The Biological Clock of Liver Metabolism in Metabolic Dysfunction-Associated Steatohepatitis Progression to Hepatocellular Carcinoma
Abstract
1. Introduction
2. The Regulators of the Cellular Circadian/Biological Clock
3. Circadian Variations and Cell Differentiation
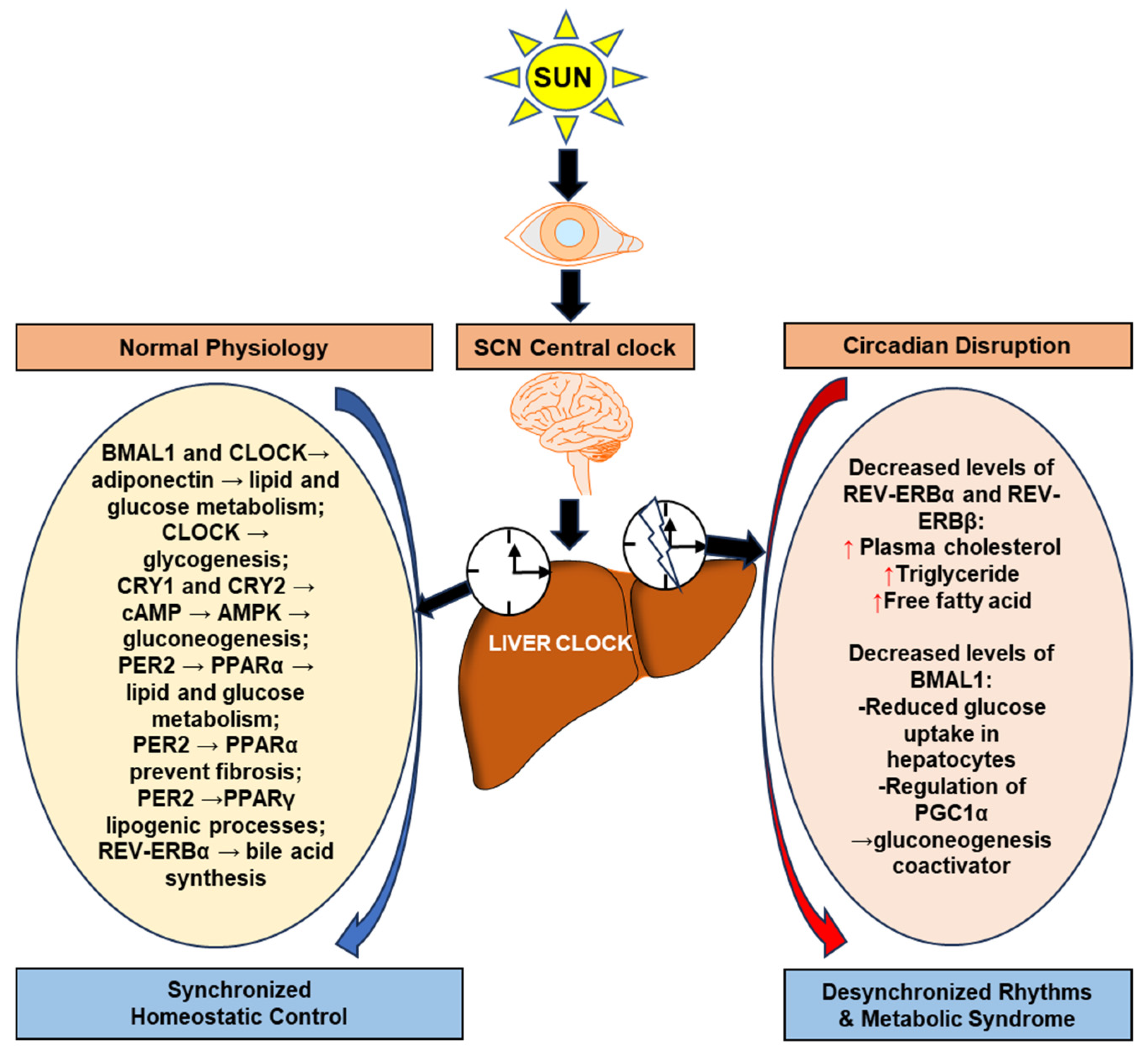
4. The Association of Cell Biological Clock Regulators with Liver Metabolism
5. Cell Circadian Clock Regulators and Cell Apoptosis
6. Cell Circadian Clock Regulators and Cell Autophagy
7. Epigenetic and Posttranslational Regulation of Liver Circadian Rhythms
8. The Role of Circadian Rhythms in HCC Progression
9. Circadian Genes and Therapeutic Targets in HCC
10. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AFP | α-fetoprotein |
| AIF | apoptosis-inducing factor |
| AMPK | adenosine monophosphate-activated protein kinase |
| APAF-1 | apoptotic protease activating factor-1 |
| ARID | AT-rich interactive domain-containing proteins |
| ARNTL | arylhydrocarbon receptor nuclear translocator-like |
| BMAL1 | brain and muscle Arnt-like protein-1 |
| cAMP | cyclic adenosine monophosphate |
| CAR | constitutive androstane receptor |
| CCGs | clock-controlled genes |
| CLOCK | circadian locomotor output cycles kaput |
| CRY1 | cryptochrome 1 |
| CRY2 | cryptochrome 2 |
| CSNK1D | casein kinase 1 delta f-box |
| CSNK1E | casein kinase 1 epsilon |
| CYCS | cytochrome C somatic |
| CYP7A1 | cholesterol 7α-hydroxylase |
| DEN | Diethylnitrosamine |
| DNMT1 | DNA methyltransferase1 |
| FBXL21 | leucine-rich repeat protein 21 |
| FGF21 | fibroblast growth factor 21 |
| FXR | farnesoid X receptor |
| lncRNAs | long noncoding RNAs |
| MAFLD | metabolic dysfunction-associated fatty liver disease |
| MAPK | mitogen-activated protein kinase |
| MASH | metabolic dysfunction-associated steatohepatitis |
| MLL | mixed lineage leukemia |
| MRTs | malignant rhabdoid tumors |
| mTOR | mammalian target of rapamycin |
| MWA | microwave ablation |
| NJKs | c-Jun N-terminal kinases |
| NOCT | Nocturnin |
| NPAS2/MOP4 | neuronal PAS domain protein 2 |
| NR1D1/REV-ERBα | nuclear receptor subfamily 1, group D, member 1 |
| NR1F1/RORα | nuclear receptor subfamily 1, group F, member 1 |
| PER1/2/3 | period-circadian protein homolog 1/2/3 |
| PGC-1α | Peroxisome proliferator activated receptor gamma coactivator1 alpha |
| PPAR-α | peroxisome proliferator-activated receptors-alpha |
| PPAR-γ | peroxisome proliferator-activated receptors-gamma |
| RFA | radiofrequency ablation |
| RHT | retino-hypothalamic tract |
| ROR | retinoic acid-related orphan nuclear receptor |
| ROS | reactive oxygen species |
| SBRT | stereotactic body radiation therapy |
| SCN | suprachiasmatic nucleus |
| SIRT-1 | Sirtuin-1 |
| SMAC | second mitochondria-derived activator of caspases |
| SNPs | single nucleotide polymorphisms |
| TACE | trans arterial chemoembolization |
| TFEB | transcription factor EB |
| TIM | Timeless |
| TNM | tumor node metastasis |
| TTFL | transcriptional translational feedback loop |
References
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef]
- Davis, S.; Mirick, D.K. Circadian disruption, shift work and the risk of cancer: A summary of the evidence and studies in Seattle. Cancer Causes Control 2006, 17, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Filipski, E.; King, V.M.; Li, X.; Granda, T.G.; Mormont, M.C.; Claustrat, B.; Hastings, M.H.; Levi, F. Disruption of circadian coordination accelerates malignant growth in mice. Pathol. Biol. 2003, 51, 216–219. [Google Scholar] [CrossRef]
- Fu, L.; Pelicano, H.; Liu, J.; Huang, P.; Lee, C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in Vivo. Cell 2002, 111, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Penev, P.D.; Kolker, D.E.; Zee, P.C.; Turek, F.W. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am. J. Physiol. 1998, 275, H2334–H2337. [Google Scholar] [CrossRef]
- Reddy, S.; Reddy, V.; Sharma, S. Physiology, Circadian Rhythm. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef]
- Panda, S.; Antoch, M.P.; Miller, B.H.; Su, A.I.; Schook, A.B.; Straume, M.; Schultz, P.G.; Kay, S.A.; Takahashi, J.S.; Hogenesch, J.B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 2002, 109, 307–320. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.; Xiao, W.; Fan, X. A review of MASLD-related hepatocellular carcinoma: Progress in pathogenesis, early detection, and therapeutic interventions. Front. Med. 2024, 11, 1410668. [Google Scholar] [CrossRef]
- Portincasa, P.; Khalil, M.; Mahdi, L.; Perniola, V.; Idone, V.; Graziani, A.; Baffy, G.; Di Ciaula, A. Metabolic Dysfunction-Associated Steatotic Liver Disease: From Pathogenesis to Current Therapeutic Options. Int. J. Mol. Sci. 2024, 25, 5640. [Google Scholar] [CrossRef]
- Qu, M.; Zhang, G.; Qu, H.; Vu, A.; Wu, R.; Tsukamoto, H.; Jia, Z.; Huang, W.; Lenz, H.J.; Rich, J.N.; et al. Circadian regulator BMAL1::CLOCK promotes cell proliferation in hepatocellular carcinoma by controlling apoptosis and cell cycle. Proc. Natl. Acad. Sci. USA 2023, 120, e2214829120. [Google Scholar] [CrossRef] [PubMed]
