Combination of Anti-CD40 and Anti-CD40L Antibodies as Co-Stimulation Blockade in Preclinical Cardiac Xenotransplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Virological Screening
2.3. Anesthesia, Surgical Procedures and Heart Preservation
2.4. Immunosuppression
2.5. Laboratory Tests
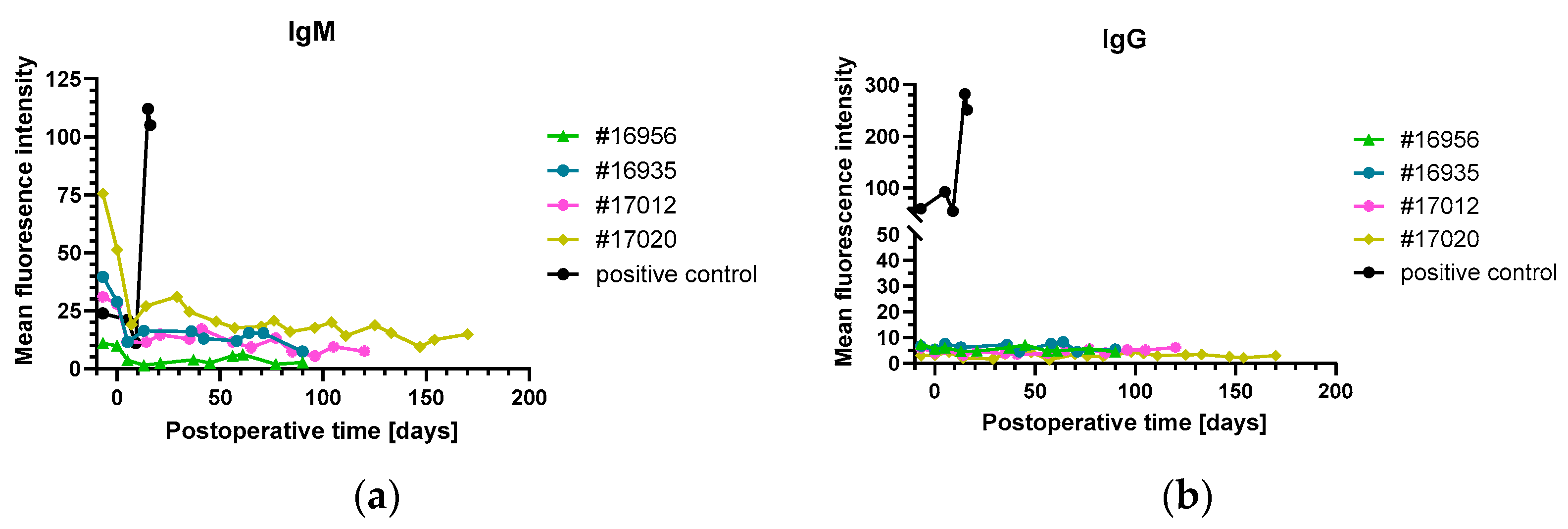
2.6. Assessment of Non-Gal-α(1,3)Gal Xenoreactive Antibody Levels
2.7. Cytokine Secretion Profile
2.8. Necropsy and Histology
2.9. Immunohistochemical Staining
2.10. Immunofluorescence Staining
2.11. Statistics
3. Results
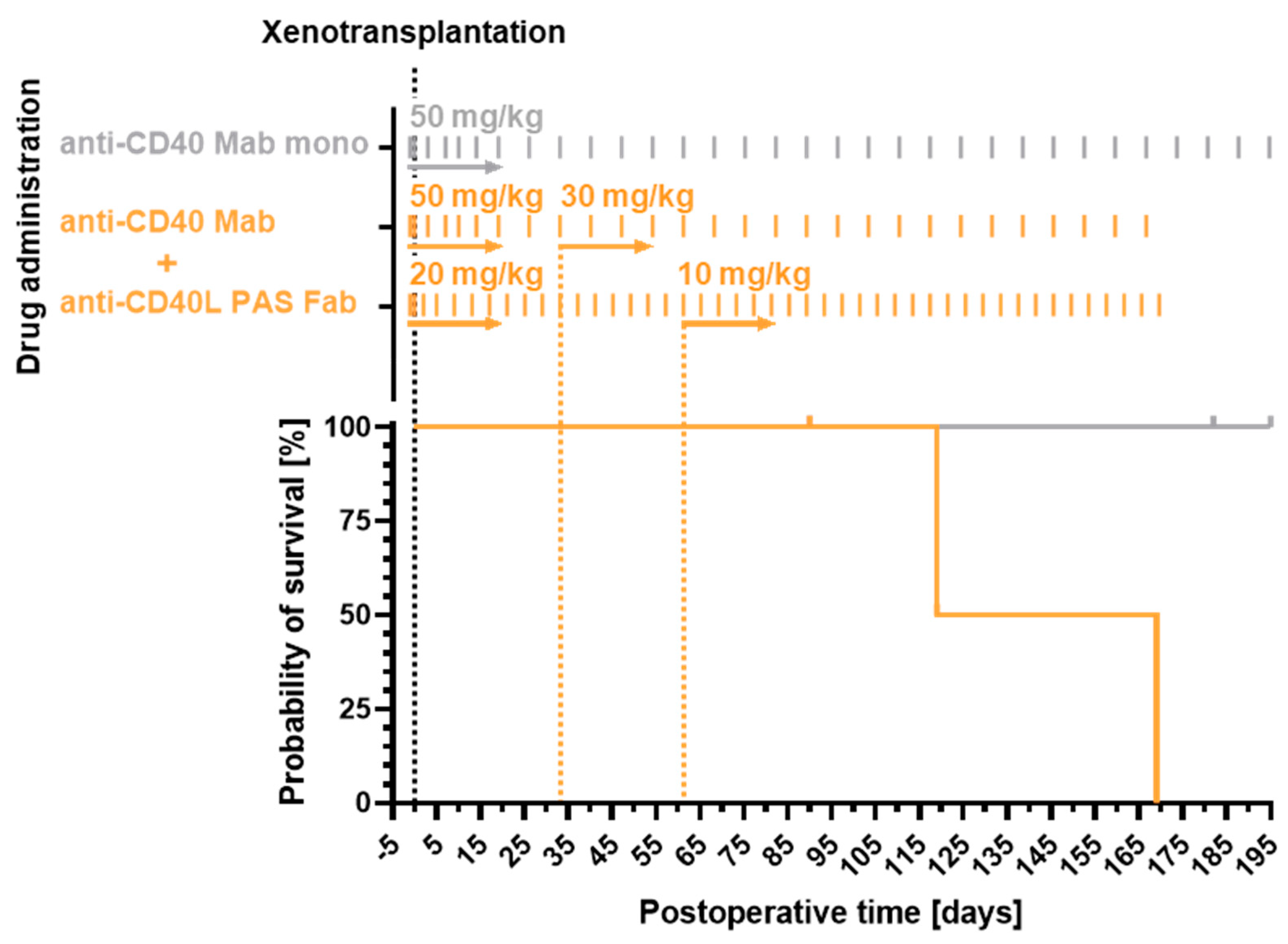
3.1. Recipient Survival Times
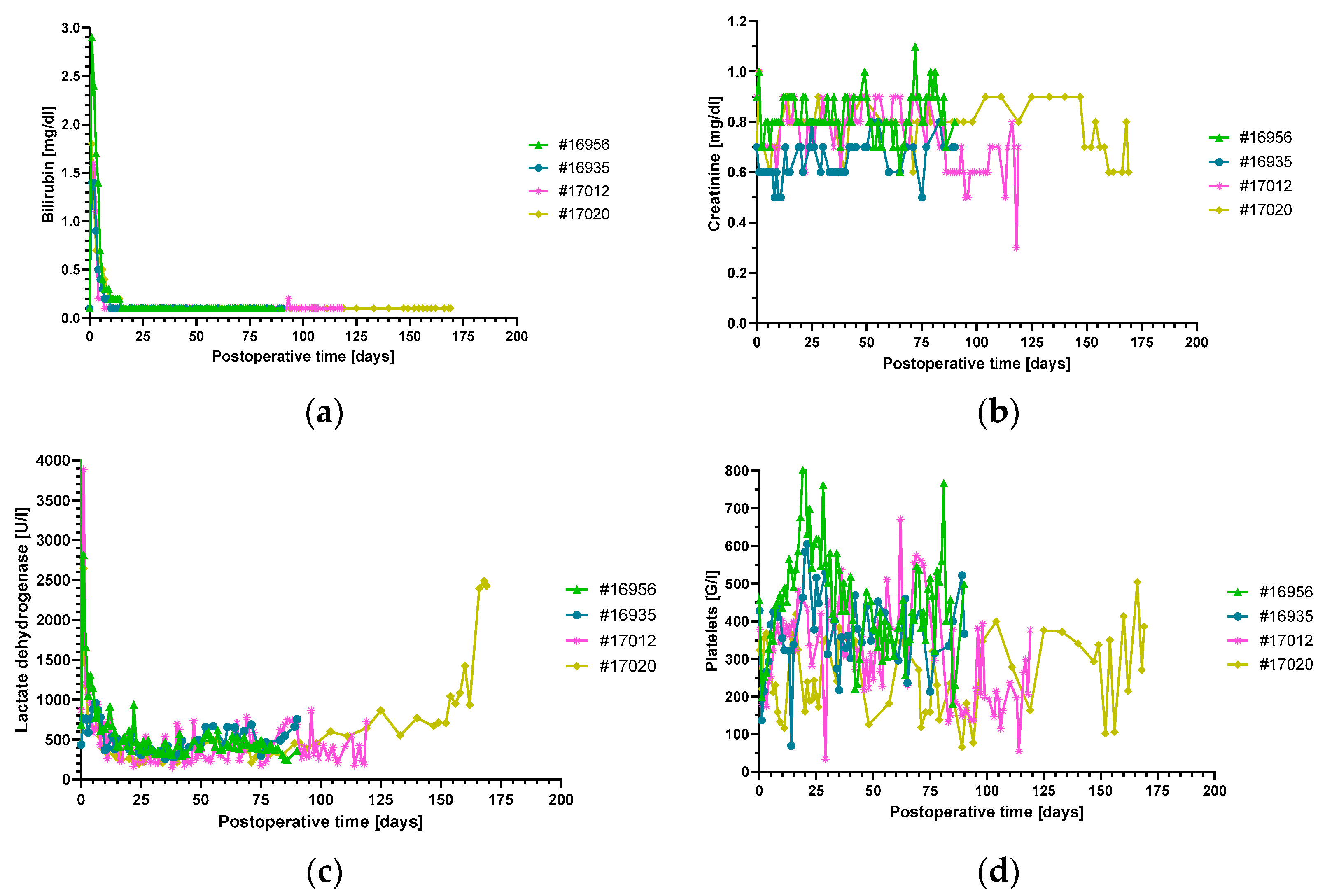
3.2. Organ Blood Parameters and Non-Gal-α(1,3)-Gal Xenoreactive Antibody Levels
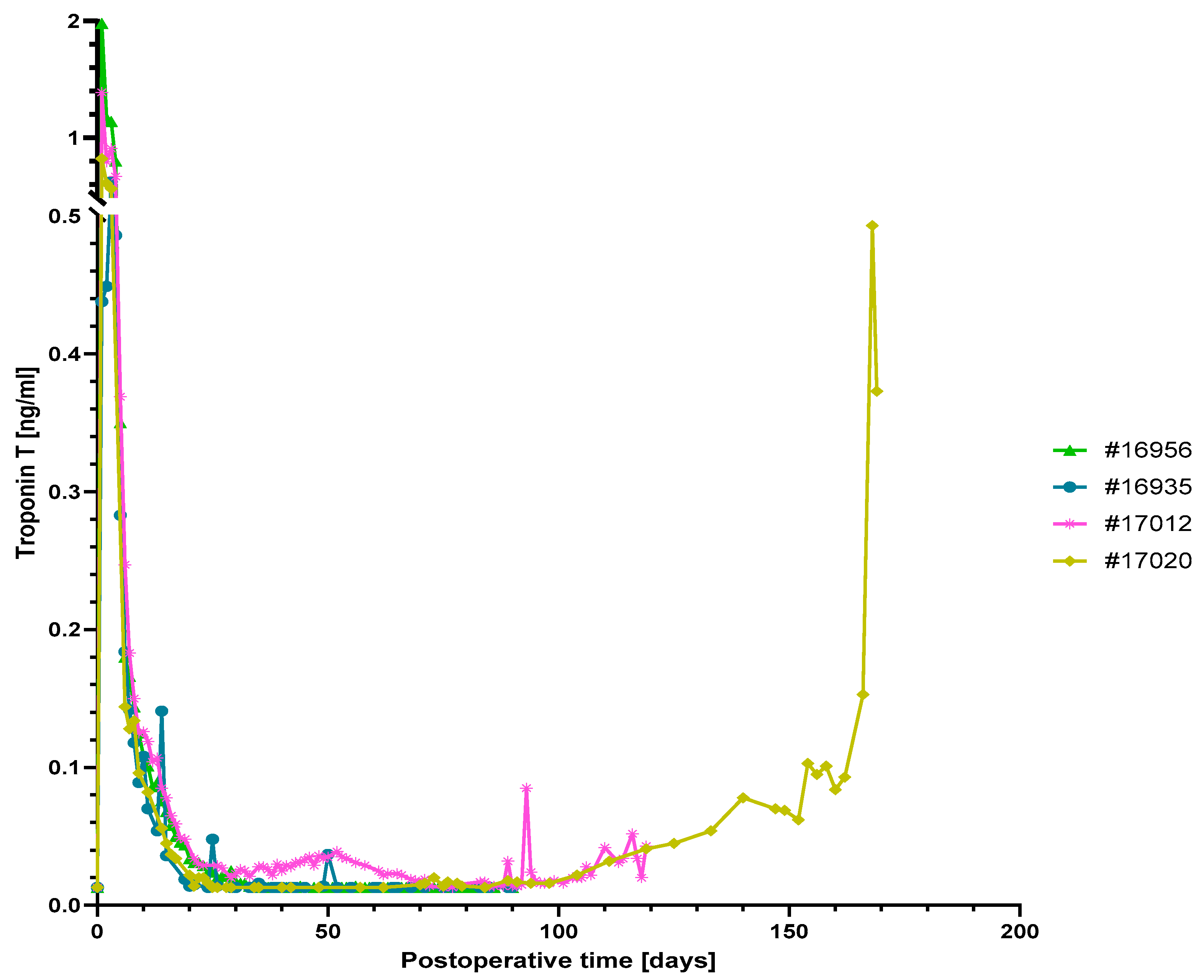
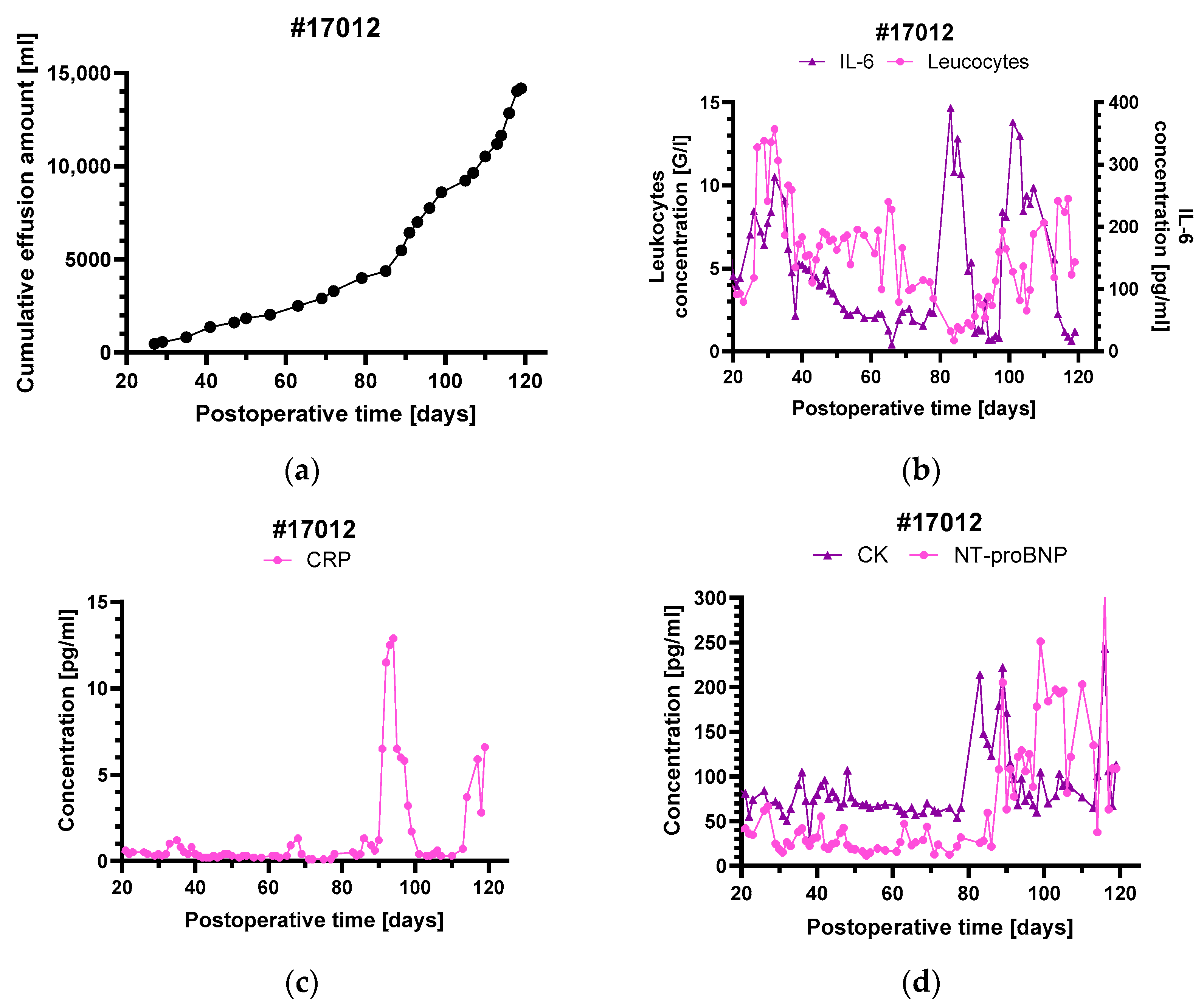
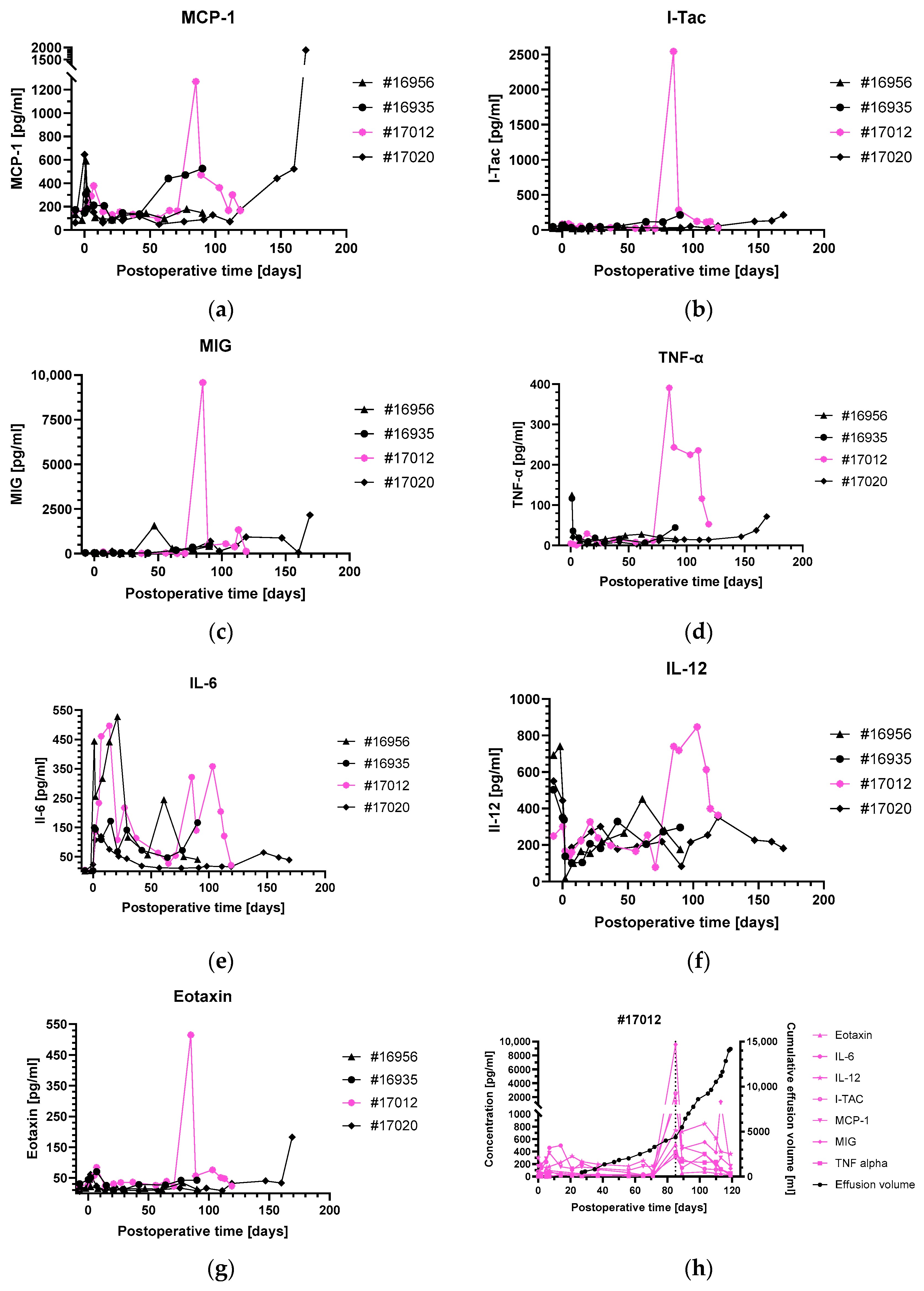
3.3. Levels of Inflammatory and Injury Markers
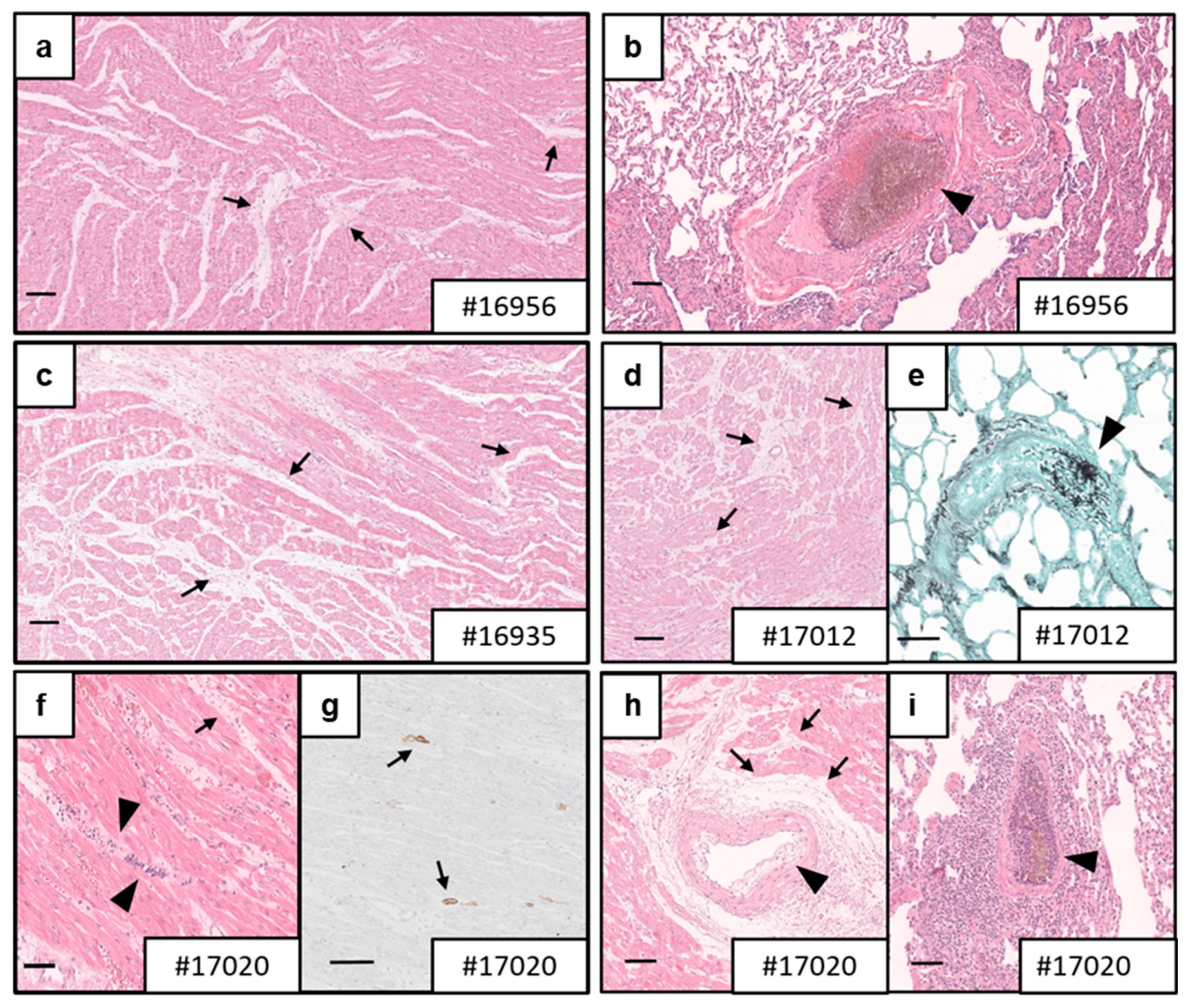
3.4. Necropsy and Histology
3.5. Analyses of Complement System, Coagulation System, Tissue Structure and Innate Immune Cell Infiltration
3.6. Virus Testing

4. Discussion
4.1. Rationale for a Combination Therapy with Anti-CD40 and Anti-CD40L Antibodies
4.2. Outcome of a Combination Therapy of Anti-CD40 IgG4 and Anti-CD40L PAS-Fab
4.3. Myocardial Integrity of the Xenografts
4.4. Pleural Effusions in Animal #17012
4.5. Fungal Infections
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reichart, B.; Cooper, D.K.C.; Langin, M.; Tonjes, R.R.; Pierson, R.N.; Wolf, E. Cardiac xenotransplantation: From concept to clinic. Cardiovasc. Res. 2023, 118, 3499–3516. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.M.; Reichart, B.; Byrne, G.W.; McGregor, C.G. Current status of pig heart xenotransplantation. Int. J. Surg. 2015, 23 Pt B, 234–239. [Google Scholar] [CrossRef]
- Längin, M.; Mayr, T.; Reichart, B.; Michel, S.; Buchholz, S.; Guethoff, S.; Dashkevich, A.; Baehr, A.; Egerer, S.; Bauer, A.; et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018, 564, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Reichart, B.; Längin, M.; Radan, J.; Mokelke, M.; Buttgereit, I.; Ying, J.; Fresch, A.K.; Mayr, T.; Issl, L.; Buchholz, S.; et al. Pig-to-non-human primate heart transplantation: The final step toward clinical xenotransplantation? J. Heart Lung Transplant. 2020, 39, 751–757. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Goerlich, C.E.; Singh, A.K.; Zhang, T.; Tatarov, I.; Lewis, B.; Sentz, F.; Hershfeld, A.; Braileanu, G.; Odonkor, P.; et al. Progressive genetic modifications of porcine cardiac xenografts extend survival to 9 months. Xenotransplantation 2022, 29, e12744. [Google Scholar] [CrossRef]
- Reichart, B.; Langin, M.; Denner, J.; Schwinzer, R.; Cowan, P.J.; Wolf, E. Pathways to Clinical Cardiac Xenotransplantation. Transplantation 2021, 105, 1930–1943. [Google Scholar] [CrossRef]
