Postoperative Atrial Fibrillation: A Review
Abstract
1. Introduction
Incidence
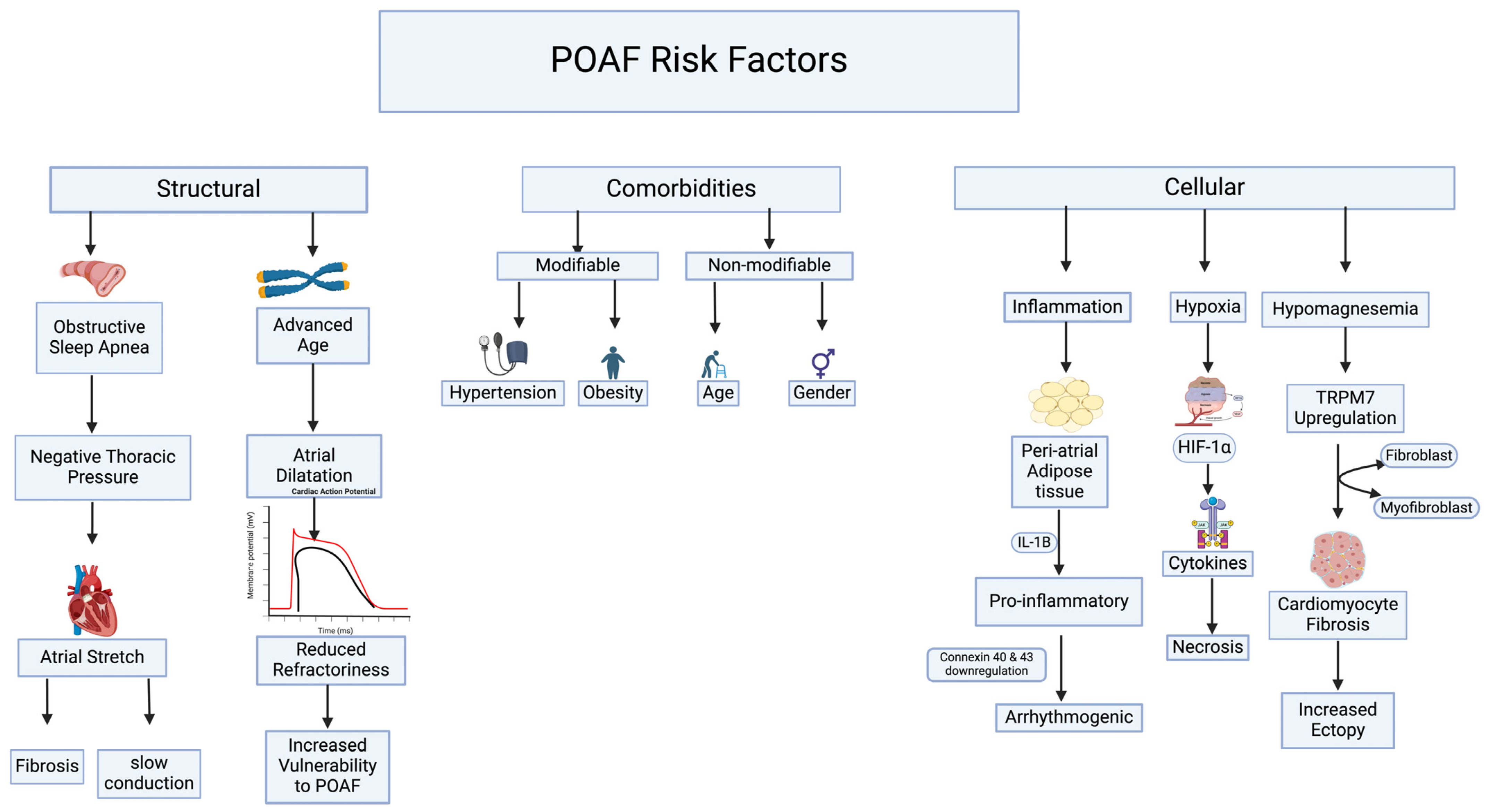
2. Risk Factors
3. Prediction
4. Pathophysiology
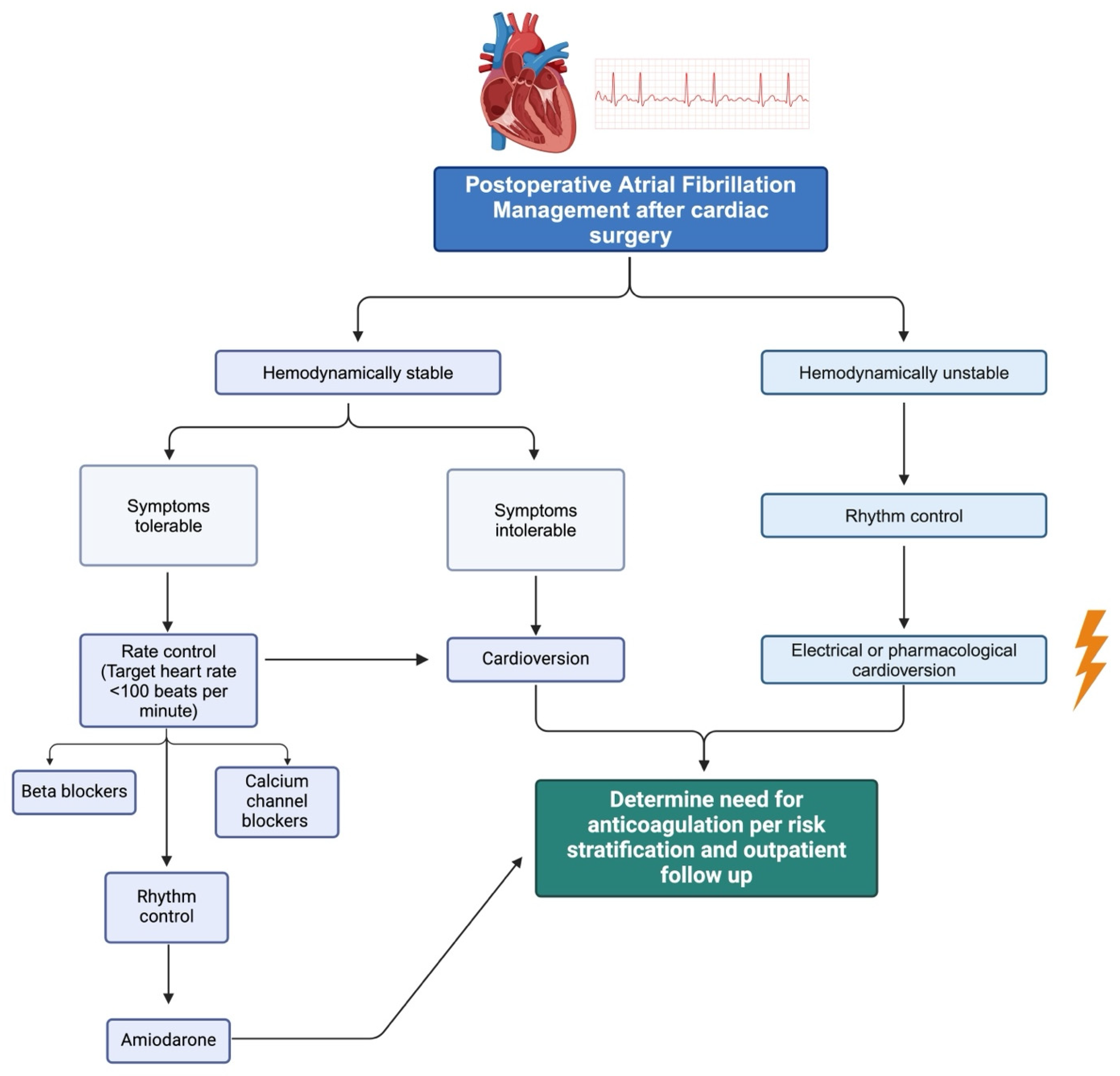
5. Treatment
Prevention
6. Management of Anticoagulation
7. Periprocedural Interruption and Postprocedural Bridging
8. Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| POAF | Post-operative atrial fibrillation |
| CABG | Coronary artery bypass surgery |
| TAVR | Transcatheter aortic valve replacement |
| CPB | Cardiopulmonary bypass |
| BB | Beta-blockers |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| OSA | Obstructive Sleep Apnea |
| EKG | Electrocardiogram |
| HIF | Hypoxia-inducible factor-1α |
| IL-1β | Interleukin 1β |
| NTP | Negative thoracic pressure |
| DOACs | Direct oral anticoagulants |
| LMWH | Low-molecular-weight heparin |
| UFH | Unfractionated heparin |
| AI | Artificial Intelligence |
| ML | Machine Learning |
References
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Joshi, K.K.; Tiru, M.; Chin, T.; Fox, M.T.; Stefan, M.S. Postoperative Atrial fibrillation in Patients undergoing Non-cardiac Non-thoracic Surgery: A Practical Approach for the Hospitalist. Hosp. Pract. 2015, 43, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Egbe, A.C.; Miranda, W.R.; Anderson, J.H.; DeSimone, C.V.; Andi, K.; Goda, A.Y.; Stephens, E.H.; Dearani, J.A.; Crestanello, J.; Connolly, H.M.; et al. Outcome of New-Onset Postoperative Atrial Fibrillation after Cardiac Surgery in Adults with Congenital Heart Disease. JACC Clin. Electrophysiol. 2022, 8, 1407–1416. [Google Scholar] [CrossRef]
- McIntyre, W.F. Post-operative atrial fibrillation after cardiac surgery: Challenges throughout the patient journey. Front. Cardiovasc. Med. 2023, 10, 1156626. [Google Scholar] [CrossRef]
- Gaudino, M.; Di Franco, A.; Rong, L.Q.; Piccini, J.; Mack, M. Postoperative atrial fibrillation: From mechanisms to treatment. Eur. Heart J. 2023, 44, 1020–1039. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.A.; Agrawal, D.K. Post-Operative Atrial Fibrillation: Current Treatments and Etiologies for a Persistent Surgical Complication. J. Surg. Res. 2022, 5, 159–172. [Google Scholar] [CrossRef]
- Meenashi Sundaram, D.; Vasavada, A.M.; Ravindra, C.; Rengan, V.; Meenashi Sundaram, P. The Management of Postoperative Atrial Fibrillation (POAF): A Systematic Review. Cureus 2023, 15, e42880. [Google Scholar] [CrossRef]
- Jagadish, P.S.; Kirolos, I.; Khare, S.; Rawal, A.; Lin, V.; Khouzam, R.N. Post-operative atrial fibrillation: Should we anticoagulate? Ann. Transl. Med. 2019, 7, 407. [Google Scholar] [CrossRef]
- Bidar, E.; Bramer, S.; Maesen, B.; Maessen, J.G.; Schotten, U. Post-operative Atrial Fibrillation—Pathophysiology, Treatment and Prevention. J. Atr. Fibrillation 2013, 5, 781. [Google Scholar] [CrossRef]
- Bessissow, A.; Khan, J.; Devereaux, P.J.; Alvarez-Garcia, J.; Alonso-Coello, P. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: An overview. J. Thromb. Haemost. 2015, 13, S304–S312. [Google Scholar] [CrossRef]
- Bhave, P.D.; Goldman, L.E.; Vittinghoff, E.; Maselli, J.; Auerbach, A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am. Heart J. 2012, 164, 918–924. [Google Scholar] [CrossRef]