- Farshadi, E.; Yan, J.; Leclere, P.; Goldbeter, A.; Chaves, I.; van der Horst, G.T.J. The positive circadian regulators CLOCK and BMAL1 control G2/M cell cycle transition through Cyclin B1. Cell Cycle 2019, 18, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Garbazza, C.; Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 2019, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Reinke, H.; Asher, G. Circadian Clock Control of Liver Metabolic Functions. Gastroenterology 2016, 150, 574–580. [Google Scholar] [CrossRef]
- Gallego, M.; Virshup, D.M. Post-translational modifications regulate the ticking of the circadian clock. Nat. Rev. Mol. Cell. Biol. 2007, 8, 139–148. [Google Scholar] [CrossRef]
- Masri, S.; Sassone-Corsi, P. The circadian clock: A framework linking metabolism, epigenetics and neuronal function. Nat. Rev. Neurosci. 2013, 14, 69–75. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Harmer, S.L.; Panda, S.; Kay, S.A. Molecular bases of circadian rhythms. Annu. Rev. Cell Dev. Biol. 2001, 17, 215–253. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef]
- Lowrey, P.L.; Takahashi, J.S. Genetics of circadian rhythms in Mammalian model organisms. Adv. Genet. 2011, 74, 175–230. [Google Scholar] [CrossRef]
- Aggarwal, A.; Costa, M.J.; Rivero-Gutierrez, B.; Ji, L.; Morgan, S.L.; Feldman, B.J. The Circadian Clock Regulates Adipogenesis by a Per3 Crosstalk Pathway to Klf15. Cell Rep. 2017, 21, 2367–2375. [Google Scholar] [CrossRef]
- Kawai, M.; Green, C.B.; Lecka-Czernik, B.; Douris, N.; Gilbert, M.R.; Kojima, S.; Ackert-Bicknell, C.; Garg, N.; Horowitz, M.C.; Adamo, M.L.; et al. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-gamma nuclear translocation. Proc. Natl. Acad. Sci. USA 2010, 107, 10508–10513. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Rollins, D.; Ruhn, K.A.; Stubblefield, J.J.; Green, C.B.; Kashiwada, M.; Rothman, P.B.; Takahashi, J.S.; Hooper, L.V. TH17 cell differentiation is regulated by the circadian clock. Science 2013, 342, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Katoku-Kikyo, N.; Paatela, E.; Houtz, D.L.; Lee, B.; Munson, D.; Wang, X.; Hussein, M.; Bhatia, J.; Lim, S.; Yuan, C.; et al. Per1/Per2-Igf2 axis-mediated circadian regulation of myogenic differentiation. J. Cell. Biol. 2021, 220, e20210105. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, E.; Moriggi, E.; Bauer, C.; Dibner, C.; Brown, S.A. The circadian clock starts ticking at a developmentally early stage. J. Biol. Rhythms 2010, 25, 442–449. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Umemura, Y.; Yagita, K. Circadian clock and cancer: From a viewpoint of cellular differentiation. Int. J. Urol. 2020, 27, 518–524. [Google Scholar] [CrossRef]
- Perez-Villa, A.; Echeverria-Garces, G.; Ramos-Medina, M.J.; Prathap, L.; Martinez-Lopez, M.; Ramirez-Sanchez, D.; Garcia-Cardenas, J.M.; Armendariz-Castillo, I.; Guerrero, S.; Paz, C.; et al. Integrated multi-omics analysis reveals the molecular interplay between circadian clocks and cancer pathogenesis. Sci. Rep. 2023, 13, 14198. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Sinha, J.; Bahrami-Nejad, Z.; Teruel, M.N. The circadian clock mediates daily bursts of cell differentiation by periodically restricting cell-differentiation commitment. Proc. Natl. Acad. Sci. USA 2022, 119, e2204470119. [Google Scholar] [CrossRef]
- Umemura, Y.; Koike, N.; Matsumoto, T.; Yoo, S.H.; Chen, Z.; Yasuhara, N.; Takahashi, J.S.; Yagita, K. Transcriptional program of Kpna2/Importin-alpha2 regulates cellular differentiation-coupled circadian clock development in mammalian cells. Proc. Natl. Acad. Sci. USA 2014, 111, E5039–E5048. [Google Scholar] [CrossRef]
- Daley, G.Q. Common themes of dedifferentiation in somatic cell reprogramming and cancer. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 171–174. [Google Scholar] [CrossRef]
- Friedmann-Morvinski, D.; Verma, I.M. Dedifferentiation and reprogramming: Origins of cancer stem cells. EMBO Rep. 2014, 15, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P.; Koldobskiy, M.A.; Gondor, A. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat. Rev. Genet. 2016, 17, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Brien, G.L.; Valerio, D.G.; Armstrong, S.A. Exploiting the Epigenome to Control Cancer-Promoting Gene-Expression Programs. Cancer Cell 2016, 29, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Baylin, S.B.; Jones, P.A. A decade of exploring the cancer epigenome—Biological and translational implications. Nat. Rev. Cancer 2011, 11, 726–734. [Google Scholar] [CrossRef]
- Versteege, I.; Sevenet, N.; Lange, J.; Rousseau-Merck, M.F.; Ambros, P.; Handgretinger, R.; Aurias, A.; Delattre, O. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 1998, 394, 203–206. [Google Scholar] [CrossRef]
