Toxicological Assessment of Biodegradable Poli-ε-Caprolactone Polymer Composite Materials Containing Hydroxyapatite, Bioglass, and Chitosan as Potential Biomaterials for Bone Regeneration Scaffolds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomaterials
2.1.1. Materials
2.1.2. Production of PCL Composite Materials
2.1.3. Preparation of PCL Powder with Added Hydroxyapatite, Bioglass, and Chitosan
2.1.4. X-ray Diffraction Studies
2.1.5. Calorimetric Studies
2.1.6. Surface Area Studies
2.1.7. Scanning Electron Microscopy of Biomaterials
2.1.8. Radiation Sterilization
2.2. Toxicological Examination
2.2.1. Preparation of Extracts for Elution Test
2.2.2. Cell Culture
2.2.3. MTT Assay
2.2.4. XTT Assay
2.2.5. Scratch Assay
2.2.6. Flow Cytometry
2.2.7. Fluorescence Microscopy
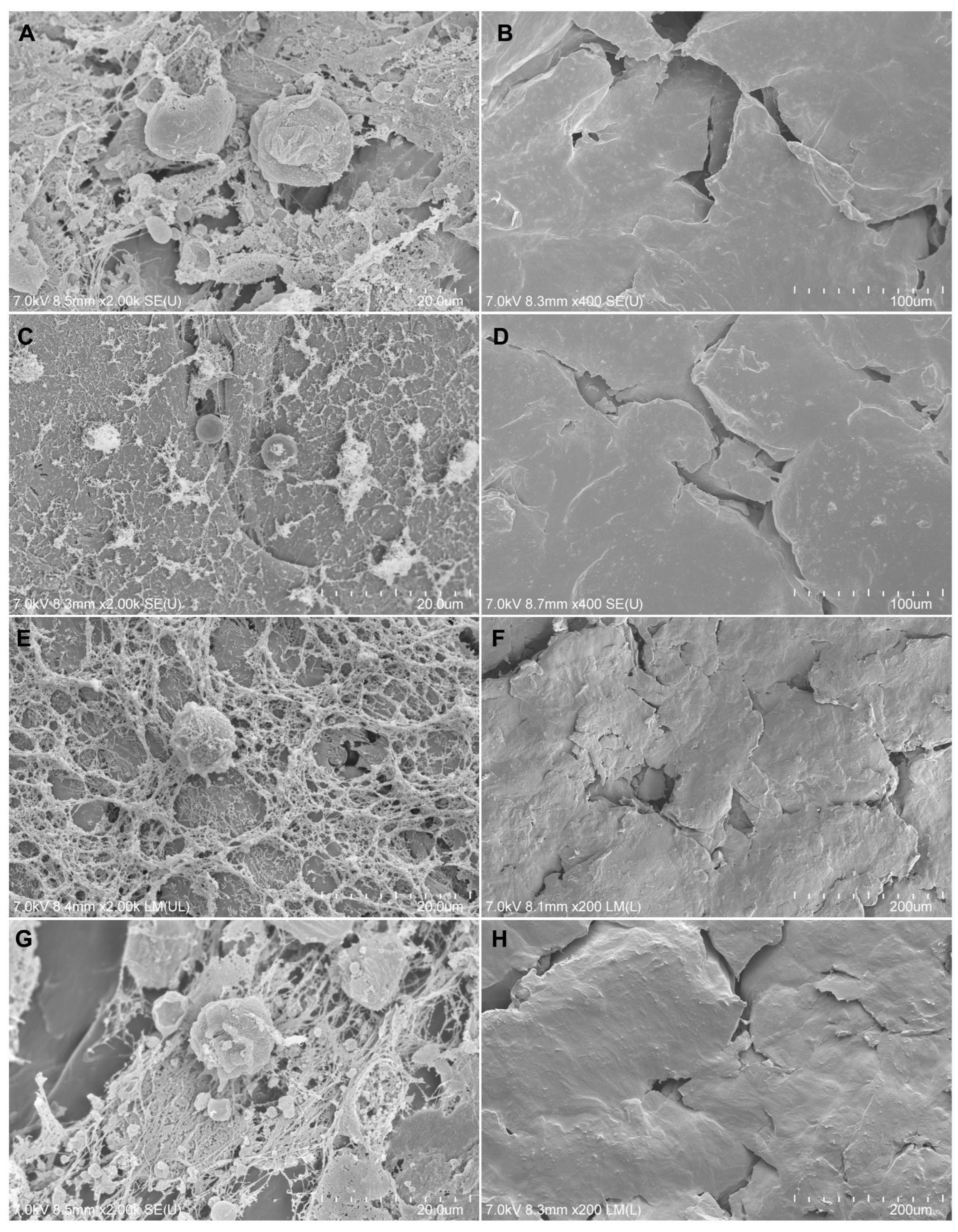
2.2.8. Scanning Electron Microscopy of Biomaterials Populated by Cells
2.2.9. Statistical Analysis
3. Results
3.1. Biomaterial Analysis
3.1.1. X-ray Diffraction
3.1.2. Thermogravimetric Analysis
3.1.3. BET
3.1.4. Initial Sample Assessment with SEM
3.1.5. Elemental Analysis
3.2. Impact of Biomaterials on MG-63 Cells
3.2.1. Morphology of Cells
3.2.2. Viability of MG-63 Cells
3.2.3. Proliferation and Migration of Cells
3.2.4. Cell Cycle Analysis
3.2.5. Live/Dead Cells Visualized on Composite Materials
3.2.6. Adherence of Cells to Composite Materials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaparro, O.; Linero, I. Regenerative Medicine: A New Paradigm in Bone Regeneration. In Advanced Techniques in Bone Regeneration; Zorzi, A.R., de Miranda, J.B., Eds.; InTech: Rijeka, Croatia, 2016; pp. 253–274. [Google Scholar]
- Sakkas, A.; Wilde, F.; Heufelder, M.; Winter, K.; Schramm, A. Autogenous Bone Grafts in Oral Implantology—Is It Still a “Gold Standard”? A Consecutive Review of 279 Patients with 456 Clinical Procedures. Int. J. Implant Dent. 2017, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Kurd, M.; Cohick, S.; Park, A.; Ahmadinia, K.; Lee, J.; An, H. Fusion in Degenerative Spondylolisthesis: Comparison of Osteoconductive and Osteoinductive Bone Graft Substitutes. Eur. Spine J. 2015, 24, 1066–1073. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the Art and New Perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Ortuño-Costela, M.d.C.; García-López, M.; Cerrada, V.; Gallardo, M.E. iPSCs: A Powerful Tool for Skeletal Muscle Tissue Engineering. J. Cell. Mol. Med. 2019, 23, 3784–3794. [Google Scholar] [CrossRef]