- Tang, T.; Cheng, X.; Truong, B.; Sun, L.; Yang, X.; Wang, H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol. Ther. 2021, 219, 107709. [Google Scholar] [CrossRef] [PubMed]
- Griffith, B.P.; Goerlich, C.E.; Singh, A.K.; Rothblatt, M.; Lau, C.L.; Shah, A.; Lorber, M.; Grazioli, A.; Saharia, K.K.; Hong, S.N.; et al. Genetically Modified Porcine-to-Human Cardiac Xenotransplantation. N. Engl. J. Med. 2022, 387, 35–44. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Singh, A.K.; Scobie, L.; Goerlich, C.E.; Grazioli, A.; Saharia, K.; Crossan, C.; Burke, A.; Drachenberg, C.; Oguz, C.; et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: A case report. Lancet 2023, 402, 397–410. [Google Scholar] [CrossRef]
- Singh, A.K.; Goerlich, C.E.; Zhang, T.; Lewis, B.G.T.; Hershfeld, A.; Mohiuddin, M.M. CD40-CD40L Blockade: Update on Novel Investigational Therapeutics for Transplantation. Transplantation 2023, 107, 1472–1481. [Google Scholar] [CrossRef]
- Bourgeois, C.; Rocha, B.; Tanchot, C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 2002, 297, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Dubois, B.; Fayette, J.; Burdin, N.; Brière, F.; Miossec, P.; Rissoan, M.C.; van Kooten, C.; Caux, C. Functional CD40 antigen on B cells, dendritic cells and fibroblasts. Adv. Exp. Med. Biol. 1995, 378, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.; Ruggiero, G.; Terrazzano, G.; Palomba, C.; Manzo, C.; Fontana, S.; Spits, H.; Kärre, K.; Zappacosta, S. A new mechanism of NK cell cytotoxicity activation: The CD40-CD40 ligand interaction. J. Exp. Med. 1997, 185, 2053–2060. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Singh, A.K.; Corcoran, P.C.; Thomas Iii, M.L.; Clark, T.; Lewis, B.G.; Hoyt, R.F.; Eckhaus, M.; Pierson Iii, R.N.; Belli, A.J.; et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat. Commun. 2016, 7, 11138. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, M.M.; Singh, A.K.; Corcoran, P.C.; Hoyt, R.F.; Thomas, M.L., 3rd; Ayares, D.; Horvath, K.A. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J. Thorac. Cardiovasc. Surg. 2014, 148, 1106–1113; discussion 1113–1114. [Google Scholar] [CrossRef]
- Kawai, T.; Andrews, D.; Colvin, R.B.; Sachs, D.H.; Cosimi, A.B. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat. Med. 2000, 6, 114. [Google Scholar] [CrossRef]
- Robles-Carrillo, L.; Meyer, T.; Hatfield, M.; Desai, H.; Davila, M.; Langer, F.; Amaya, M.; Garber, E.; Francis, J.L.; Hsu, Y.M.; et al. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2A transgenic mice. J. Immunol. 2010, 185, 1577–1583. [Google Scholar] [CrossRef]
- Xu, H.; Tadaki, D.K.; Elster, E.A.; Burkly, L.C.; Berning, J.D.; Cruzata, F.; Kampen, R.L.; Montgomery, S.P.; Patterson, N.B.; Harlan, D.M.; et al. Humanized anti-CD154 antibody therapy for the treatment of allograft rejection in nonhuman primates. Transplantation 2002, 74, 940–943. [Google Scholar] [CrossRef]