- Danelich, I.M.; Lose, J.M.; Wright, S.S.; Asirvatham, S.J.; Ballinger, B.A.; Larson, D.W.; Lovely, J.K. Practical management of postoperative atrial fibrillation after noncardiac surgery. J. Am. Coll. Surg. 2014, 219, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Maisel, W.H.; Rawn, J.D.; Stevenson, W.G. Atrial fibrillation after cardiac surgery. Ann. Intern. Med. 2001, 135, 1061–1073. [Google Scholar] [CrossRef]
- Echahidi, N.; Pibarot, P.; O’Hara, G.; Mathieu, P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J. Am. Coll. Cardiol. 2008, 51, 793–801. [Google Scholar] [CrossRef]
- Zaman, A.G.; Archbold, R.A.; Helft, G.; Paul, E.A.; Curzen, N.P.; Mills, P.G. Atrial fibrillation after coronary artery bypass surgery: A model for preoperative risk stratification. Circulation 2000, 101, 1403–1408. [Google Scholar] [CrossRef]
- Asher, C.R.; Miller, D.P.; Grimm, R.A.; Cosgrove, D.M.; Chung, M.K. Analysis of risk factors for development of atrial fibrillation early after cardiac valvular surgery. Am. J. Cardiol. 1998, 82, 892–895. [Google Scholar] [CrossRef]
- Helgadottir, S.; Sigurdsson, M.I.; Ingvarsdottir, I.L.; Arnar, D.O.; Gudbjartsson, T. Atrial fibrillation following cardiac surgery: Risk analysis and long-term survival. J. Cardiothorac. Surg. 2012, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Ascione, R.; Caputo, M.; Calori, G.; Lloyd, C.T.; Underwood, M.J.; Angelini, G.D. Predictors of atrial fibrillation after conventional and beating heart coronary surgery: A prospective, randomized study. Circulation 2000, 102, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.P.; Fontes, M.L.; Tudor, I.C.; Ramsay, J.; Duke, P.; Mazer, C.D.; Barash, P.G.; Hsu, P.H.; Mangano, D.T.; Foundation, E. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004, 291, 1720–1729. [Google Scholar] [CrossRef]
- Abdelmoneim, S.S.; Rosenberg, E.; Meykler, M.; Patel, B.; Reddy, B.; Ho, J.; Klem, I.; Singh, J.; Worku, B.; Tranbaugh, R.F.; et al. The Incidence and Natural Progression of New-Onset Postoperative Atrial Fibrillation. JACC Clin. Electrophysiol. 2021, 7, 1134–1144. [Google Scholar] [CrossRef]
- Greenberg, J.W.; Lancaster, T.S.; Schuessler, R.B.; Melby, S.J. Postoperative atrial fibrillation following cardiac surgery: A persistent complication. Eur. J. Cardio Thorac. Surg. 2017, 52, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Chandy, J.; Nakai, T.; Lee, R.J.; Bellows, W.H.; Dzankic, S.; Leung, J.M. Increases in P-wave dispersion predict postoperative atrial fibrillation after coronary artery bypass graft surgery. Anesth. Analg. 2004, 98, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Holtstrand-Hjälm, H.; Celik, Y.; Glantz, H.; Thunström, E. Postoperative Atrial Fibrillation in Adults with Obstructive Sleep Apnea Undergoing Coronary Artery Bypass Grafting in the RICCADSA Cohort. J. Clin. Med. 2022, 11, 2459. [Google Scholar] [CrossRef] [PubMed]
- Burgos, L.M.; Seoane, L.; Parodi, J.B.; Espinoza, J.; Brito, V.G.; Benzadón, M.; Navia, D. Postoperative atrial fibrillation is associated with higher scores on predictive indices. J. Thorac. Cardiovasc. Surg. 2019, 157, 2279–2286. [Google Scholar] [CrossRef]
- Xi, Y.; Cheng, J. Dysfunction of the autonomic nervous system in atrial fibrillation. J. Thorac. Dis. 2015, 7, 193–198. [Google Scholar] [CrossRef]