- Guduric, V.; Metz, C.; Siadous, R.; Bareille, R.; Levato, R.; Engel, E.; Fricain, J.-C.; Devillard, R.; Luzanin, O.; Catros, S. Layer-by-Layer Bioassembly of Cellularized Polylactic Acid Porous Membranes for Bone Tissue Engineering. J. Mater. Sci. Mater. Med. 2017, 28, 78. [Google Scholar] [CrossRef]
- Morelli, S.; Salerno, S.; Holopainen, J.; Ritala, M.; De Bartolo, L. Osteogenic and Osteoclastogenic Differentiation of Co-Cultured Cells in Polylactic Acid–Nanohydroxyapatite Fiber Scaffolds. J. Biotechnol. 2015, 204, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Abazari, M.F.; Soleimanifar, F.; Enderami, S.E.; Nematzadeh, M.; Nasiri, N.; Nejati, F.; Saburi, E.; Khodashenas, S.; Darbasizadeh, B.; Khani, M.M.; et al. Incorporated-bFGF Polycaprolactone/Polyvinylidene Fluoride Nanocomposite Scaffold Promotes Human Induced Pluripotent Stem Cells Osteogenic Differentiation. J. Cell. Biochem. 2019, 120, 16750–16759. [Google Scholar] [CrossRef]
- Hosseini, F.S.; Soleimanifar, F.; Ardeshirylajimi, A.; Vakilian, S.; Mossahebi-Mohammadi, M.; Enderami, S.E.; Khojasteh, A.; Zare Karizi, S. In Vitro Osteogenic Differentiation of Stem Cells with Different Sources on Composite Scaffold Containing Natural Bioceramic and Polycaprolactone. Artif. Cells Nanomed. Biotechnol. 2019, 47, 300–307. [Google Scholar] [CrossRef]
- Soleimanifar, F.; Hosseini, F.S.; Atabati, H.; Behdari, A.; Kabiri, L.; Enderami, S.E.; Khani, M.; Ardeshirylajimi, A.; Saburi, E. Adipose-derived Stem Cells-conditioned Medium Improved Osteogenic Differentiation of Induced Pluripotent Stem Cells When Grown on Polycaprolactone Nanofibers. J. Cell. Physiol. 2019, 234, 10315–10323. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, M.; Xie, Q.; Sun, H.; Huang, Y.; Zhang, D.; Yu, Z.; Bi, X.; Chen, J.; Wang, J.; et al. Electrospun Silk Fibroin/Poly(Lactide-Co-ε-Caprolactone) Nanofibrous Scaffolds for Bone Regeneration. Int. J. Nanomed. 2016, 11, 1483–1500. [Google Scholar] [CrossRef]