- Lee, R.S.; Stewart, C.; Carter, S.L.; Ambrogio, L.; Cibulskis, K.; Sougnez, C.; Lawrence, M.S.; Auclair, D.; Mora, J.; Golub, T.R.; et al. A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J. Clin. Investig. 2012, 122, 2983–2988. [Google Scholar] [CrossRef]
- Widschwendter, M.; Fiegl, H.; Egle, D.; Mueller-Holzner, E.; Spizzo, G.; Marth, C.; Weisenberger, D.J.; Campan, M.; Young, J.; Jacobs, I.; et al. Epigenetic stem cell signature in cancer. Nat. Genet. 2007, 39, 157–158. [Google Scholar] [CrossRef]
- Kim, J.; Orkin, S.H. Embryonic stem cell-specific signatures in cancer: Insights into genomic regulatory networks and implications for medicine. Genome Med. 2011, 3, 75. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Finley, L.W.S. Metabolic signatures of cancer cells and stem cells. Nat. Metab. 2019, 1, 177–188. [Google Scholar] [CrossRef]
- Sato, S.; Hishida, T.; Kinouchi, K.; Hatanaka, F.; Li, Y.; Nguyen, Q.; Chen, Y.; Wang, P.H.; Kessenbrock, K.; Li, W.; et al. The circadian clock CRY1 regulates pluripotent stem cell identity and somatic cell reprogramming. Cell Rep. 2023, 42, 112590. [Google Scholar] [CrossRef] [PubMed]
- Aschoff, J. Circadian Rhythms in Man. Science 1965, 148, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Yoshida, Y.; Minamino, T. A role for circadian clock in metabolic disease. Hypertens. Res. 2016, 39, 483–491. [Google Scholar] [CrossRef]
- Mukherji, A.; Bailey, S.M.; Staels, B.; Baumert, T.F. The circadian clock and liver function in health and disease. J. Hepatol. 2019, 71, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y. Regulation of bile acid synthesis. Front. Biosci. 1998, 3, d176–d193. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Lazar, M.A. Circadian Regulation of Gene Expression and Metabolism in the Liver. In Seminars in Liver Disease; Thieme Medical Publishers: New York, NY, USA, 2022; Volume 42, pp. 113–121. [Google Scholar] [CrossRef]
- Shi, D.; Chen, J.; Wang, J.; Yao, J.; Huang, Y.; Zhang, G.; Bao, Z. Circadian Clock Genes in the Metabolism of Non-alcoholic Fatty Liver Disease. Front. Physiol. 2019, 10, 423. [Google Scholar] [CrossRef]
- Saran, A.R.; Dave, S.; Zarrinpar, A. Circadian Rhythms in the Pathogenesis and Treatment of Fatty Liver Disease. Gastroenterology 2020, 158, 1948–1966.e1941. [Google Scholar] [CrossRef]
- Baust, G. Emergency medicine, a medical and social responsibility. Z. Fur Arztl. Fortbild. 1989, 83, 665–667. [Google Scholar]
- Greco, C.M.; Koronowski, K.B.; Smith, J.G.; Shi, J.; Kunderfranco, P.; Carriero, R.; Chen, S.; Samad, M.; Welz, P.S.; Zinna, V.M.; et al. Integration of feeding behavior by the liver circadian clock reveals network dependency of metabolic rhythms. Sci. Adv. 2021, 7, eabi7828. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.S.; Li, R.; Liu, V.Y.; Fu, L.; Moore, D.D.; Ma, K.; Yechoor, V.K. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in beta-cells. Islets 2011, 3, 381–388. [Google Scholar] [CrossRef]
- Shimba, S.; Ogawa, T.; Hitosugi, S.; Ichihashi, Y.; Nakadaira, Y.; Kobayashi, M.; Tezuka, M.; Kosuge, Y.; Ishige, K.; Ito, Y.; et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS ONE 2011, 6, e25231. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Zhong, X.; Yu, J.; Frazier, K.; Weng, X.; Li, Y.; Cham, C.M.; Dolan, K.; Zhu, X.; Hubert, N.; Tao, Y.; et al. Circadian Clock Regulation of Hepatic Lipid Metabolism by Modulation of m(6)A mRNA Methylation. Cell Rep. 2018, 25, 1816–1828. [Google Scholar] [CrossRef]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; DiTacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R.; et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012, 485, 62–68. [Google Scholar] [CrossRef]
- Bugge, A.; Feng, D.; Everett, L.J.; Briggs, E.R.; Mullican, S.E.; Wang, F.; Jager, J.; Lazar, M.A. Rev-erbalpha and Rev-erbbeta coordinately protect the circadian clock and normal metabolic function. Genes. Dev. 2012, 26, 657–667. [Google Scholar] [CrossRef]
- Crespo, M.; Gonzalez-Teran, B.; Nikolic, I.; Mora, A.; Folgueira, C.; Rodriguez, E.; Leiva-Vega, L.; Pintor-Chocano, A.; Fernandez-Chacon, M.; Ruiz-Garrido, I.; et al. Neutrophil infiltration regulates clock-gene expression to organize daily hepatic metabolism. Elife 2020, 9, e59258. [Google Scholar] [CrossRef] [PubMed]
- Manieri, E.; Folgueira, C.; Rodriguez, M.E.; Leiva-Vega, L.; Esteban-Lafuente, L.; Chen, C.; Cubero, F.J.; Barrett, T.; Cavanagh-Kyros, J.; Seruggia, D.; et al. JNK-mediated disruption of bile acid homeostasis promotes intrahepatic cholangiocarcinoma. Proc. Natl. Acad. Sci. USA 2020, 117, 16492–16499. [Google Scholar] [CrossRef] [PubMed]
- Robles, M.S.; Cox, J.; Mann, M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014, 10, e1004047. [Google Scholar] [CrossRef]
- Mauvoisin, D.; Wang, J.; Jouffe, C.; Martin, E.; Atger, F.; Waridel, P.; Quadroni, M.; Gachon, F.; Naef, F. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc. Natl. Acad. Sci. USA 2014, 111, 167–172. [Google Scholar] [CrossRef]