- Shock, A.; Burkly, L.; Wakefield, I.; Peters, C.; Garber, E.; Ferrant, J.; Taylor, F.R.; Su, L.; Hsu, Y.M.; Hutto, D.; et al. CDP7657, an anti-CD40L antibody lacking an Fc domain, inhibits CD40L-dependent immune responses without thrombotic complications: An in vivo study. Arthritis Res. Ther. 2015, 17, 234. [Google Scholar] [CrossRef]
- Langer, F.; Ingersoll, S.B.; Amirkhosravi, A.; Meyer, T.; Siddiqui, F.A.; Ahmad, S.; Walker, J.M.; Amaya, M.; Desai, H.; Francis, J.L. The role of CD40 in CD40L- and antibody-mediated platelet activation. Thromb. Haemost. 2005, 93, 1137–1146. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Singh, A.K.; Corcoran, P.C.; Hoyt, R.F.; Thomas, M.L., 3rd; Lewis, B.G.; Eckhaus, M.; Dabkowski, N.L.; Belli, A.J.; Reimann, K.A.; et al. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO.hCD46Tg pig-to-baboon model. Xenotransplantation 2014, 21, 35–45. [Google Scholar] [CrossRef]
- Liu, D.; Ford, M.L. CD11b is a novel alternate receptor for CD154 during alloimmunity. Am. J. Transplant. 2020, 20, 2216–2225. [Google Scholar] [CrossRef]
- O’Neill, N.A.; Zhang, T.; Braileanu, G.; Sun, W.; Cheng, X.; Hershfeld, A.; Laird, C.T.; Kronfli, A.; Hock, L.A.; Dahi, S.; et al. Comparative Evaluation of alphaCD40 (2C10R4) and alphaCD154 (5C8H1 and IDEC-131) in a Nonhuman Primate Cardiac Allotransplant Model. Transplantation 2017, 101, 2038–2047. [Google Scholar] [CrossRef] [PubMed]
- Mehlhop, P.D.; van de Rijn, M.; Brewer, J.P.; Kisselgof, A.B.; Geha, R.S.; Oettgen, H.C.; Martin, T.R. CD40L, but not CD40, is required for allergen-induced bronchial hyperresponsiveness in mice. Am. J. Respir. Cell Mol. Biol. 2000, 23, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Léveillé, C.; Bouillon, M.; Guo, W.; Bolduc, J.; Sharif-Askari, E.; El-Fakhry, Y.; Reyes-Moreno, C.; Lapointe, R.; Merhi, Y.; Wilkins, J.A.; et al. CD40 ligand binds to alpha5beta1 integrin and triggers cell signaling. J. Biol. Chem. 2007, 282, 5143–5151. [Google Scholar] [CrossRef]
- Zirlik, A.; Maier, C.; Gerdes, N.; MacFarlane, L.; Soosairajah, J.; Bavendiek, U.; Ahrens, I.; Ernst, S.; Bassler, N.; Missiou, A.; et al. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation 2007, 115, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.S.; Salti, S.; Mourad, W. Novel Functions of Integrins as Receptors of CD154: Their Role in Inflammation and Apoptosis. Cells 2022, 11, 1747. [Google Scholar] [CrossRef]
- Bachsais, M.; Salti, S.; Zaoui, K.; Hassan, G.S.; Aoudjit, F.; Mourad, W. CD154 inhibits death of T cells via a Cis interaction with the α5β1 integrin. PLoS ONE 2020, 15, e0235753. [Google Scholar] [CrossRef]
- An, H.J.; Kim, Y.J.; Song, D.H.; Park, B.S.; Kim, H.M.; Lee, J.D.; Paik, S.G.; Lee, J.O.; Lee, H. Crystallographic and mutational analysis of the CD40-CD154 complex and its implications for receptor activation. J. Biol. Chem. 2011, 286, 11226–11235. [Google Scholar] [CrossRef]
