Analysis of Peri-Implantitis Photothermal Therapy Effect According to Laser Irradiation Location and Angle: A Numerical Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Laser-Induced Heat Transfer Analysis
2.2. Inflamed Tissue Elimination Analysis
2.3. Numerical Conditions
2.4. Numerical Model Validation
3. Results and Discussion
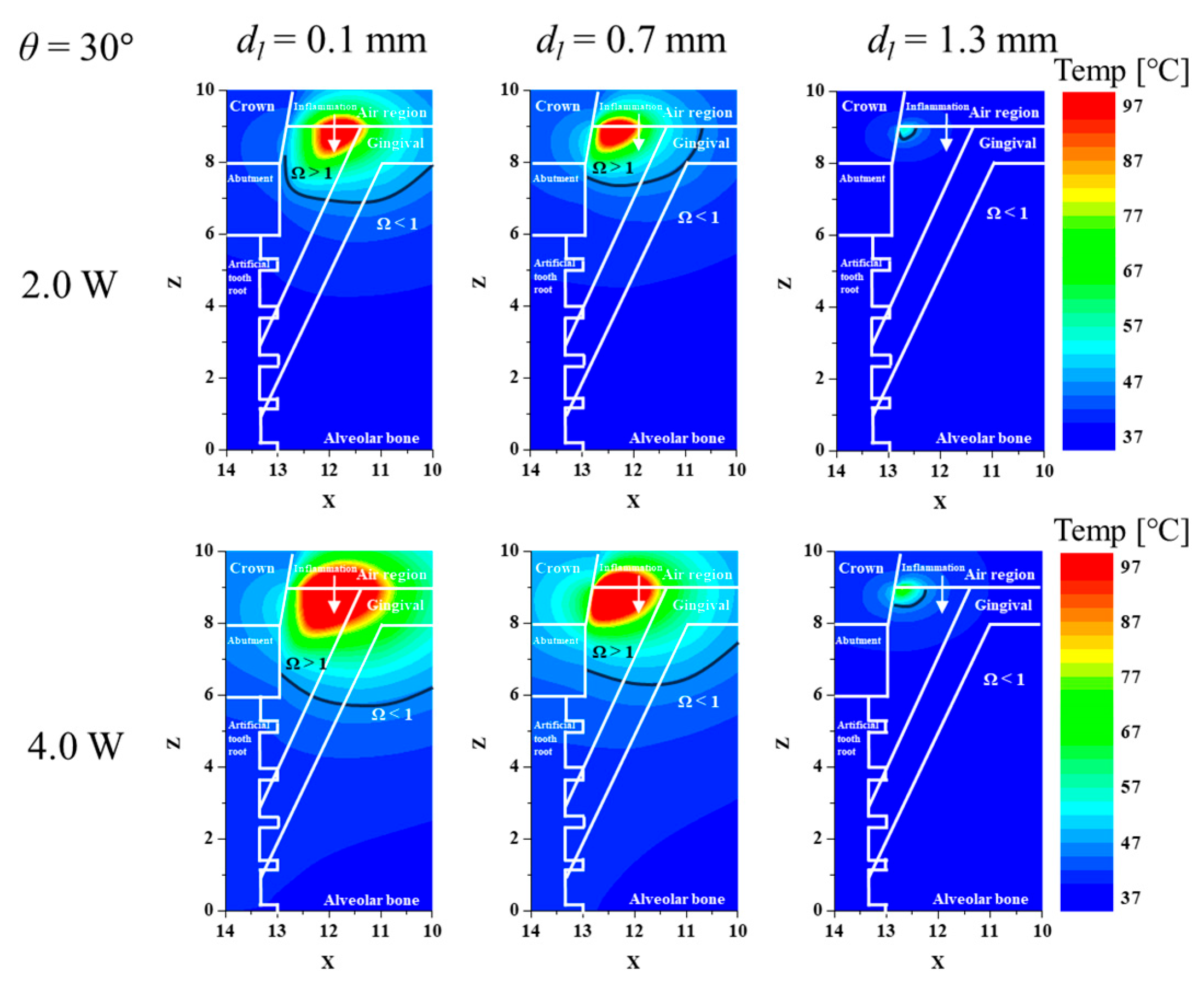
3.1. Confirmation of Inflamed Tissue Elimination for Various Laser Irradiation Positions and Powers
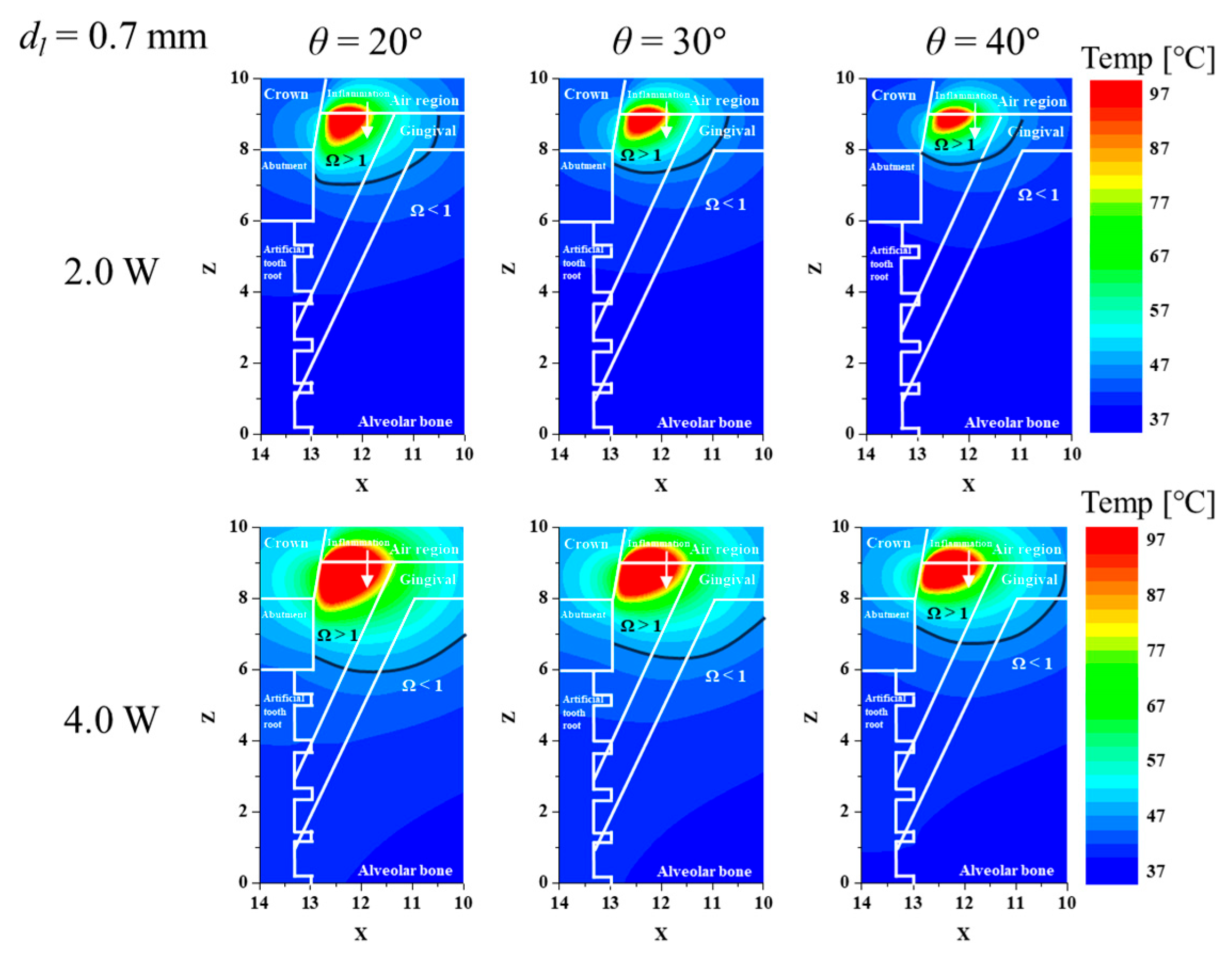
3.2. Confirmation of Inflamed Tissue Elimination for Various Laser Irradiation Angles and Powers
3.3. Elimination Ratio for Inflamed Tissue and Normal Tissue
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | frequency factor (1/s) |
| cp | specific heat (J/(kg∙K)) |
| dl | laser irradiation location (m) |
| Ea | activation energy (J/mol) |
| k | thermal conductivity (W/(m∙K)) |
| n1 | refractive index of air |
| n2 | refractive index of inflamed tissue |
| Pl | laser power (W) |
| q | volumetric heat source (W/m3) |
| rl | laser radius (m) |
| R1 | specular reflection value |
| R2 | diffuse reflection value |
| Rt | total reflectivity |
| R | ideal gas constant (J/(mol∙K)) |
| T | temperature (K) |
| t | time (s) |
| Greek symbols | |
| θ | irradiation angle (°) |
| μ | optical coefficient (1/m) |
| μ’ | reduced optical coefficient (1/m) |
| ρ | density (kg/m3) |
| ϕArrh | Arrhenius thermal damage ratio |
| ϕNArrh | normal tissue Arrhenius thermal damage ratio |
| Ω | Arrhenius damage integral value |
| ωβ | blood perfusion rate (1/s) |
| Subscripts | |
| a | absorption |
| b | blood |
| l | laser |
| m | metabolic |
| tot | attenuation |
