baldus, C.D. The Role of Microrna-196a-1 and Microrna-196b in Acute T-Lymphoblastic Leukemia and Acute Myeloid Leukemia. Blood 2009, 114, 1607. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Zhang, L.; Weakley, S.M.; Yao, Q. MicroRNA-196: Critical roles and clinical applications in development and cancer. J. Cell. Mol. Med. 2011, 15, 14–23. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Hekmatirad, S.; Mofarahe, Z.S.; Asghari, M.H. Exosomal microRNA panels as biomarkers for hematological malignancies. Curr. Probl. Cancer 2021, 45, 100726. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Qie, J.; Fu, Q.; Chen, J.; Jin, Y.; Ding, Z. miR-20a-5p/TGFBR2 Axis Affects Pro-inflammatory Macrophages and Aggravates Liver Fibrosis. Front. Oncol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, D.; Li, S.; Feng, Y.; Li, X.; Chang, W.; Zhang, J.; Sun, Y.; Qing, D.; Chen, G.; et al. Licochalcone A reverses NNK-induced ectopic miRNA expression to elicit in vitro and in vivo chemopreventive effects. Phytomed. Int. J. Phytother. Phytopharm. 2020, 76, 153245. [Google Scholar] [CrossRef]
- Li, J.; Ye, D.; Shen, P.; Liu, X.; Zhou, P.; Zhu, G.; Xu, Y.; Fu, Y.; Li, X.; Sun, J.; et al. Mir-20a-5p induced WTX deficiency promotes gastric cancer progressions through regulating PI3K/AKT signaling pathway. J. Exp. Clin. Cancer Res. 2020, 39, 212. [Google Scholar] [CrossRef] [PubMed]
- Khare, D.; Goldschmidt, N.; Bardugo, A.; Gur-Wahnon, D.; Ben-Dov, I.Z.; Avni, B. Plasma microRNA profiling: Exploring better biomarkers for lymphoma surveillance. PLoS ONE 2017, 12, e0187722. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Ding, Y.; Li, Q.; Wang, R.; Liu, T.; Sun, Q.; Yang, H.; Peng, S.; Wang, W.; et al. MiR-20a Induces Cell Radioresistance by Activating the PTEN/PI3K/Akt Signaling Pathway in Hepatocellular Carcinoma. Int. J. Radiat. Oncol. 2015, 92, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, J.; Janiszewska, J.; Ustaszewski, A.; Bein, J.; Skalski, M.; Dzikiewicz-Krawczyk, A.; Rozwadowska, N.; Hansmann, M.L.; Hartmann, S.; Giefing, M.; et al. Deregulated mirnas contribute to silencing of b-cell specific transcription factors and activation of nf-κb in classical hodgkin lymphoma. Cancers 2021, 13, 3131. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, Z.; Jiang, P.; Zhang, X.; Xu, Y.; Chen, K.; Li, X. MicroRNA-23a promotes pancreatic cancer metastasis by targeting epithelial splicing regulator protein 1. Oncotarget 2017, 8, 82854–82871. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Liang, H.; Fan, Q.; Zhu, R.; Cui, J.; Zhang, W.; Zen, K.; Zhang, C.Y.; Hou, D.; et al. MiR-23a/b promote tumor growth and suppress apoptosis by targeting PDCD4 in gastric cancer. Cell Death Dis. 2017, 8, e3059. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.-Y.; Feng, Y.-G.; Zhang, C.; Chen, F.; Feng, Y. microRNA-23a in Human Cancer: Its Roles, Mechanisms and Therapeutic Relevance. Cancers. 2018, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Yong Jin, H.; Oda, H.; Lai, M.; Skalsky, R.L.; Bethel, K.; Shepherd, J.; Kang, S.G.; Liu, W.H.; Sabouri-Ghomi, M.; Cullen, B.R.; et al. MicroRNA-17B92 plays a causative role in lymphomagenesis by coordinating multiple oncogenic pathways. EMBO J. 2013, 32, 2377–2391. [Google Scholar]
- Du, Y.; Miao, Z.; Wang, K.; Lv, Y.; Qiu, L.; Guo, L. Expression levels and clinical values of miR-92b-3p in breast cancer. World J. Surg. Oncol. 2021, 19, 239. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shan, W.; Hua, Y.; Chao, F.; Cui, Y.; Lv, L.; Dou, X.; Bian, X.; Zou, J.; Li, H.; et al. Exosomal miR-92b-3p Promotes Chemoresistance of Small Cell Lung Cancer Through the PTEN/AKT Pathway. Front. Cell Dev. Biol. 2021, 9, 661602. [Google Scholar] [CrossRef] [PubMed]



| miRNA | Type of Disease | Stage and Fold Change | Trend | |
|---|---|---|---|---|
| hsa-miR-223-3p | Leukemia | Healthy control | ND | Upregulation |
| 1 | 5.97 | |||
| hsa-miR-24-3p | Lymphoma | Healthy control | ND | Upregulation |
| 1 | 2.41 | |||
| miRNA | p-Value | Disease Type | Stage and Fold Change Compared to ND Control | Trend | Potential Clinical Implications | Suggested Prognosis | ||
|---|---|---|---|---|---|---|---|---|
| hsa-miR-1 | 0.059 | Leukemia | ND | Res | Rem | Up regulation | Higher in Rem and ND compared to Res, and higher in Rem compared to ND | Good |
| 1 | 0.38 | 2.43 | ||||||
| hsa-miR-196a-5p | 0.059 | Leukemia | ND | Res | Rem | Up regulation | Very high in Rem compared to Res, also higher in Rem compared to ND, and higher in ND compared to Res | Good |
| 1 | 0.51 | 1161.2 | ||||||