- Antoniades, C.; Bakogiannis, C.; Tousoulis, D.; Antonopoulos, A.S.; Stefanadis, C. The CD40/CD40 ligand system: Linking inflammation with atherothrombosis. J. Am. Coll. Cardiol. 2009, 54, 669–677. [Google Scholar] [CrossRef]
- Michaels, A.J.; Stoppato, M.; Flores, W.J.; Reimann, K.A.; Engelman, K.D. Anti-CD40 antibody 2C10 binds to a conformational epitope at the CD40-CD154 interface that is conserved among primate species. Am. J. Transplant. 2020, 20, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chan, H.T.C.; Orr, C.M.; Dadas, O.; Booth, S.G.; Dahal, L.N.; Penfold, C.A.; O’Brien, L.; Mockridge, C.I.; French, R.R.; et al. Complex Interplay between Epitope Specificity and Isotype Dictates the Biological Activity of Anti-human CD40 Antibodies. Cancer Cell 2018, 33, 664–675.e664. [Google Scholar] [CrossRef] [PubMed]
- Binder, U.; Skerra, A. PASylation®: A versatile technology to extend drug delivery. Curr. Opin. Colloid Interface Sci. 2017, 31, 10–17. [Google Scholar] [CrossRef]
- Jhelum, H.; Grand, N.; Jacobsen, K.R.; Halecker, S.; Salerno, M.; Prate, R.; Krüger, L.; Kristiansen, Y.; Krabben, L.; Möller, L.; et al. First virological and pathological study of Göttingen Minipigs with Dippity Pig Syndrome (DPS). PLoS ONE 2023, 18, e0281521. [Google Scholar] [CrossRef]
- Fischer, N.; Gulich, B.; Keßler, B.; Längin, M.; Fishman, J.A.; Wolf, E.; Boller, K.; Tönjes, R.R.; Godehardt, A.W. PCR and peptide based PCMV detection in pig—Development and application of a combined testing procedure differentiating newly from latent infected pigs. Xenotransplantation 2023, 30, e12803. [Google Scholar] [CrossRef] [PubMed]
- Krüger, L.; Längin, M.; Reichart, B.; Fiebig, U.; Kristiansen, Y.; Prinz, C.; Kessler, B.; Egerer, S.; Wolf, E.; Abicht, J.M.; et al. Transmission of Porcine Circovirus 3 (PCV3) by Xenotransplantation of Pig Hearts into Baboons. Viruses 2019, 11, 650. [Google Scholar] [CrossRef]
- Mayr, T.; Bauer, A.; Reichart, B.; Guethoff, S.; Schoenmann, U.; Längin, M.; Panelli, A.; Kind, A.; Brenner, P.; Abicht, J.M. Hemodynamic and perioperative management in two different preclinical pig-to-baboon cardiac xenotransplantation models. Xenotransplantation 2017, 24, 12295. [Google Scholar] [CrossRef]
- Steen, S.; Paskevicius, A.; Liao, Q.; Sjöberg, T. Safe orthotopic transplantation of hearts harvested 24 hours after brain death and preserved for 24 hours. Scand. Cardiovasc. J. 2016, 50, 193–200. [Google Scholar] [CrossRef]
- Längin, M.; Reichart, B.; Steen, S.; Sjöberg, T.; Paskevicius, A.; Liao, Q.; Qin, G.; Mokelke, M.; Mayr, T.; Radan, J.; et al. Cold non-ischemic heart preservation with continuous perfusion prevents early graft failure in orthotopic pig-to-baboon xenotransplantation. Xenotransplantation 2021, 28, e12636. [Google Scholar] [CrossRef]
- Lower, R.R.; Shumway, N.E. Studies on orthotopic homotransplantation of the canine heart. Surg. Forum 1960, 11, 18–19. [Google Scholar]