- Lu, F.; Zhao, Y.; Xie, W.; Guo, Q.; Wang, S.-Q.; Wang, X.; Cheng, H. Imaging Sarcoplasmic Reticulum Ca2+ Signaling in Intact Cardiac Myocytes. Circulation 2020, 142, 1503–1505. [Google Scholar] [CrossRef]
- Maesen, B.; Nijs, J.; Maessen, J.; Allessie, M.; Schotten, U. Post-operative atrial fibrillation: A maze of mechanisms. Europace 2012, 14, 159–174. [Google Scholar] [CrossRef]
- Saleeb-Mousa, J.; Nathanael, D.; Coney, A.M.; Kalla, M.; Brain, K.L.; Holmes, A.P. Mechanisms of Atrial Fibrillation in Obstructive Sleep Apnoea. Cells 2023, 12, 1661. [Google Scholar] [CrossRef]
- Fontes, M.L.; Amar, D.; Kulak, A.; Koval, K.; Zhang, H.; Shi, W.; Thaler, H. Increased preoperative white blood cell count predicts postoperative atrial fibrillation after coronary artery bypass surgery. J. Cardiothorac. Vasc. Anesth. 2009, 23, 484–487. [Google Scholar] [CrossRef]
- Nelson, J.A.; Gue, Y.X.; Christensen, J.M.; Lip, G.Y.H.; Ramakrishna, H. Analysis of the ESC/EACTS 2020 Atrial Fibrillation Guidelines with Perioperative Implications. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2177–2195. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, C.; Gao, D.; Zhang, C.; Zhang, Y.; Lu, Y.; Gao, Y. Meta-analysis of amiodarone versus β-blocker as a prophylactic therapy against atrial fibrillation following cardiac surgery. Intern. Med. J. 2012, 42, 1078–1087. [Google Scholar] [CrossRef]
- Turagam, M.K.; Downey, F.X.; Kress, D.C.; Sra, J.; Tajik, A.J.; Jahangir, A. Pharmacological strategies for prevention of postoperative atrial fibrillation. Expert. Rev. Clin. Pharmacol. 2015, 8, 233–250. [Google Scholar] [CrossRef]
- Chen, S.; Acou, W.-J.; Kiuchi, M.G.; Meyer, C.; Sommer, P.; Martinek, M.; Schratter, A.; Andrea, B.R.; Ling, Z.; Liu, S.; et al. Association of Preoperative Renin-Angiotensin System Inhibitors with Prevention of Postoperative Atrial Fibrillation and Adverse Events: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e194934. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, B.; Burrage, P.S.; Ngai, J.Y.; Prutkin, J.M.; Huang, C.-C.; Xu, X.; Chae, S.H.; Bollen, B.A.; Piccini, J.P.; Schwann, N.M.; et al. Society of Cardiovascular Anesthesiologists/European Association of Cardiothoracic Anaesthetists Practice Advisory for the Management of Perioperative Atrial Fibrillation in Patients Undergoing Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019, 33, 12–26. [Google Scholar] [CrossRef]
- Horbach, S.J.; Lopes, R.D.; Guaragna, J.C.d.C.; Martini, F.; Mehta, R.H.; Petracco, J.B.; Bodanese, L.C.; Filho, A.C.; Cirenza, C.; de Paola, A.A. Naproxen as prophylaxis against atrial fibrillation after cardiac surgery: The NAFARM randomized trial. Am. J. Med. 2011, 124, 1036–1042. [Google Scholar] [CrossRef]
- Conen, D.; Wang, M.K.; Popova, E.; Chan, M.T.V.; Landoni, G.; Reimer, C.; Reyes, J.C.T.; Grande, A.M.; Tallada, A.G.; I Sessler, D.; et al. Effect of colchicine on perioperative atrial fibrillation and myocardial injury after non-cardiac surgery in patients undergoing major thoracic surgery (COP-AF): An international randomised trial. Lancet 2023, 402, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Herrera, L.A.; Rojas-Velasco, G.; Márquez-Murillo, M.F.; Portillo-Romero, A.d.R.; Medina-Paz, L.; Álvarez-Álvarez, R.J.; Ramos-Enríquez, Á.; Baranda-Tovar, F.M. Atrial fibrillation in cardiac surgery. Arch. Cardiol. Mex. 2019, 89, 348–359. [Google Scholar] [CrossRef] [PubMed]