| x, y, z | notation of direction |
References
- Alghamdi, H.S.; Jansen, J.A. The development and future of dental implants. Dent. Mater. J. 2020, 39, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Takaichi, A.; Fueki, K.; Murakami, N.; Ueno, T.; Inamochi, Y.; Wada, J.; Arai, Y.; Wakabayashi, N. A systematic review of digital removable partial dentures. Part II: CAD/CAM framework, artificial teeth, and denture base. J. Prosthodont. Res. 2022, 66, 53–67. [Google Scholar] [CrossRef]
- Sailer, I.; Karasan, D.; Todorovic, A.; Ligoutsikou, M.; Pjetursson, B.E. Prosthetic failures in dental implant therapy. Periodontology 2000 2022, 88, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Sehring, J.; Beltsos, A.; Jeelani, R. Human implantation: The complex interplay between endometrial receptivity, inflammation, and the microbiome. Placenta 2022, 117, 179–186. [Google Scholar] [CrossRef]
- Monje, A.; Nart, J. Management and sequelae of dental implant removal. Periodontology 2000 2022, 88, 182–200. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Wisitrasameewon, W.; Humagain, M.; Thunyakitpisal, P. Peri-implantitis update: Risk indicators, diagnosis, and treatment. Eur. J. Dent. 2020, 14, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.H.; Russi, K.L.; Rocha, N.C.; Bastos, F.; Darrieux, M.; Parisotto, T.M.; Girardello, R. Host-microbiome interactions regarding peri-implantitis and dental implant loss. J. Transl. Med. 2022, 20, 425. [Google Scholar] [CrossRef]
- Oh, S.L.; Shiau, H.J.; Reynolds, M.A. Survival of dental implants at sites after implant failure: A systematic review. J. Prosthet. Dent. 2020, 123, 54–60. [Google Scholar] [CrossRef]