- Fu, C.; Bai, H.; Zhu, J.; Niu, Z.; Wang, Y.; Li, J.; Yang, X.; Bai, Y. Enhanced Cell Proliferation and Osteogenic Differentiation in Electrospun PLGA/Hydroxyapatite Nanofibre Scaffolds Incorporated with Graphene Oxide. PLoS ONE 2017, 12, e0188352. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wang, S.; Qi, Z.; Zhang, W.; Sun, Y. BMP-2-Releasing Gelatin Microspheres/PLGA Scaffolds for Bone Repairment of X-Ray-Radiated Rabbit Radius Defects. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Toosi, S.; Naderi-Meshkin, H.; Kalalinia, F.; Peivandi, M.T.; HosseinKhani, H.; Bahrami, A.R.; Heirani-Tabasi, A.; Mirahmadi, M.; Behravan, J. PGA-Incorporated Collagen: Toward a Biodegradable Composite Scaffold for Bone-Tissue Engineering. J. Biomed. Mater. Res. A 2016, 104, 2020–2028. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Cao, Y.; Nanda, A.; Xu, C.; Ye, Q. 3D Printed β-TCP Scaffold with Sphingosine 1-Phosphate Coating Promotes Osteogenesis and Inhibits Inflammation. Biochem. Biophys. Res. Commun. 2019, 512, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Liu, L.; Wang, Y.; Yang, R.; Hu, C.; Rung, S.; Man, Y.; Qu, Y. Evaluation of Epigallocatechin-3-Gallate (EGCG)-Modified Scaffold Determines Macrophage Recruitment. Mater. Sci. Eng. C 2019, 100, 505–513. [Google Scholar] [CrossRef]
- Lee, D.J.; Kwon, J.; Kim, Y.; Wang, X.; Wu, T.; Lee, Y.; Kim, S.; Miguez, P.; Ko, C. Effect of Pore Size in Bone Regeneration Using Polydopamine-laced Hydroxyapatite Collagen Calcium Silicate Scaffolds Fabricated by 3D Mould Printing Technology. Orthod. Craniofac. Res. 2019, 22, 127–133. [Google Scholar] [CrossRef]
- Tamburaci, S.; Kimna, C.; Tihminlioglu, F. Bioactive Diatomite and POSS Silica Cage Reinforced Chitosan/Na-Carboxymethyl Cellulose Polyelectrolyte Scaffolds for Hard Tissue Regeneration. Mater. Sci. Eng. C 2019, 13, 196–208. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, L.; Xie, X.; Ren, J.; Ma, W. Three-Dimensional Printing Titanium Plate Bone-Anchored Maxillary Protraction Combined with Multidisciplinary Treatment of Skeletal Class III Malocclusion with Nonsyndromic Oligodontia. Am. J. Orthod. Dentofacial. Orthop. 2023, 3, 335–347. [Google Scholar] [CrossRef]
- Budharaju, H.; Suresh, S.; Sekar, M.P.; De Vega, B.; Sethuraman, S.; Sundaramurthi, D.; Kalaskar, D.M. Ceramic Materials for 3D Printing of Biomimetic Bone Scaffolds–Current State-of-the-Art & Future Perspectives. Mater. Des. 2023, 231, 112064. [Google Scholar] [CrossRef]