- Adamovich, Y.; Rousso-Noori, L.; Zwighaft, Z.; Neufeld-Cohen, A.; Golik, M.; Kraut-Cohen, J.; Wang, M.; Han, X.; Asher, G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014, 19, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Yoshitane, H.; Ozaki, H.; Terajima, H.; Du, N.H.; Suzuki, Y.; Fujimori, T.; Kosaka, N.; Shimba, S.; Sugano, S.; Takagi, T.; et al. CLOCK-controlled polyphonic regulation of circadian rhythms through canonical and noncanonical E-boxes. Mol. Cell. Biol. 2014, 34, 1776–1787. [Google Scholar] [CrossRef]
- Yamada, M.; Nagatomo, J.; Setoguchi, Y.; Kuroki, N.; Higashi, S.; Setoguchi, T. Circadian rhythms of sterol 12alpha-hydroxylase, cholesterol 7alpha-hydroxylase and DBP involved in rat cholesterol catabolism. Biol. Chem. 2000, 381, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Kovar, J.; Lenicek, M.; Zimolova, M.; Vitek, L.; Jirsa, M.; Pitha, J. Regulation of diurnal variation of cholesterol 7alpha-hydroxylase (CYP7A1) activity in healthy subjects. Physiol. Res. 2010, 59, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Noshiro, M.; Usui, E.; Kawamoto, T.; Kubo, H.; Fujimoto, K.; Furukawa, M.; Honma, S.; Makishima, M.; Honma, K.; Kato, Y. Multiple mechanisms regulate circadian expression of the gene for cholesterol 7alpha-hydroxylase (Cyp7a), a key enzyme in hepatic bile acid biosynthesis. J. Biol. Rhythms 2007, 22, 299–311. [Google Scholar] [CrossRef]
- Edwards, P.A.; Muroya, H.; Gould, R.G. In vivo demonstration of the circadian thythm of cholesterol biosynthesis in the liver and intestine of the rat. J. Lipid Res. 1972, 13, 396–401. [Google Scholar] [CrossRef]
- Galman, C.; Angelin, B.; Rudling, M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology 2005, 129, 1445–1453. [Google Scholar] [CrossRef]
- Duez, H.; van der Veen, J.N.; Duhem, C.; Pourcet, B.; Touvier, T.; Fontaine, C.; Derudas, B.; Bauge, E.; Havinga, R.; Bloks, V.W.; et al. Regulation of bile acid synthesis by the nuclear receptor Rev-erbalpha. Gastroenterology 2008, 135, 689–698. [Google Scholar] [CrossRef]
- Ma, K.; Xiao, R.; Tseng, H.T.; Shan, L.; Fu, L.; Moore, D.D. Circadian dysregulation disrupts bile acid homeostasis. PLoS ONE 2009, 4, e6843. [Google Scholar] [CrossRef]
- Ruiter, M.; La Fleur, S.E.; van Heijningen, C.; van der Vliet, J.; Kalsbeek, A.; Buijs, R.M. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes 2003, 52, 1709–1715. [Google Scholar] [CrossRef]
- Boden, G.; Ruiz, J.; Urbain, J.L.; Chen, X. Evidence for a circadian rhythm of insulin secretion. Am. J. Physiol. 1996, 271, E246–E252. [Google Scholar] [CrossRef] [PubMed]
- La Fleur, S.E.; Kalsbeek, A.; Wortel, J.; Buijs, R.M. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. J. Neuroendocrinol. 1999, 11, 643–652. [Google Scholar] [CrossRef] [PubMed]
- la Fleur, S.E.; Kalsbeek, A.; Wortel, J.; Fekkes, M.L.; Buijs, R.M. A daily rhythm in glucose tolerance: A role for the suprachiasmatic nucleus. Diabetes 2001, 50, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Sadacca, L.A.; Lamia, K.A.; deLemos, A.S.; Blum, B.; Weitz, C.J. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 2011, 54, 120–124. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef]
- Emilsson, V.; Liu, Y.L.; Cawthorne, M.A.; Morton, N.M.; Davenport, M. Expression of the functional leptin receptor mRNA in pancreatic islets and direct inhibitory action of leptin on insulin secretion. Diabetes 1997, 46, 313–316. [Google Scholar] [CrossRef]
- Meinders, A.E.; Toornvliet, A.C.; Pijl, H. Leptin. Neth. J. Med. 1996, 49, 247–252. [Google Scholar] [CrossRef]
- Ando, H.; Kumazaki, M.; Motosugi, Y.; Ushijima, K.; Maekawa, T.; Ishikawa, E.; Fujimura, A. Impairment of peripheral circadian clocks precedes metabolic abnormalities in ob/ob mice. Endocrinology 2011, 152, 1347–1354. [Google Scholar] [CrossRef]
- Ishikawa-Kobayashi, E.; Ushijima, K.; Ando, H.; Maekawa, T.; Takuma, M.; Furukawa, Y.; Fujimura, A. Reduced histone H3K9 acetylation of clock genes and abnormal glucose metabolism in ob/ob mice. Chronobiol. Int. 2012, 29, 982–993. [Google Scholar] [CrossRef]
- Machicao, F.; Peter, A.; Machann, J.; Konigsrainer, I.; Bohm, A.; Lutz, S.Z.; Heni, M.; Fritsche, A.; Schick, F.; Konigsrainer, A.; et al. Glucose-Raising Polymorphisms in the Human Clock Gene Cryptochrome 2 (CRY2) Affect Hepatic Lipid Content. PLoS ONE 2016, 11, e0145563. [Google Scholar] [CrossRef]
- Matsuo, T.; Yamaguchi, S.; Mitsui, S.; Emi, A.; Shimoda, F.; Okamura, H. Control mechanism of the circadian clock for timing of cell division in Vivo. Science 2003, 302, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Kondratov, R.V.; Antoch, M.P. Circadian proteins in the regulation of cell cycle and genotoxic stress responses. Trends Cell Biol. 2007, 17, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.A.; McNair, C.M.; McCann, J.J.; Alshalalfa, M.; Shostak, A.; Severson, T.M.; Zhu, Y.; Bergman, A.; Gordon, N.; Mandigo, A.C.; et al. The circadian cryptochrome, CRY1, is a pro-tumorigenic factor that rhythmically modulates DNA repair. Nat. Commun. 2021, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, Y.; Wang, S.; Liu, H. Circadian Clock Genes Modulate Immune, Cell Cycle and Apoptosis in the Diagnosis and Prognosis of Pan-Renal Cell Carcinoma. Front. Mol. Biosci. 2021, 8, 747629. [Google Scholar] [CrossRef]