- Fragkioudakis, I.; Tseleki, G.; Doufexi, A.E.; Sakellari, D. Current concepts on the pathogenesis of peri-implantitis: A narrative review. Eur. J. Dent. 2021, 15, 379–387. [Google Scholar] [CrossRef]
- Muthukuru, M.; Zainvi, A.; Esplugues, E.O.; Flemmig, T.F. Non-surgical therapy for the management of peri-implantitis: A systematic review. Clin. Oral. Implants Res. 2012, 23, 77–83. [Google Scholar] [CrossRef]
- Toledano, M.; Osorio, M.T.; Vallecillo-Rivas, M.; Toledano-Osorio, M.; Rodríguez-Archilla, A.; Toledano, R.; Osorio, R. Efficacy of local antibiotic therapy in the treatment of peri-implantitis: A systematic review and meta-analysis. J. Dent. 2021, 113, 103790. [Google Scholar] [CrossRef] [PubMed]
- Montero, E.; Roccuzzo, A.; Molina, A.; Monje, A.; Herrera, D.; Roccuzzo, M. Minimal invasiveness in the reconstructive treatment of peri-implantitis defects. Periodontology 2000 2023, 91, 199–216. [Google Scholar] [CrossRef]
- Øen, M.; Leknes, K.N.; Lund, B.; Bunæs, D.F. The efficacy of systemic antibiotics as an adjunct to surgical treatment of peri-implantitis: A systematic review. BMC Oral Health 2021, 21, 666. [Google Scholar] [CrossRef]
- Mattar, H.; Bahgat, M.; Ezzat, A.; Bahaa El-Din, B.; Keraa, K.; El Taftazany, I. Management of peri-implantitis using a diode laser (810 nm) vs conventional treatment: A systematic review. Lasers Med. Sci. 2021, 36, 13–23. [Google Scholar] [CrossRef]
- Lu, J.; Shi, Y.; Chen, Z.; Sun, X.; Yuan, H.; Guo, F.; Shi, W. Photothermal effect of carbon dots for boosted photothermal-assisted photocatalytic water/seawater splitting into hydrogen. Chem. Eng. J. 2023, 453, 139834. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; O’hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal therapy. J. Control. Release 2020, 325, 52–71. [Google Scholar] [CrossRef]