- Azimzadeh, A.M.; Byrne, G.W.; Ezzelarab, M.; Welty, E.; Braileanu, G.; Cheng, X.; Robson, S.C.; McGregor, C.G.; Cooper, D.K.; Pierson, R.N., 3rd. Development of a consensus protocol to quantify primate anti-non-Gal xenoreactive antibodies using pig aortic endothelial cells. Xenotransplantation 2014, 21, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Denner, J.; Längin, M.; Reichart, B.; Krüger, L.; Fiebig, U.; Mokelke, M.; Radan, J.; Mayr, T.; Milusev, A.; Luther, F.; et al. Impact of porcine cytomegalovirus on long-term orthotopic cardiac xenotransplant survival. Sci. Rep. 2020, 10, 17531. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.J.; Sulling, K.; Gollackner, B.; Yamamoto, S.; Knosalla, C.; Wilkinson, R.A.; Kaur, A.; Sachs, D.H.; Yamada, K.; Cooper, D.K.; et al. Reduced efficacy of ganciclovir against porcine and baboon cytomegalovirus in pig-to-baboon xenotransplantation. Am. J. Transplant. 2003, 3, 1057–1064. [Google Scholar] [CrossRef]
- Fryer, J.F.; Griffiths, P.D.; Emery, V.C.; Clark, D.A. Susceptibility of porcine cytomegalovirus to antiviral drugs. J. Antimicrob. Chemother. 2004, 53, 975–980. [Google Scholar] [CrossRef]
- Denner, J. First transplantation of a pig heart from a multiple gene-modified donor, porcine cytomegalovirus/roseolovirus, and antiviral drugs. Xenotransplantation 2023, 30, e12800. [Google Scholar] [CrossRef]
- Xie, J.H.; Yamniuk, A.P.; Borowski, V.; Kuhn, R.; Susulic, V.; Rex-Rabe, S.; Yang, X.; Zhou, X.; Zhang, Y.; Gillooly, K.; et al. Engineering of a novel anti-CD40L domain antibody for treatment of autoimmune diseases. J. Immunol. 2014, 192, 4083–4092. [Google Scholar] [CrossRef]
- Knosalla, C.; Gollackner, B.; Buhler, L.; Mueller, N.J.; Houser, S.; Mauiyyedi, S.; Sachs, D.H.; Robson, S.C.; Fishman, J.; Schuurman, H.J.; et al. Correlation of biochemical and hematological changes with graft failure following pig heart and kidney transplantation in baboons. Am. J. Transplant. 2003, 3, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y. Macrophages in xenotransplantation. Korean J. Transplant. 2019, 33, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, C.; Zhuang, Q.; Peng, B.; Zhu, Y.; Ye, Q.; Ming, Y. The Evolving Roles of Macrophages in Organ Transplantation. J. Immunol. Res. 2019, 2019, 5763430. [Google Scholar] [CrossRef]
- Längin, M.; Buttgereit, I.; Reichart, B.; Panelli, A.; Radan, J.; Mokelke, M.; Neumann, E.; Bender, M.; Michel, S.; Ellgass, R.; et al. Xenografts Show Signs of Concentric Hypertrophy and Dynamic Left Ventricular Outflow Tract Obstruction After Orthotopic Pig-to-baboon Heart Transplantation. Transplantation 2023, 107, e328–e338. [Google Scholar] [CrossRef]
- Hussain, N. Elevated cardiac troponins in setting of systemic inflammatory response syndrome, sepsis, and septic shock. ISRN Cardiol. 2013, 2013, 723435. [Google Scholar] [CrossRef]
- Altmann, D.R.; Korte, W.; Maeder, M.T.; Fehr, T.; Haager, P.; Rickli, H.; Kleger, G.R.; Rodriguez, R.; Ammann, P. Elevated cardiac troponin I in sepsis and septic shock: No evidence for thrombus associated myocardial necrosis. PLoS ONE 2010, 5, e9017. [Google Scholar] [CrossRef]