- Lee, J.H.; Sancar, A. Regulation of apoptosis by the circadian clock through NF-kappaB signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 12036–12041. [Google Scholar] [CrossRef]
- Matsunaga, N.; Kohno, Y.; Kakimoto, K.; Hayashi, A.; Koyanagi, S.; Ohdo, S. Influence of CLOCK on cytotoxicity induced by diethylnitrosamine in mouse primary hepatocytes. Toxicology 2011, 280, 144–151. [Google Scholar] [CrossRef]
- Yuan, P.; Li, J.; Zhou, F.; Huang, Q.; Zhang, J.; Guo, X.; Lyu, Z.; Zhang, H.; Xing, J. NPAS2 promotes cell survival of hepatocellular carcinoma by transactivating CDC25A. Cell Death Dis. 2017, 8, e2704. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Guo, Y.; Liu, R.; Qi, F.; Li, X.; Yu, H.; Cheng, S.; Wang, Z. Circadian gene Clock participates in mitochondrial apoptosis pathways by regulating mitochondrial membrane potential, mitochondria out membrane permeablization and apoptosis factors in AML12 hepatocytes. Mol. Cell Biochem. 2020, 467, 65–75. [Google Scholar] [CrossRef]
- Gu, X.; Xing, L.; Shi, G.; Liu, Z.; Wang, X.; Qu, Z.; Wu, X.; Dong, Z.; Gao, X.; Liu, G.; et al. The circadian mutation PER2(S662G) is linked to cell cycle progression and tumorigenesis. Cell Death Differ. 2012, 19, 397–405. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Z.; Cai, Y.; Zeng, S.; Peng, B.; Ren, X.; Yan, Y.; Gong, Z. Rheostatic Balance of Circadian Rhythm and Autophagy in Metabolism and Disease. Front. Cell Dev. Biol. 2020, 8, 616434. [Google Scholar] [CrossRef]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef]
- McKee, C.A.; Polino, A.J.; King, M.W.; Musiek, E.S. Circadian clock protein BMAL1 broadly influences autophagy and endolysosomal function in astrocytes. Proc. Natl. Acad. Sci. USA 2023, 120, e2220551120. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Y.; Fan, H.; Wang, Y.; Fan, S.; Hu, S.; Shen, H.; Li, H.; Xue, Q.; Ni, J.; et al. GluA1 Degradation by Autophagy Contributes to Circadian Rhythm Effects on Cerebral Ischemia Injury. J. Neurosci. 2023, 43, 2381–2397. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich-Nikitin, I.; Love, M.; Kirshenbaum, L.A. Intersection of autophagy regulation and circadian rhythms in the heart. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166354. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cornelissen-Guillaume, G.G.; He, J.; Kastin, A.J.; Harrison, L.M.; Pan, W. Circadian rhythm of autophagy proteins in hippocampus is blunted by sleep fragmentation. Chronobiol. Int. 2016, 33, 553–560. [Google Scholar] [CrossRef]
- Maiese, K. Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, mTOR, and SIRT1 in Degenerative Disease and Cancer. Curr. Neurovasc. Res. 2017, 14, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, E.; Luo, B.; Cai, D.; Zhao, X.; Luo, Q.; Jin, Y.; Chen, L.; Wang, Q.; Liu, C.; et al. Methamphetamine exposure triggers apoptosis and autophagy in neuronal cells by activating the C/EBPbeta-related signaling pathway. FASEB J. 2018, 32, 6737–6759. [Google Scholar] [CrossRef]
- Ma, D.; Lin, J.D. Circadian regulation of autophagy rhythm through transcription factor C/EBPbeta. Autophagy 2012, 8, 124–125. [Google Scholar] [CrossRef]
- Ma, D.; Panda, S.; Lin, J.D. Temporal orchestration of circadian autophagy rhythm by C/EBPbeta. EMBO J. 2011, 30, 4642–4651. [Google Scholar] [CrossRef]
- Nierenberg, A.A.; Ghaznavi, S.A.; Sande Mathias, I.; Ellard, K.K.; Janos, J.A.; Sylvia, L.G. Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 Alpha as a Novel Target for Bipolar Disorder and Other Neuropsychiatric Disorders. Biol. Psychiatry 2018, 83, 761–769. [Google Scholar] [CrossRef]
- Sonoda, J.; Mehl, I.R.; Chong, L.W.; Nofsinger, R.R.; Evans, R.M. PGC-1beta controls mitochondrial metabolism to modulate circadian activity, adaptive thermogenesis, and hepatic steatosis. Proc. Natl. Acad. Sci. USA 2007, 104, 5223–5228. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, S.; Liu, T.; Borjigin, J.; Lin, J.D. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature 2007, 447, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, J.D. Transcriptional control of circadian metabolic rhythms in the liver. Diabetes Obes. Metab. 2015, 17 (Suppl. 1), 33–38. [Google Scholar] [CrossRef]
- Annunziata, I.; van de Vlekkert, D.; Wolf, E.; Finkelstein, D.; Neale, G.; Machado, E.; Mosca, R.; Campos, Y.; Tillman, H.; Roussel, M.F.; et al. MYC competes with MiT/TFE in regulating lysosomal biogenesis and autophagy through an epigenetic rheostat. Nat. Commun. 2019, 10, 3623. [Google Scholar] [CrossRef]
- Yang, M.; Liu, E.; Tang, L.; Lei, Y.; Sun, X.; Hu, J.; Dong, H.; Yang, S.M.; Gao, M.; Tang, B. Emerging roles and regulation of MiT/TFE transcriptional factors. Cell Commun. Signal 2018, 16, 31. [Google Scholar] [CrossRef]