- European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery; Camm, A.J.; Kirchhof, P.; Lip, G.Y.; Schotten, U.; Savelieva, I.; Ernst, S.; Van Gelder, I.C.; Al-Attar, N.; et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 2010, 31, 2369–2429. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, K.; Wang, W.; Xie, G.; Fang, X. Dexmedetomidine sedation reduces atrial fibrillation after cardiac surgery compared to propofol: A randomized controlled trial. Crit. Care 2016, 20, 298. [Google Scholar] [CrossRef]
- Lin, M.H.; Kamel, H.; Singer, D.E.; Wu, Y.L.; Lee, M.; Ovbiagele, B. Perioperative/Postoperative Atrial Fibrillation and Risk of Subsequent Stroke and/or Mortality. Stroke 2019, 50, 1364–1371. [Google Scholar] [CrossRef]
- Joglar, J.A.; Chung, M.K.; Armbruster, A.L.; Benjamin, E.J.; Chyou, J.Y.; Cronin, E.M.; Deswal, A.; Eckhardt, L.L.; Goldberger, Z.D.; Gopinathannair, R.; et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1–e156. [Google Scholar] [CrossRef] [PubMed]
- Lubitz, S.A.; Yin, X.; Rienstra, M.; Schnabel, R.B.; Walkey, A.J.; Magnani, J.W.; Rahman, F.; McManus, D.D.; Tadros, T.M.; Levy, D.; et al. Long-term outcomes of secondary atrial fibrillation in the community: The Framingham Heart Study. Circulation 2015, 131, 1648–1655. [Google Scholar] [CrossRef]
- Steinberg, B.A.; Peterson, E.D.; Kim, S.; Thomas, L.; Gersh, B.J.; Fonarow, G.C.; Kowey, P.R.; Mahaffey, K.W.; Sherwood, M.W.; Chang, P.; et al. Use and Outcomes Associated with Bridging during Anticoagulation Interruptions in Patients with Atrial Fibrillation: Findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation 2015, 131, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.J.; Allen, A.L.; Minichello, T. A Call to Reduce the Use of Bridging Anticoagulation. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 64–67. [Google Scholar] [CrossRef]
- Douketis, J.D.; Spyropoulos, A.C.; Kaatz, S.; Becker, R.C.; Caprini, J.A.; Dunn, A.S.; Garcia, D.A.; Jacobson, A.; Jaffer, A.K.; Kong, D.F.; et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N. Engl. J. Med. 2015, 373, 823–833. [Google Scholar] [CrossRef]
- Karri, R.; Kawai, A.; Thong, Y.J.; Ramson, D.M.; Perry, L.A.; Segal, R.; Smith, J.A.; Penny-Dimri, J.C. Machine Learning Outperforms Existing Clinical Scoring Tools in the Prediction of Postoperative Atrial Fibrillation During Intensive Care Unit Admission After Cardiac Surgery. Heart Lung Circ. 2021, 30, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, Q.; Zhang, H.; Huang, M.; Yao, Y.; Ming, Y.; Yan, M.; Yu, Y.; Yu, L. Machine Learning Models of Postoperative Atrial Fibrillation Prediction after Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2023, 37, 360–366. [Google Scholar] [CrossRef]
- Magee, M.J.; Herbert, M.A.; Dewey, T.M.; Edgerton, J.R.; Ryan, W.H.; Prince, S.; Mack, M.J. Atrial Fibrillation After Coronary Artery Bypass Grafting Surgery: Development of a Predictive Risk Algorithm. Ann. Thorac. Surg. 2007, 83, 1707–1712. [Google Scholar] [CrossRef]
- He, K.; Liang, W.; Liu, S.; Bian, L.; Xu, Y.; Luo, C.; Li, Y.; Yue, H.; Yang, C.; Wu, Z. Long-term single-lead electrocardiogram monitoring to detect new-onset postoperative atrial fibrillation in patients after cardiac surgery. Front. Cardiovasc. Med. 2022, 9, 1001883. [Google Scholar] [CrossRef]