- Holm, T.; Aukrust, P.; Andreassen, A.K.; Ueland, T.; Brosstad, F.; Froland, S.S.; Simonsen, S.; Gullestad, L. Peripheral endothelial dysfunction in heart transplant recipients: Possible role of proinflammatory cytokines. Clin. Transplant. 2000, 14, 218–225. [Google Scholar] [CrossRef]
- Kong, D.; Huang, S.; Miao, X.; Li, J.; Wu, Z.; Shi, Y.; Liu, H.; Jiang, Y.; Yu, X.; Xie, M.; et al. The dynamic cellular landscape of grafts with acute rejection after heart transplantation. J. Heart Lung Transplant. 2023, 42, 160–172. [Google Scholar] [CrossRef]
- Burns, W.R.; Wang, Y.; Tang, P.C.; Ranjbaran, H.; Iakimov, A.; Kim, J.; Cuffy, M.; Bai, Y.; Pober, J.S.; Tellides, G. Recruitment of CXCR3+ and CCR5+ T cells and production of interferon-gamma-inducible chemokines in rejecting human arteries. Am. J. Transplant. 2005, 5, 1226–1236. [Google Scholar] [CrossRef]
- Li, B.; Xu, W.; Xu, L.; Jiang, Z.; Wen, Z.; Li, K.; Xiong, S. I-TAC is a dominant chemokine in controlling skin intragraft inflammation via recruiting CXCR3+ cells into the graft. Cell Immunol. 2010, 260, 83–91. [Google Scholar] [CrossRef]
- Vanderheyden, M.; Kersschot, E.; Paulus, W.J. Pro-inflammatory cytokines and endothelium-dependent vasodilation in the forearm. Serial assessment in patients with congestive heart failure. Eur. Heart J. 1998, 19, 747–752. [Google Scholar] [CrossRef]
- Katz, S.D.; Rao, R.; Berman, J.W.; Schwarz, M.; Demopoulos, L.; Bijou, R.; LeJemtel, T.H. Pathophysiological correlates of increased serum tumor necrosis factor in patients with congestive heart failure. Relation to nitric oxide-dependent vasodilation in the forearm circulation. Circulation 1994, 90, 12–16. [Google Scholar] [CrossRef]
- Hodge, G.; Hodge, S.; Reynolds, P.N.; Holmes, M. Up-regulation of interleukin-8, interleukin-10, monocyte chemotactic protein-1, and monocyte chemotactic protein-3 in peripheral blood monocytes in stable lung transplant recipients: Are immunosuppression regimens working? Transplantation 2005, 79, 387–391. [Google Scholar] [CrossRef]
- Pham, S.M.; Yoshida, Y.; Aeba, R.; Hattler, B.G.; Iwaki, Y.; Zeevi, A.; Hardesty, R.L.; Griffith, B.P. Interleukin-6, a marker of preservation injury in clinical lung transplantation. J. Heart Lung Transplant. 1992, 11, 1017–1024. [Google Scholar]
- Andrade, C.F.; Kaneda, H.; Der, S.; Tsang, M.; Lodyga, M.; Chimisso Dos Santos, C.; Keshavjee, S.; Liu, M. Toll-like receptor and cytokine gene expression in the early phase of human lung transplantation. J. Heart Lung Transplant. 2006, 25, 1317–1323. [Google Scholar] [CrossRef]
- Xie, A.; Wang, S.; Zhang, K.; Wang, G.; Ye, P.; Li, J.; Chen, W.; Xia, J. Treatment with interleukin-12/23p40 antibody attenuates acute cardiac allograft rejection. Transplantation 2011, 91, 27–34. [Google Scholar] [CrossRef]