- Hu, M.L.; Zheng, G.; Lin, H.; Li, N.; Zhao, P.F.; Han, J.M. Network meta-analysis of the treatment efficacy of different lasers for peri-implantitis. Lasers Med. Sci. 2021, 36, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Lin, Y.C.; Kung, J.C.; Yan, D.Y.; Chen, I.H.; Jheng, Y.S.; Lai, C.H.; Wu, Y.M.; Lee, K.T. Efficacy of Er: YAG laser for the peri-implantitis treatment and microbiological changes: A randomized controlled trial. Lasers Med. Sci. 2022, 37, 3517–3525. [Google Scholar] [CrossRef]
- Kim, D.; Paik, J.; Kim, H. Effect of gold nanoparticles distribution radius on photothermal therapy efficacy. Sci. Rep. 2023, 13, 12135. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.K.; Ooi, E.H.; Chiew, Y.S.; Menichetti, L.; Armanetti, P.; Franchini, M.C.; Alchera, E.; Locatelli, I.; Canu, T.; Maturi, M.; et al. Gold nanorods assisted photothermal therapy of bladder cancer in mice: A computational study on the effects of gold nanorods distribution at the centre, periphery, and surface of bladder cancer. Comput. Methods Programs Biomed. 2023, 230, 107363. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.; Kim, D.; Kim, H.; Kim, H.S. Numerical study on the three-dimensional temperature distribution according to laser conditions in photothermal therapy of peri-implantitis. Int. J. Implant Dent. 2024, 10, 19. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef]
- Chang, W.S.; NA, S.J. A study on heat source equations for the prediction of weld shape and thermal deformation in laser microwelding. Metall. Mater. Trans. B 2002, 33, 757–764. [Google Scholar] [CrossRef]