- Hiraoka, D.; Inui, T.; Kawakami, E.; Oya, M.; Tsuji, A.; Honma, K.; Kawasaki, Y.; Ozawa, Y.; Shiko, Y.; Ueda, H.; et al. Diagnosis of Atrial Fibrillation Using Machine Learning with Wearable Devices after Cardiac Surgery: Algorithm Development Study. JMIR Form. Res. 2022, 6, e35396. [Google Scholar] [CrossRef]
- Parise, O.; Parise, G.; Vaidyanathan, A.; Occhipinti, M.; Gharaviri, A.; Tetta, C.; Bidar, E.; Maesen, B.; Maessen, J.G.; La Meir, M.; et al. Machine Learning to Identify Patients at Risk of Developing New-Onset Atrial Fibrillation after Coronary Artery Bypass. J. Cardiovasc. Dev. Dis. 2023, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, T.; Ide, T.; Ikeda, M.; Nagata, T.; Tagawa, K.; Hirose, M.; Funakoshi, K.; Sakamoto, K.; Kishimoto, J.; Todaka, K.; et al. Deep Learning of ECG for the Prediction of Postoperative Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2023, 16, e011579. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.R.; Park, J.; Shin, S.J.; Choi, B.; Lee, J.-H.; Yang, K.; Kim, H.Y.; Sung, J.D.; Lee, S.-H. Prediction model for postoperative atrial fibrillation in non-cardiac surgery using machine learning. Front. Med. 2023, 9, 983330. [Google Scholar] [CrossRef]
- Gruwez, H.; Vandenberghe, E.; Barthels, M.; Vermunicht, P.; Ezzat, D.; Lamberigts, M.; Rodrigus, I.; Van Kerrebroeck, C.; Pierlet, N.; Heidbuchel, H.; et al. Predicting post-operative atrial fibrillation after cardiac surgery using an artificial intelligence-enabled electrocardiogram algorithm. EP Eur. 2024, 26 (Suppl. S1), euae102.571. [Google Scholar] [CrossRef]
- Rublev, V.; Geltser, B.; Shakhgeldyan, K.; Tsivanyuk, M. Machine Learning Prediction Models for Atrial Fibrillation after Isolated on-Pump Coronary Artery Bypass Grafting. Chest 2022, 161, A24. [Google Scholar] [CrossRef]
- Chamberlain, A.M.; Bergeron, N.P.; Al-Abcha, A.K.; Weston, S.A.; Jiang, R.; Attia, Z.I.; Friedman, P.A.; Gersh, B.J.; Noseworthy, P.A.; Siontis, K.C. Postoperative atrial fibrillation: Prediction of subsequent recurrences with clinical risk modeling and artificial intelligence electrocardiography. Cardiovasc. Digit. Health J. 2024, 5, 111–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Xing, W.; Chen, Q.; Liu, X.; Pu, Y.; Xin, F.; Jiang, H.; Yin, Z.; Tao, D.; et al. Robust Artificial Intelligence Tool for Atrial Fibrillation Diagnosis: Novel Development Approach Incorporating Both Atrial Electrograms and Surface ECG and Evaluation by Head-to-Head Comparison with Hospital-Based Physician ECG Readers. J. Am. Heart Assoc. 2024, 13, e032100. [Google Scholar] [CrossRef]
- Siontis, K.C.; Noseworthy, P.A.; Arghami, A.; Weston, S.A.; Attia, Z.I.; Crestanello, J.A.; Friedman, P.A.; Chamberlain, A.M.; Gersh, B.J. Use of artificial intelligence tools across different clinical settings: A cautionary tale. Circ. Cardiovasc. Qual. Outcomes 2021, 14, e008153. [Google Scholar] [CrossRef]
- Levy, A.E.; Biswas, M.; Weber, R.; Tarakji, K.; Chung, M.; Noseworthy, P.A.; Newton-Cheh, C.; Rosenberg, M.A. Applications of machine learning in decision analysis for dose management for dofetilide. PLoS ONE 2019, 14, e0227324. [Google Scholar] [CrossRef]