- Pastore, N.; Vainshtein, A.; Herz, N.J.; Huynh, T.; Brunetti, L.; Klisch, T.J.; Mutarelli, M.; Annunziata, P.; Kinouchi, K.; Brunetti-Pierri, N.; et al. Nutrient-sensitive transcription factors TFEB and TFE3 couple autophagy and metabolism to the peripheral clock. EMBO J. 2019, 38, e101347. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Chen, L.; Ge, L.; Huang, W. A Novel Loop: Mutual Regulation Between Epigenetic Modification and the Circadian Clock. Front. Plant Sci. 2019, 10, 22. [Google Scholar] [CrossRef]
- Hudec, M.; Dankova, P.; Solc, R.; Bettazova, N.; Cerna, M. Epigenetic Regulation of Circadian Rhythm and Its Possible Role in Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 3005. [Google Scholar] [CrossRef]
- Saad, L.; Zwiller, J.; Kalsbeek, A.; Anglard, P. Epigenetic Regulation of Circadian Clocks and Its Involvement in Drug Addiction. Genes 2021, 12, 1263. [Google Scholar] [CrossRef]
- Fujimoto, A.; Totoki, Y.; Abe, T.; Boroevich, K.A.; Hosoda, F.; Nguyen, H.H.; Aoki, M.; Hosono, N.; Kubo, M.; Miya, F.; et al. Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat. Genet. 2012, 44, 760–764. [Google Scholar] [CrossRef]
- Valekunja, U.K.; Edgar, R.S.; Oklejewicz, M.; van der Horst, G.T.; O’Neill, J.S.; Tamanini, F.; Turner, D.J.; Reddy, A.B. Histone methyltransferase MLL3 contributes to genome-scale circadian transcription. Proc. Natl. Acad. Sci. USA 2013, 110, 1554–1559. [Google Scholar] [CrossRef]
- Doyen, C.M.; An, W.; Angelov, D.; Bondarenko, V.; Mietton, F.; Studitsky, V.M.; Hamiche, A.; Roeder, R.G.; Bouvet, P.; Dimitrov, S. Mechanism of polymerase II transcription repression by the histone variant macroH2A. Mol. Cell Biol. 2006, 26, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Pazienza, V.; Panebianco, C.; Rappa, F.; Memoli, D.; Borghesan, M.; Cannito, S.; Oji, A.; Mazza, G.; Tamburrino, D.; Fusai, G.; et al. Histone macroH2A1.2 promotes metabolic health and leanness by inhibiting adipogenesis. Epigenetics Chromatin 2016, 9, 45. [Google Scholar] [CrossRef]
- Carbone, A.; De Santis, E.; Cela, O.; Giambra, V.; Miele, L.; Marrone, G.; Grieco, A.; Buschbeck, M.; Capitanio, N.; Mazza, T.; et al. The Histone Variant MacroH2A1 Impacts Circadian Gene Expression and Cell Phenotype in an In Vitro Model of Hepatocellular Carcinoma. Biomedicines 2021, 9, 1057. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Schoonjans, K.; Auwerx, J. Sirtuin functions in health and disease. Mol. Endocrinol. 2007, 21, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.J.; Humphries, J.R.; Niemeyer, D.J.; Sindram, D.; McKillop, I.H. The effect of alcohol on Sirt1 expression and function in animal and human models of hepatocellular carcinoma (HCC). Adv. Exp. Med. Biol. 2015, 815, 361–373. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Alenghat, T.; Meyers, K.; Mullican, S.E.; Leitner, K.; Adeniji-Adele, A.; Avila, J.; Bucan, M.; Ahima, R.S.; Kaestner, K.H.; Lazar, M.A. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature 2008, 456, 997–1000. [Google Scholar] [CrossRef]
- Feng, D.; Liu, T.; Sun, Z.; Bugge, A.; Mullican, S.E.; Alenghat, T.; Liu, X.S.; Lazar, M.A. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 2011, 331, 1315–1319. [Google Scholar] [CrossRef]
- Wilting, R.H.; Dannenberg, J.H. Epigenetic mechanisms in tumorigenesis, tumor cell heterogeneity and drug resistance. Drug Resist. Updat. 2012, 15, 21–38. [Google Scholar] [CrossRef]
- Lin, Y.M.; Chang, J.H.; Yeh, K.T.; Yang, M.Y.; Liu, T.C.; Lin, S.F.; Su, W.W.; Chang, J.G. Disturbance of circadian gene expression in hepatocellular carcinoma. Mol. Carcinog. 2008, 47, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Boumans, I.; de Boer, I.J.M.; Hofstede, G.J.; la Fleur, S.E.; Bokkers, E.A.M. The importance of hormonal circadian rhythms in daily feeding patterns: An illustration with simulated pigs. Horm. Behav. 2017, 93, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Fustin, J.M.; Doi, M.; Yamaguchi, Y.; Hida, H.; Nishimura, S.; Yoshida, M.; Isagawa, T.; Morioka, M.S.; Kakeya, H.; Manabe, I.; et al. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell 2013, 155, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, X.; Zheng, L.; Zhuang, C. Gene Signatures and Prognostic Values of m6A Regulators in Hepatocellular Carcinoma. Front. Genet. 2020, 11, 540186. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Chen, J.; Peng, C.; Zhang, Y.; Tong, R.; Cheng, Q.; Yang, B.; Feng, X.; Lu, Y.; et al. ALKBH5 suppresses malignancy of hepatocellular carcinoma via m(6)A-guided epigenetic inhibition of LYPD1. Mol. Cancer 2020, 19, 123. [Google Scholar] [CrossRef]
- Guo, X.; Li, K.; Jiang, W.; Hu, Y.; Xiao, W.; Huang, Y.; Feng, Y.; Pan, Q.; Wan, R. RNA demethylase ALKBH5 prevents pancreatic cancer progression by posttranscriptional activation of PER1 in an m6A-YTHDF2-dependent manner. Mol. Cancer 2020, 19, 91. [Google Scholar] [CrossRef]