- Swokowski, E.W. Calculus with Analytic Geometry; Taylor & Francis: Abingdon, UK, 1979. [Google Scholar]
- Li, J.; Xu, J.; Lian, Z.; Yu, Z.; Yu, H. Fabrication of antireflection surfaces with superhydrophobic property for titanium alloy by nanosecond laser irradiation. Opt. Laser Technol. 2020, 126, 106129. [Google Scholar] [CrossRef]
- Gowda, R.B.; Saara, K.; Sharan, P. Detection of oral cancerous cells using highly sensitive one-dimensional distributed Bragg’s Reflector Fabry Perot Microcavity. Optik 2021, 244, 167599. [Google Scholar] [CrossRef]
- Einstein, G.; Udayakumar, K.; Aruna, P.R.; Koteeswaran, D.; Ganesan, S. Diffuse reflectance spectroscopy for monitoring physiological and morphological changes in oral cancer. Optik 2016, 127, 1479–1485. [Google Scholar] [CrossRef]
- Ren, Y.; Qi, H.; Chen, Q.; Ruan, L. Thermal dosage investigation for optimal temperature distribution in gold nanoparticle enhanced photothermal therapy. Int. J. Heat Mass Transf. 2017, 106, 212–221. [Google Scholar] [CrossRef]
- Pearce, J.A. Comparative analysis of mathematical models of cell death and thermal damage processes. Int. J. Hyperth. 2013, 29, 262–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, H. Induction of apoptotic temperature in photothermal therapy under various heating conditions in multi-layered skin structure. Int. J. Mol. Sci. 2021, 22, 11091. [Google Scholar] [CrossRef]
- Jaglarz, J.; Szewczenko, J.; Marszałek, K.; Basiaga, M.; Marszałek, M.; Gaweł, R. Nonstandard optical methods as a tool for rough surface analysis. Mater. Today Proc. 2015, 2, 4046–4052. [Google Scholar] [CrossRef]