- Abicht, J.M.; Mayr, T.; Reichart, B.; Buchholz, S.; Werner, F.; Lutzmann, I.; Schmoeckel, M.; Bauer, A.; Thormann, M.; Langenmayer, M.; et al. Pre-clinical heterotopic intrathoracic heart xenotransplantation: A possibly useful clinical technique. Xenotransplantation 2015, 22, 427–442. [Google Scholar] [CrossRef]
- Bauer, A.; Postrach, J.; Thormann, M.; Blanck, S.; Faber, C.; Wintersperger, B.; Michel, S.; Abicht, J.M.; Christ, F.; Schmitz, C.; et al. First experience with heterotopic thoracic pig-to-baboon cardiac xenotransplantation. Xenotransplantation 2010, 17, 243–249. [Google Scholar] [CrossRef]
- Mohiuddin, M.M.; Corcoran, P.C.; Singh, A.K.; Azimzadeh, A.; Hoyt, R.F., Jr.; Thomas, M.L.; Eckhaus, M.A.; Seavey, C.; Ayares, D.; Pierson, R.N., 3rd; et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am. J. Transplant. 2012, 12, 763–771. [Google Scholar] [CrossRef]
- Muñoz, P.; Fernández, N.S.; Fariñas, M.C. Epidemiology and risk factors of infections after solid organ transplantation. Enferm. Infecc. Microbiol. Clin. 2012, 30 (Suppl. S2), 10–18. [Google Scholar] [CrossRef]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Uribe, L.G.; Cortés, J.A.; Granados, C.E.; Montoya, J.G. Antifungal prophylaxis following heart transplantation: Systematic review. Mycoses 2014, 57, 429–436. [Google Scholar] [CrossRef]
- Bitterman, R.; Marinelli, T.; Husain, S. Strategies for the Prevention of Invasive Fungal Infections after Lung Transplant. J. Fungi 2021, 7, 122. [Google Scholar] [CrossRef]
- Senoner, T.; Breitkopf, R.; Treml, B.; Rajsic, S. Invasive Fungal Infections after Liver Transplantation. J. Clin. Med. 2023, 12, 3238. [Google Scholar] [CrossRef]
- Gibson, R.H.; Evans, R.J.; Hotham, R.; Bojarczuk, A.; Lewis, A.; Bielska, E.; May, R.C.; Elks, P.M.; Renshaw, S.A.; Johnston, S.A. Mycophenolate mofetil increases susceptibility to opportunistic fungal infection independent of lymphocytes. bioRxiv 2018, bioRxiv:10.1101/131540. [Google Scholar] [CrossRef]
- Ritter, M.L.; Pirofski, L. Mycophenolate mofetil: Effects on cellular immune subsets, infectious complications, and antimicrobial activity. Transpl. Infect. Dis. 2009, 11, 290–297. [Google Scholar] [CrossRef]
- Meier-Kriesche, H.U.; Friedman, G.; Jacobs, M.; Mulgaonkar, S.; Vaghela, M.; Kaplan, B. Infectious complications in geriatric renal transplant patients: Comparison of two immunosuppressive protocols. Transplantation 1999, 68, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Meer, J.W.; Verschueren, I.; Kullberg, B.J. CD40/CD40 ligand interactions in the host defense against disseminated Candida albicans infection: The role of macrophage-derived nitric oxide. Eur. J. Immunol. 2002, 32, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yang, B.; Suen, W.C. Prospects for modulating the CD40/CD40L pathway in the therapy of the hyper-IgM syndrome. Innate Immun. 2018, 24, 4–10. [Google Scholar] [CrossRef]