| hsa-miR-20a-5p | 0.077 | Lymphoma | ND | Res | Rem | Up regulation | Higher in Res compared to Rem, and higher in ND compared to Rem, and Res | Poor |
| 1 | 0.87 | 0.00 | ||||||
| hsa-miR-23a-3p | 0.077 | Lymphoma | ND | Res | Rem | Up regulation | Higher in ND compared to Res and Rem, but higher in Res compared to Rem | Poor |
| 1 | 0.44 | 0.01 | ||||||
| hsa-miR-92b-3p | 0.064 | Lymphoma | ND | Res | Rem | Up regulation | Higher in Res compared to ND and Rem, and higher in ND compared to Rem | Poor |
| 1 | 3.67 | 0.38 | ||||||
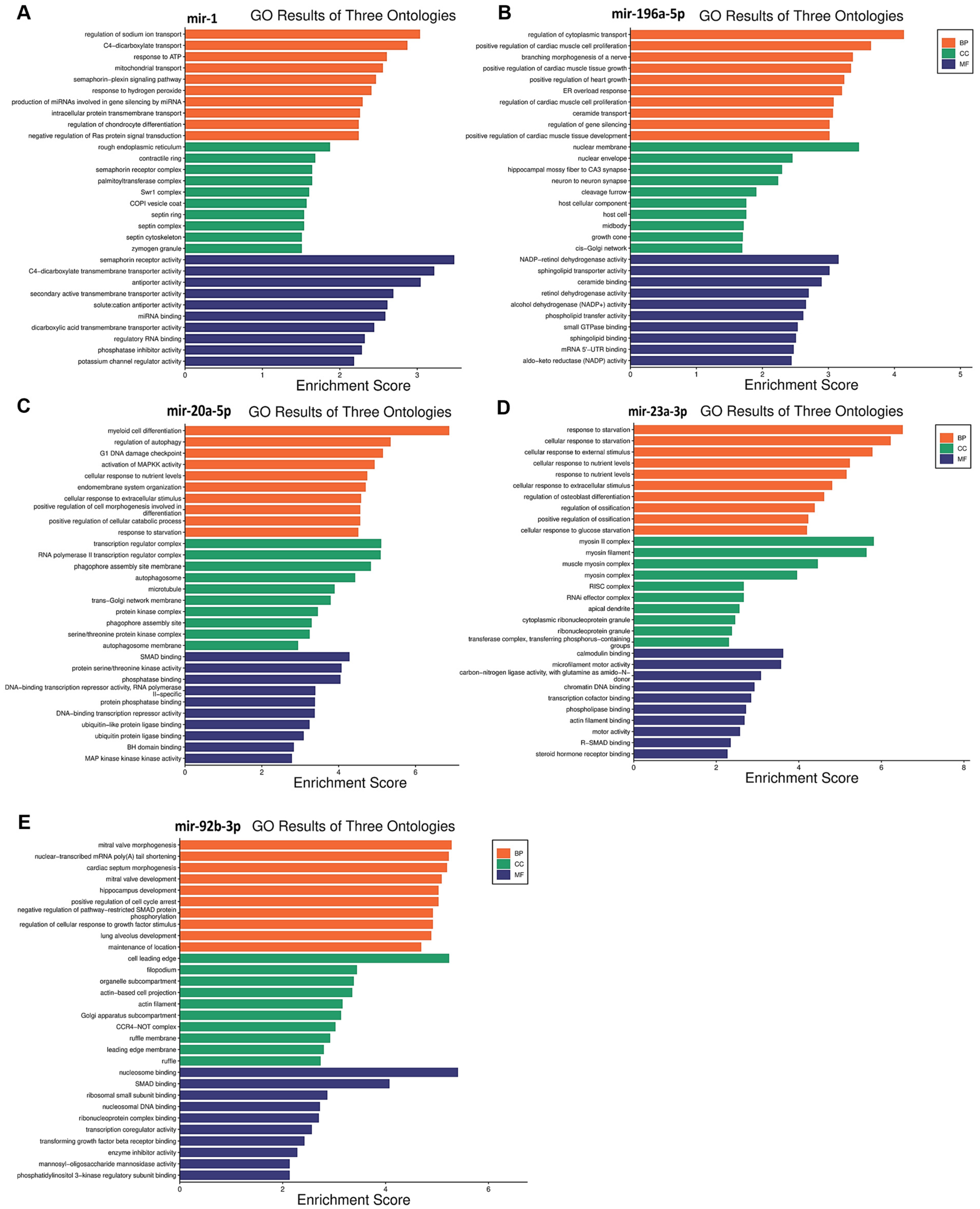
| miRNA | Pathways | p-Value | Target Genes |
|---|---|---|---|
| hsa-miR-1 | semaphorin receptor activity | 0.0003 | PLXNA4/MET |
| RAS protein signal transduction | 0.0390 | G3BP1/RASA1/MET | |
| hsa-miR-196a-5p | small GTPase binding | 0.0029 | RAB29/RCC2/EXOC8/RGL2/SMCR8/GGA3 |
| activation of MAPKK activity | 0.0115 | MAPK1/MAP3K21 | |
| hsa-miR-20a-5p | transforming growth factor beta receptor signaling pathway | 0.0103 | SPRED1/CREB1/CAV1/SMAD7/BAMBI/SKIL/SMAD5/SMAD4/SKI/ZBTB7A |
| regulation of apoptotic signaling pathway | 0.0281 | DNM1L/ZNF385A/BCL2L2/BCL2L11/CAV1/SGMS1/MAPK9/SKIL/FEM1B/TRIM32/HIF1A/MCL1/RB1/RB1CC1/PLAGL2 | |
| hsa-miR-23a-3p | SMAD binding | 0.0465 | SMAD3/DDX5 |
| response to starvation | 0.00003 | TNRC6A/PPARGC1A/FNIP1/TBL2/FAS/FOXO3/KLF10/SESN2/ASNS | |
| hsa-miR-92b-3p | extrinsic apoptotic signaling pathway | 0.0001 | FASLG/SGK3/DAB2IP/BCL2L11/MOAP1/ITGAV/BAK1/DDX3X/ITGA6/MCL1/SGPP1 |
| TORC2 complex | 0.0093 | SMG1/SESN3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashem, J.; Alkhalaileh, L.; Abushukair, H.; Ayesh, M. miRNA Profiles in Patients with Hematological Malignancy at Different Stages of the Disease: A Preliminary Study. Biomedicines 2024, 12, 1924. https://doi.org/10.3390/biomedicines12081924
Hashem J, Alkhalaileh L, Abushukair H, Ayesh M. miRNA Profiles in Patients with Hematological Malignancy at Different Stages of the Disease: A Preliminary Study. Biomedicines. 2024; 12(8):1924. https://doi.org/10.3390/biomedicines12081924
Chicago/Turabian StyleHashem, Jood, Lujain Alkhalaileh, Hassan Abushukair, and Mahmoud Ayesh. 2024. "miRNA Profiles in Patients with Hematological Malignancy at Different Stages of the Disease: A Preliminary Study" Biomedicines 12, no. 8: 1924. https://doi.org/10.3390/biomedicines12081924