- Liu, G.M.; Zeng, H.D.; Zhang, C.Y.; Xu, J.W. Identification of METTL3 as an Adverse Prognostic Biomarker in Hepatocellular Carcinoma. Dig. Dis. Sci. 2021, 66, 1110–1126. [Google Scholar] [CrossRef]
- Liu, C.; Chung, M. Genetics and epigenetics of circadian rhythms and their potential roles in neuropsychiatric disorders. Neurosci. Bull. 2015, 31, 141–159. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.; Diao, Y.; Liu, J.; Li, J.; Li, R.; Zheng, L.; Zhang, K.; Ma, Y.; Hao, X. Circulating tumour DNA methylation in hepatocellular carcinoma diagnosis using digital droplet PCR. J. Int. Med. Res. 2021, 49, 300060521992962. [Google Scholar] [CrossRef]
- Han, T.S.; Ban, H.S.; Hur, K.; Cho, H.S. The Epigenetic Regulation of HCC Metastasis. Int. J. Mol. Sci. 2018, 19, 3978. [Google Scholar] [CrossRef]
- Rumgay, H.; Ferlay, J.; de Martel, C.; Georges, D.; Ibrahim, A.S.; Zheng, R.; Wei, W.; Lemmens, V.; Soerjomataram, I. Global, regional and national burden of primary liver cancer by subtype. Eur. J. Cancer 2022, 161, 108–118. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Foglia, B.; Turato, C.; Cannito, S. Hepatocellular Carcinoma: Latest Research in Pathogenesis, Detection and Treatment. Int. J. Mol. Sci. 2023, 24, 12224. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Kanwal, F.; Llovet, J.M. Global trends in hepatocellular carcinoma epidemiology: Implications for screening, prevention and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 864–884. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Crespo, M.; Leiva, M.; Sabio, G. Circadian Clock and Liver Cancer. Cancers 2021, 13, 3631. [Google Scholar] [CrossRef]
- Yang, Y.; Abdo, A.N.; Kawara, H.; Selby, C.P.; Sancar, A. Preservation of circadian rhythm in hepatocellular cancer. J. Biol. Chem. 2023, 299, 105251. [Google Scholar] [CrossRef]
- Jiang, Y.; Shen, X.; Fasae, M.B.; Zhi, F.; Chai, L.; Ou, Y.; Feng, H.; Liu, S.; Liu, Y.; Yang, S. The Expression and Function of Circadian Rhythm Genes in Hepatocellular Carcinoma. Oxid. Med. Cell Longev. 2021, 2021, 4044606. [Google Scholar] [CrossRef]
- Coomans, C.P.; van den Berg, S.A.; Lucassen, E.A.; Houben, T.; Pronk, A.C.; van der Spek, R.D.; Kalsbeek, A.; Biermasz, N.R.; Willems van Dijk, K.; Romijn, J.A.; et al. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes 2013, 62, 1102–1108. [Google Scholar] [CrossRef]
- Lee, S.; Donehower, L.A.; Herron, A.J.; Moore, D.D.; Fu, L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS ONE 2010, 5, e10995. [Google Scholar] [CrossRef] [PubMed]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef] [PubMed]
- Tentolouris, N.; Liatis, S.; Katsilambros, N. Sympathetic system activity in obesity and metabolic syndrome. Ann. N. Y. Acad. Sci. 2006, 1083, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Kettner, N.M.; Voicu, H.; Finegold, M.J.; Coarfa, C.; Sreekumar, A.; Putluri, N.; Katchy, C.A.; Lee, C.; Moore, D.D.; Fu, L. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell 2016, 30, 909–924. [Google Scholar] [CrossRef]
- Kettner, N.M.; Katchy, C.A.; Fu, L. Circadian gene variants in cancer. Ann. Med. 2014, 46, 208–220. [Google Scholar] [CrossRef]
- Filipski, E.; Levi, F. Circadian disruption in experimental cancer processes. Integr. Cancer Ther. 2009, 8, 298–302. [Google Scholar] [CrossRef]
- Mteyrek, A.; Filipski, E.; Guettier, C.; Okyar, A.; Levi, F. Clock gene Per2 as a controller of liver carcinogenesis. Oncotarget 2016, 7, 85832–85847. [Google Scholar] [CrossRef]
- Fleet, T.; Stashi, E.; Zhu, B.; Rajapakshe, K.; Marcelo, K.L.; Kettner, N.M.; Gorman, B.K.; Coarfa, C.; Fu, L.; O’Malley, B.W.; et al. Genetic and Environmental Models of Circadian Disruption Link SRC-2 Function to Hepatic Pathology. J. Biol. Rhythms 2016, 31, 443–460. [Google Scholar] [CrossRef]
- Zhao, B.; Lu, J.; Yin, J.; Liu, H.; Guo, X.; Yang, Y.; Ge, N.; Zhu, Y.; Zhang, H.; Xing, J. A functional polymorphism in PER3 gene is associated with prognosis in hepatocellular carcinoma. Liver Int. 2012, 32, 1451–1459. [Google Scholar] [CrossRef]
- Parikh, N.D.; Pillai, A. Recent Advances in Hepatocellular Carcinoma Treatment. Clin. Gastroenterol. Hepatol. 2021, 19, 2020–2024. [Google Scholar] [CrossRef]
- Singal, A.G.; Kudo, M.; Bruix, J. Breakthroughs in Hepatocellular Carcinoma Therapies. Clin. Gastroenterol. Hepatol. 2023, 21, 2135–2149. [Google Scholar] [CrossRef]
- Yarchoan, M.; Agarwal, P.; Villanueva, A.; Rao, S.; Dawson, L.A.; Llovet, J.M.; Finn, R.S.; Groopman, J.D.; El-Serag, H.B.; Monga, S.P.; et al. Recent Developments and Therapeutic Strategies against Hepatocellular Carcinoma. Cancer Res. 2019, 79, 4326–4330. [Google Scholar] [CrossRef] [PubMed]
- Blum, H.E. Hepatocellular carcinoma: Therapy and prevention. World J. Gastroenterol. 2005, 11, 7391–7400. [Google Scholar] [CrossRef] [PubMed]
- Ramai, D.; Singh, J.; Lester, J.; Khan, S.R.; Chandan, S.; Tartaglia, N.; Ambrosi, A.; Serviddio, G.; Facciorusso, A. Systematic review with meta-analysis: Bariatric surgery reduces the incidence of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2021, 53, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Casper, R.F.; Gladanac, B. Introduction: Circadian rhythm and its disruption: Impact on reproductive function. Fertil. Steril. 2014, 102, 319–320. [Google Scholar] [CrossRef]