- Vinter, N.; Frederiksen, A.S.; Albertsen, A.E.; Lip, G.Y.H.; Fenger-Grøn, M.; Trinquart, L.; Frost, L.; Møller, D.S. Role for machine learning in sex-specific prediction of successful electrical cardioversion in atrial fibrillation? Open Heart 2020, 7, e001297. [Google Scholar] [CrossRef]
- Alhusseini, M.I.; Abuzaid, F.; Rogers, A.J.; Zaman, J.A.; Baykaner, T.; Clopton, P.; Bailis, P.; Zaharia, M.; Wang, P.J.; Rappel, W.-J.; et al. Machine Learning to Classify Intracardiac Electrical Patterns During Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2020, 13, e008160. [Google Scholar] [CrossRef] [PubMed]
- Ghrissi, A.; Almonfrey, D.; de Almeida, R.C.; Squara, F.; Montagnat, J.; Zarzoso, V. Data Augmentation for Automatic Identification of Spatiotemporal Dispersion Electrograms in Persistent Atrial Fibrillation Ablation Using Machine Learning. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020, 2020, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Luongo, G.; Azzolin, L.; Schuler, S.; Rivolta, M.W.; Almeida, T.P.; Martínez, J.P.; Soriano, D.C.; Luik, A.; Müller-Edenborn, B.; Jadidi, A.; et al. Machine learning enables noninvasive prediction of atrial fibrillation driver location and acute pulmonary vein ablation success using the 12-lead ECG. Cardiovasc. Digit. Health J. 2021, 2, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Suero, O.R.; Ali, A.K.; Barron, L.R.; Segar, M.W.; Moon, M.R.; Chatterjee, S. Postoperative atrial fibrillation (POAF) after cardiac surgery: Clinical practice review. J. Thorac. Dis. 2024, 16, 1503–1520. [Google Scholar] [CrossRef] [PubMed]
- Ha, A.C.T.; Verma, S.; Mazer, C.D.; Quan, A.; Yanagawa, B.; Latter, D.A.; Yau, T.M.; Jacques, F.; Brown, C.D.; Singal, R.K.; et al. Effect of Continuous Electrocardiogram Monitoring on Detection of Undiagnosed Atrial Fibrillation after Hospitalization for Cardiac Surgery: A Randomized Clinical Trial. JAMA Network Open 2021, 4, e2121867. [Google Scholar] [CrossRef]
- Olier, I.; Ortega-Martorell, S.; Pieroni, M.; Lip, G.Y.H. How machine learning is impacting research in atrial fibrillation: Implications for risk prediction and future management. Cardiovasc. Res. 2021, 117, 1700–1717. [Google Scholar] [CrossRef]



| Author | Aim of Study | Outcome |
|---|---|---|
| Karri et al. [46] | Compared the performance of a ML model with the established gold standard POAF Score in predicting POAF following cardiac surgery. | A study of 6040 patients found POAF in 21.5% (1364 admissions). ML models demonstrated superior predictive performance for POAF during ICU admission after cardiac surgery compared to the POAF Score, with AUCs as follows: GBM (0.74), LR (0.73), RF (0.72), KNN (0.68), SVM (0.67), and DT (0.59). The POAF Score achieved an AUC of 0.63. |
| Lu et al. [47] | Used ML algorithms to develop an efficient forecasting model for atrial fibrillation following cardiac surgery and compare the predictive performance of these algorithms with traditional logistic regression. | The study included 1400 patients who underwent valve and/or CABG with cardiopulmonary bypass. Postoperative atrial fibrillation occurred in 519 patients (37.1%). Predictive model AUCs were 0.777 (SVM), 0.767 (LR), and 0.765 (GBDT), with decision curve analysis showing appropriate net benefit for all models. |