- Hudecki, A.; Łyko-Morawska, D.; Likus, W.; Skonieczna, M.; Markowski, J.; Wilk, R.; Kolano-Burian, A.; Maziarz, W.; Adamska, J.; Łos, M.J. Composite Nanofibers Containing Multiwall Carbon Nanotubes as Biodegradable Membranes in Reconstructive Medicine. Nanomaterials 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Yahay, Z.; Moein Farsani, N.; Mirhadi, M.; Tavangarian, F. Fabrication of Highly Ordered Willemite/PCL Bone Scaffolds by 3D Printing: Nanostructure Effects on Compressive Strength and in Vitro Behavior. J. Mech. Behav. Biomed. Mater. 2023, 144, 105996. [Google Scholar] [CrossRef] [PubMed]
- Lau, H.Y.; Hussin, M.S.; Hamat, S.; Abdul Manan, M.S.; Ibrahim, M.; Zakaria, H. Effect of Kenaf Fiber Loading on the Tensile Properties of 3D Printing PLA Filament. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Govindasamy, K.; Dahlan, N.A.; Janarthanan, P.; Goh, K.L.; Chai, S.-P.; Pasbakhsh, P. Electrospun Chitosan/Polyethylene-Oxide (PEO)/Halloysites (HAL) Membranes for Bone Regeneration Applications. Appl. Clay Sci. 2020, 190, 105601. [Google Scholar] [CrossRef]
- Cestari, F.; Petretta, M.; Yang, Y.; Motta, A.; Grigolo, B.; Sglavo, V.M. 3D Printing of PCL/Nano-Hydroxyapatite Scaffolds Derived from Biogenic Sources for Bone Tissue Engineering. Sustain. Mater. Technol. 2021, 29, e00318. [Google Scholar] [CrossRef]
- D’Angelo, N.A.; Câmara, M.C.C.; Noronha, M.A.; Grotto, D.; Chorilli, M.; Lourenço, F.R.; Rangel-Yagui, C.d.O.; Lopes, A.M. Development of PEG-PCL-Based Polymersomes through Design of Experiments for Co-Encapsulation of Vemurafenib and Doxorubicin as Chemotherapeutic Drugs. J. Mol. Liq. 2022, 349, 118166. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, K.; Liu, Y.; Zhang, C.; Wang, B. Textile-Based Sandwich Scaffold Using Wet Electrospun Yarns for Skin Tissue Engineering. J. Mech. Behav. Biomed. Mater. 2021, 119, 104499. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In Search of an Osteoblast Cell Model for in Vitro Research. Eur. Cell Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Jablonská, E.; Horkavcová, D.; Rohanová, D.; Brauer, D.S. A Review of In Vitro Cell Culture Testing Methods for Bioactive Glasses and Other Biomaterials for Hard Tissue Regeneration. J. Mater. Chem. B 2020, 8, 10941–10953. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009. Available online: https://www.iso.org/standard/36406.html (accessed on 23 April 2024).