- Ye, Y.; Xiang, Y.; Ozguc, F.M.; Kim, Y.; Liu, C.J.; Park, P.K.; Hu, Q.; Diao, L.; Lou, Y.; Lin, C.; et al. The Genomic Landscape and Pharmacogenomic Interactions of Clock Genes in Cancer Chronotherapy. Cell Syst. 2018, 6, 314–328.e312. [Google Scholar] [CrossRef]
- Li, X.M.; Mohammad-Djafari, A.; Dumitru, M.; Dulong, S.; Filipski, E.; Siffroi-Fernandez, S.; Mteyrek, A.; Scaglione, F.; Guettier, C.; Delaunay, F.; et al. A circadian clock transcription model for the personalization of cancer chronotherapy. Cancer Res. 2013, 73, 7176–7188. [Google Scholar] [CrossRef]
- Levi, F.; Okyar, A.; Dulong, S.; Innominato, P.F.; Clairambault, J. Circadian timing in cancer treatments. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 377–421. [Google Scholar] [CrossRef]
- Innominato, P.F.; Giacchetti, S.; Bjarnason, G.A.; Focan, C.; Garufi, C.; Coudert, B.; Iacobelli, S.; Tampellini, M.; Durando, X.; Mormont, M.C.; et al. Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int. J. Cancer 2012, 131, 2684–2692. [Google Scholar] [CrossRef]
- Mormont, M.C.; Waterhouse, J.; Bleuzen, P.; Giacchetti, S.; Jami, A.; Bogdan, A.; Lellouch, J.; Misset, J.L.; Touitou, Y.; Levi, F. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin. Cancer Res. 2000, 6, 3038–3045. [Google Scholar]
- Wang, R.Q.; Cui, W.; Cai, J.; Sun, Y. Integrative analysis indicates the prognostic value of circadian rhythm disruption in liver cancer: Potential for therapeutic targeting. Front. Immunol. 2022, 13, 1011264. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, H.; Zhang, Q.; Wang, T.; Li, H.; Qin, Y.; Ai, X.; Yi, W.; Wei, X.; Gao, W.; et al. Four circadian rhythm-related genes predict incidence and prognosis in hepatocellular carcinoma. Front. Oncol. 2022, 12, 937403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, W.; Tan, X.; Deng, T.; Zhou, W.; Jian, H.; Zeng, P. Construction and verification of a novel circadian clock related long non-coding RNA model and prediction of treatment for survival prognosis in patients with hepatocellular carcinoma. BMC Cancer 2023, 23, 57. [Google Scholar] [CrossRef] [PubMed]


| Circadian Genes | Circadian Function |
|---|---|
| CLOCK | bHLH-PAS domain containing transcription factor, Positive Regulator, co-activator of PERs-CRYs transcription; Histone Acetyltransferase |
| BMAL1 | bHLH-PAS domain containing transcription factor, Positive Regulator |
| REV-ERBα | Nuclear Receptor, Negative Regulator; Repressor of BMAL1 transcription and regulator of clock-controlled genes |
| REV-ERBβ | Paralog of NR1D1 |
| NOCT | mRNA Deadenylase |
| PGC-1α | Transcriptional coactivator |
| PER1 | Co-repressor of CLOCK-BMAL1; PAS-domain containing negative regulator |
| PER2 | Co-repressor of CLOCK-BMAL1; PAS-domain containing negative regulator |
| PER3 | Influence chronotype |
| CRY1 | Negative Regulator/Co-repressor of CLOCK-BMAL1 |
| CRY2 | Negative Regulator/Co-repressor of CLOCK-BMAL1 |
| ARNTL | Circadian clock-regulated transcription factor promoting expression of genes involved in angiogenesis and tumor progression; Dimerization partner of CLOCK/NPAS2; co-activator of PERs-CRYs transcription |
| TIM | Role in the production of electrical oscillations output of the SCN |
| ROR | Nuclear Receptor, Positive Regulator |
| RORα | Activator of BMAL1 transcription and regulator of clock-controlled genes |
| RORβ | Paralog of NR1F1 |
| NPAS2 | Paralog of CLOCK; dimerization partner of BMAL1/2 |
| CSNK1E | Phosphorylation of PERs |
| PPAR-γ | Metabolic regulator gene, Differentiation of adipocytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajan, P.K.; Udoh, U.-A.S.; Finley, R.; Pierre, S.V.; Sanabria, J. The Biological Clock of Liver Metabolism in Metabolic Dysfunction-Associated Steatohepatitis Progression to Hepatocellular Carcinoma. Biomedicines 2024, 12, 1961. https://doi.org/10.3390/biomedicines12091961
Rajan PK, Udoh U-AS, Finley R, Pierre SV, Sanabria J. The Biological Clock of Liver Metabolism in Metabolic Dysfunction-Associated Steatohepatitis Progression to Hepatocellular Carcinoma. Biomedicines. 2024; 12(9):1961. https://doi.org/10.3390/biomedicines12091961
Chicago/Turabian StyleRajan, Pradeep Kumar, Utibe-Abasi S. Udoh, Robert Finley, Sandrine V. Pierre, and Juan Sanabria. 2024. "The Biological Clock of Liver Metabolism in Metabolic Dysfunction-Associated Steatohepatitis Progression to Hepatocellular Carcinoma" Biomedicines 12, no. 9: 1961. https://doi.org/10.3390/biomedicines12091961
APA StyleRajan, P. K., Udoh, U.-A. S., Finley, R., Pierre, S. V., & Sanabria, J. (2024). The Biological Clock of Liver Metabolism in Metabolic Dysfunction-Associated Steatohepatitis Progression to Hepatocellular Carcinoma. Biomedicines, 12(9), 1961. https://doi.org/10.3390/biomedicines12091961