| Magee et al. [48] | Developed an algorithm to predict the relative risk of developing postoperative atrial fibrillation in patients undergoing CABG. | Data from 19,083 patients undergoing CABG (1995–2006) were used to develop a logistic regression model with 14 significant indicators, including age, prolonged ventilation, cardiopulmonary bypass, and preoperative arrhythmias. The model showed 72.3% concordance, an AUC of 0.72, and a Hosmer–Lemeshow probability of 0.19. Calculated AF risk was 0.179 ± 0.116 for non-AF patients and 0.284 ± 0.153 for AF patients (p < 0.001). |
| He et al. [49] | Collected long-term single-lead ECGs of patients with preoperative sinus rhythm to develop statistical and ML models for predicting POAF. | The study of 100 cardiac surgery patients found POAF detection rates of 31% with long-term ECG and 19% with conventional monitoring. Significant differences in P-wave parameters were noted. The clinical model had an AUC of 0.86, while the clinical + ECG model had an AUC of 0.89. The SVM model achieved over 80% accuracy in the training set and over 60% in the test set. |
| Hiraoka et al. [50] | Develop an algorithm for immediate AF detection using an Apple Watch with a PPG sensor in cardiac surgery patients. ML is applied to the pulse data from the device to diagnose AF. | A total of 79 cardiac surgery patients were analyzed for POAF using telemetry-monitored ECGs and an Apple Watch. AF developed in 27 patients (34.2%), with 199 total AF events observed. The ML diagnostic algorithm on Apple Watch pulse data achieved an accuracy of 0.9416, with a sensitivity of 0.909 and specificity of 0.838. |
| Parise et al. [51] | Developed a ML prediction model of new-onset POAF following CABG. | This retrospective study of 394 patients undergoing first-time CABG developed an RF model to predict POAF, identifying key predictors: age (100%), preoperative creatinine (86.1%), aortic cross-clamping time (82.2%), body surface area (80.9%), Euro-Score (80.7%), and extracorporeal circulation time (65.7%). The RF model achieved the highest AUC values (up to 0.95), outperforming traditional logistic regression. |
| Tohyama et al. [52] | Developed a DL model using preoperative ECGs to predict POAF in patients undergoing surgery | This retrospective study analyzed 43,980 preoperative ECGs from 27,564 patients without AF. The model achieved a time-dependent C-statistic of 0.83 at 7 days, with 79.9% sensitivity, 73.5% specificity, and a 99.0% negative predictive value. The saliency map highlighted the importance of low-voltage P wave and ST regions, particularly in leads aVF, V1, V2, V5, and V6. |
| Oh et al. [53] | Developed a predictive model for POAF in non-cardiac surgery using ML. | The study used data from a cohort of 295,363. Key variables influencing POAF included age, lung operation, operation duration, history of coronary artery disease, and hypertension. The model achieved an AUC of 0.80, with 0.95 accuracy, 0.97 specificity, and 0.28 sensitivity. |
| Gruwez et al. [54] | Evaluated the usability of an AI-enabled ECG algorithm, originally trained to predict atrial fibrillation in non-surgical conditions, for predicting POAF in patients undergoing cardiac surgery. | The study analyzed 127 patients from the SURGICAL-AF trial who had no prior history of atrial fibrillation and had pre-operative 12-lead ECGs. The AI-enabled ECG algorithm predicted POAF with an AUC of 0.66, sensitivity of 64.3%, specificity of 64.7%, and accuracy of 0.65. POAF occurred in 40.4% of the high-risk group versus 21.4% of the low-risk group, indicating a hazard ratio of 2.2 (p-value = 0.020). |