- Sadat Rezaei, F.; Khorshidian, A.; Mahmoudi Beram, F.; Derakhshani, A.; Esmaeili, J.; Barati, A. 3D Printed Chitosan/Polycaprolactone Scaffold for Lung Tissue Engineering: Hope to Be Useful for COVID-19 Studies. RSC Adv. 2021, 11, 19508–19520. [Google Scholar] [CrossRef]
- Alper İşoğlu, İ.; Bölgen, N.; Korkusuz, P.; Vargel, İ.; Hamdi Çelik, H.; Güzel, E.; Çavuşoğlu, T.; Uçkan, D.; Pişkin, E. Stem Cells Combined 3D Electrospun Nanofibrous and Macrochannelled Matrices: A Preliminary Approach in Repair of Rat Cranial Bones. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1094–1110. [Google Scholar] [CrossRef] [PubMed]
- Teoh, S.H.; Goh, B.T.; Lim, J. 3D Printed Polycaprolactone Scaffolds for Bone Regeneration-Success and Future Perspective. Tissue Eng. Part A 2019, 25, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Gao, J.; Yang, R.; Yuan, C.; Wang, R.; Zou, Q.; Zuo, Y.; Zhu, M.; Li, Y.; Man, Y.; et al. A Baicalin-Loaded Coaxial Nanofiber Scaffold Regulated Inflammation and Osteoclast Differentiation for Vascularized Bone Regeneration. Bioact. Mater. 2022, 8, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Petretta, M.; Gambardella, A.; Boi, M.; Berni, M.; Cavallo, C.; Marchiori, G.; Maltarello, M.C.; Bellucci, D.; Fini, M.; Baldini, N.; et al. Composite Scaffolds for Bone Tissue Regeneration Based on PCL and Mg-Containing Bioactive Glasses. Biology 2021, 10, 398. [Google Scholar] [CrossRef]
- Hudecki, A.; Łyko-Morawska, D.; Kasprzycka, A.; Kazek-Kęsik, A.; Likus, W.; Hybiak, J.; Jankowska, K.; Kolano-Burian, A.; Włodarczyk, P.; Wolany, W.; et al. Comparison of Physicochemical, Mechanical, and (Micro-)Biological Properties of Sintered Scaffolds Based on Natural- and Synthetic Hydroxyapatite Supplemented with Selected Dopants. Int. J. Mol. Sci. 2022, 23, 4692. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating Bone with Bioactive Glass Scaffolds: A Review of in Vivo Studies in Bone Defect Models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Li, H.; Xia, W. Bioglass Could Increase Cell Membrane Fluidity with Ion Products to Develop Its Bioactivity. Cell Prolif. 2020, 53, e12906. [Google Scholar] [CrossRef]
- Deng, N.; Sun, J.; Li, Y.; Chen, L.; Chen, C.; Wu, Y.; Wang, Z.; Li, L. Experimental Study of rhBMP-2 Chitosan Nano-Sustained Release Carrier-Loaded PLGA/nHA Scaffolds to Construct Mandibular Tissue-Engineered Bone. Arch. Oral Biol. 2019, 36, 16–25. [Google Scholar] [CrossRef]
- Burden, N.; Clift, M.J.D.; Jenkins, G.J.S.; Labram, B.; Sewell, F. Opportunities and Challenges for Integrating New In Vitro Methodologies in Hazard Testing and Risk Assessment. Small 2021, 17, e2006298. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skubis-Sikora, A.; Hudecki, A.; Sikora, B.; Wieczorek, P.; Hermyt, M.; Hreczka, M.; Likus, W.; Markowski, J.; Siemianowicz, K.; Kolano-Burian, A.; et al. Toxicological Assessment of Biodegradable Poli-ε-Caprolactone Polymer Composite Materials Containing Hydroxyapatite, Bioglass, and Chitosan as Potential Biomaterials for Bone Regeneration Scaffolds. Biomedicines 2024, 12, 1949. https://doi.org/10.3390/biomedicines12091949
Skubis-Sikora A, Hudecki A, Sikora B, Wieczorek P, Hermyt M, Hreczka M, Likus W, Markowski J, Siemianowicz K, Kolano-Burian A, et al. Toxicological Assessment of Biodegradable Poli-ε-Caprolactone Polymer Composite Materials Containing Hydroxyapatite, Bioglass, and Chitosan as Potential Biomaterials for Bone Regeneration Scaffolds. Biomedicines. 2024; 12(9):1949. https://doi.org/10.3390/biomedicines12091949
Chicago/Turabian StyleSkubis-Sikora, Aleksandra, Andrzej Hudecki, Bartosz Sikora, Patrycja Wieczorek, Mateusz Hermyt, Marek Hreczka, Wirginia Likus, Jarosław Markowski, Krzysztof Siemianowicz, Aleksandra Kolano-Burian, and et al. 2024. "Toxicological Assessment of Biodegradable Poli-ε-Caprolactone Polymer Composite Materials Containing Hydroxyapatite, Bioglass, and Chitosan as Potential Biomaterials for Bone Regeneration Scaffolds" Biomedicines 12, no. 9: 1949. https://doi.org/10.3390/biomedicines12091949