- Kordzadeh-Kermani, E.; Khalili, H.; Karimzadeh, I.; Salehi, M. Prevention Strategies to Minimize the Infection Risk Associated with Biologic and Targeted Immunomodulators. Infect. Drug Resist. 2020, 13, 513–532. [Google Scholar] [CrossRef]
- Flandre, T.D.; Mansfield, K.G.; Espié, P.J.; Rubic-Schneider, T.; Ulrich, P. Immunosuppression Profile of CFZ533 (Iscalimab), a Non-Depleting Anti-CD40 Antibody, and the Presence of Opportunistic Infections in a Rhesus Monkey Toxicology Study. Toxicol. Pathol. 2022, 50, 712–724. [Google Scholar] [CrossRef]
| Experiment | #16956 | #16935 | #16950 | #17353 | #17012 | #17020 | Reference * |
|---|---|---|---|---|---|---|---|
| Bilirubin [mg/dL] | <0.1 | <0.1 | 5.8 | 5.8 | <0.1 | <0.1 | ≤1.2 |
| AST [U/L] | 27 | 64 | 1360 | 332 | 56 | 456 | ≤49 |
| PR [%] | 88 | 128 | 13 | 41 | 141 | 60 | 70–130 |
| CHE [kU/L] | 112 | 13.4 | 1.2 | 1.9 | 1.6 | 8.6 | 4.6–11.5 |
| Trop. T [ng/mL] | <0.013 | <0.013 | 10.30 | 4.85 | 0.043 | 0.373 | ≤0.014 |
| CK total [U/L] | 47 | 79 | 18689 | 5826 | 116 | 501 | ≤189 |
| LDH [U/L] | 361 | 757 | 13630 | 1028 | 729 | 2429 | ≤249 |
| Platelets [G/L] | 498 | 498 | 367 | 42 | 118 | 386 | 150–300 |
| Survival [days] | 90 | 90 | 1 | 1 | 120 | 170 | |
| Causes for euthanasia | Study endpoint | Study endpoint | Technical failure (pulmonary stenosis) | Technical failure (insufficient perfusion) | Recalcitrant pleural effusions | Graft failure (humoral rejection) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bender, M.; Abicht, J.-M.; Reichart, B.; Neumann, E.; Radan, J.; Mokelke, M.; Buttgereit, I.; Leuschen, M.; Wall, F.; Michel, S.; et al. Combination of Anti-CD40 and Anti-CD40L Antibodies as Co-Stimulation Blockade in Preclinical Cardiac Xenotransplantation. Biomedicines 2024, 12, 1927. https://doi.org/10.3390/biomedicines12081927
Bender M, Abicht J-M, Reichart B, Neumann E, Radan J, Mokelke M, Buttgereit I, Leuschen M, Wall F, Michel S, et al. Combination of Anti-CD40 and Anti-CD40L Antibodies as Co-Stimulation Blockade in Preclinical Cardiac Xenotransplantation. Biomedicines. 2024; 12(8):1927. https://doi.org/10.3390/biomedicines12081927
Chicago/Turabian StyleBender, Martin, Jan-Michael Abicht, Bruno Reichart, Elisabeth Neumann, Julia Radan, Maren Mokelke, Ines Buttgereit, Maria Leuschen, Felicia Wall, Sebastian Michel, and et al. 2024. "Combination of Anti-CD40 and Anti-CD40L Antibodies as Co-Stimulation Blockade in Preclinical Cardiac Xenotransplantation" Biomedicines 12, no. 8: 1927. https://doi.org/10.3390/biomedicines12081927
APA StyleBender, M., Abicht, J.-M., Reichart, B., Neumann, E., Radan, J., Mokelke, M., Buttgereit, I., Leuschen, M., Wall, F., Michel, S., Ellgass, R., Steen, S., Paskevicius, A., Lange, A., Kessler, B., Kemter, E., Klymiuk, N., Denner, J., Godehardt, A. W., ... Längin, M. (2024). Combination of Anti-CD40 and Anti-CD40L Antibodies as Co-Stimulation Blockade in Preclinical Cardiac Xenotransplantation. Biomedicines, 12(8), 1927. https://doi.org/10.3390/biomedicines12081927