| Rublev et al. [55] | Aimed to develop and compare ML models, particularly artificial neural networks and LR, for predicting POAF after on-pump CABG. | The study analyzed 866 patients who underwent isolated on-pump CABG surgery, excluding 85 with prior atrial fibrillation. POAF developed in 19.1% of cases. The best predictive model, an artificial neural network, identified 11 key risk factors and achieved an AUC of 0.75, specificity of 0.73, sensitivity of 0.74, and accuracy of 0.73. |
| Chamberlain [56] | Assessed whether the CHARGE-AF clinical risk score and an AI-ECG model can classify the risk of subsequent atrial fibrillation in patients with POAF after noncardiac surgery and to determine if a combined approach of both models improves risk prediction. | The study included 308 patients with POAF after noncardiac surgery. Subsequent AF rates were 87.16 and 198.51 per 1000 person-years for the lowest and highest tertiles of CHARGE-AF scores, respectively, and 90.93 and 226.00 per 1000 person-years for AI-ECG scores. The combined model had a C-statistic of 0.61, slightly improving prediction accuracy. |
| Zhang et al. [57] | Developed a robust AI-based tool for detecting AF and assessing AF burden using both surface ECG recordings and atrial electrograms in postoperative cardiac patients. | The study population consisted of 659 adult postoperative cardiac surgery patients, with data divided into training (263 patients), validation (66 patients), and testing (330 patients) sets. The AI tool achieved an AUC of 0.932 on validation and 0.953 on testing, with testing sensitivity of 97%, specificity of 81.4%, and an intraclass correlation coefficient of 0.952 for AF burden detection. |
| Siontis et al. [58] | Aimed to assess the performance of an AI-ECG algorithm in predicting POAF in patients undergoing noncardiac surgery and CABG, compared to its previous performance in a general population. | The study population included 342 patients with POAF and 255 controls from noncardiac surgery, and 4561 patients undergoing CABG, of whom 1437 had POAF. The AI-ECG model achieved a sensitivity of 75% and specificity of 49% for noncardiac surgery POAF (AUC 0.66), and a sensitivity of 50% and specificity of 61% for coronary surgery POAF (AUC 0.58), demonstrating lower performance compared to its original setting. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.; Chahil, V.; Battisha, A.; Haq, S.; Kalra, D.K. Postoperative Atrial Fibrillation: A Review. Biomedicines 2024, 12, 1968. https://doi.org/10.3390/biomedicines12091968
Shah S, Chahil V, Battisha A, Haq S, Kalra DK. Postoperative Atrial Fibrillation: A Review. Biomedicines. 2024; 12(9):1968. https://doi.org/10.3390/biomedicines12091968
Chicago/Turabian StyleShah, Sidra, Vipanpreet Chahil, Ayman Battisha, Syed Haq, and Dinesh K. Kalra. 2024. "Postoperative Atrial Fibrillation: A Review" Biomedicines 12, no. 9: 1968. https://doi.org/10.3390/biomedicines12091968
APA StyleShah, S., Chahil, V., Battisha, A., Haq, S., & Kalra, D. K. (2024). Postoperative Atrial Fibrillation: A Review. Biomedicines, 12(9), 1968. https://doi.org/10.3390/biomedicines12091968