- Abreu, A.I.; Lima, T.P.; Meireles, A.B.; Souza, B.E.; Torres, L.A.G. Evaluation of surface temperature of tongue for screening of patients with suspected oral cancer. ForScience 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Fernandez-Oliveras, A.; Rubiño, M.; Pérez, M.M. Scattering and absorption properties of biomaterials for dental restorative applications. J. Eur. Opt. Soc. Rapid Publ. 2013, 8, 13056. [Google Scholar] [CrossRef]
- Lu, X.; Lin, X.; Chiumenti, M.; Cervera, M.; Hu, Y.; Ji, X.; Ma, L.; Yang, H.; Huang, W. Residual stress and distortion of rectangular and S-shaped Ti-6Al-4V parts by Directed Energy Deposition: Modelling and experimental calibration. Addit. Manuf. 2019, 26, 166–179. [Google Scholar] [CrossRef]
- Rabbani Arshad, S.; Zoljanahi Oskui, I.; Hashemi, A. Thermal analysis of dental implants in mandibular premolar region: 3D FEM study. J. Prosthodont. 2018, 27, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Bargo, P.R.; Prahl, S.A.; Goodell, T.T.; Sleven, R.A.; Koval, G.; Blair, G.; Jacques, S.L. In vivo determination of optical properties of normal and tumor tissue with white light reflectance and an empirical light transport model during endoscopy. J. Biomed. Opt. 2005, 10, 034018. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.N.; Al-Zahawi, A.R.; Sabri, L.A. Mechanical and thermal stress behavior of a conservative proposed veneer preparation design for restoring misaligned anterior teeth: A 3D finite element analysis. Appl. Sci. 2020, 10, 5814. [Google Scholar] [CrossRef]
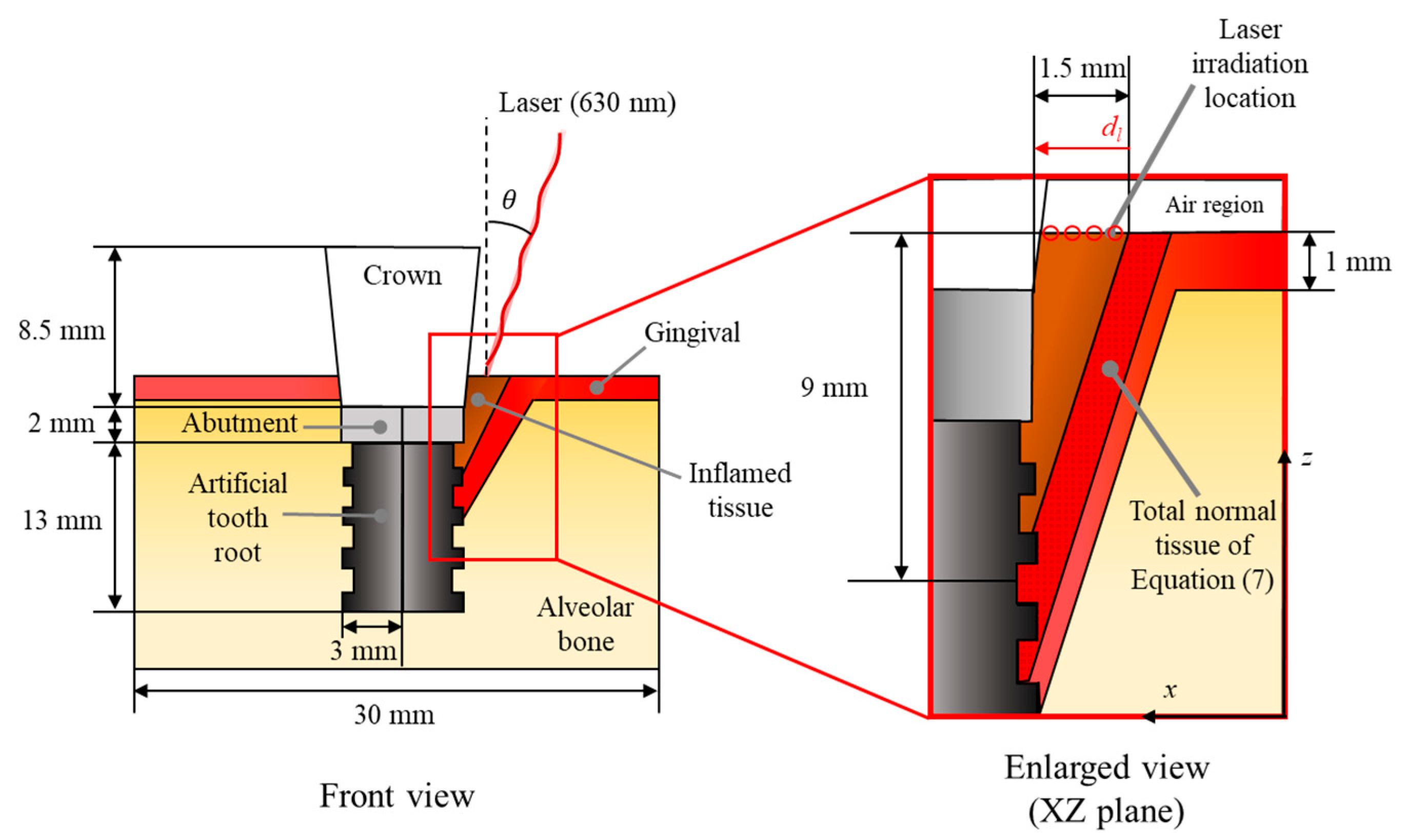


| ρ (kg/m3) | cp (J/kgK) | k (W/mK) | μa (1/cm) | μ′s (1/cm) | ωb (1/s) | qm (W/m3) | |
|---|---|---|---|---|---|---|---|
| Inflamed tissue | 1080 | 3500 | 0.48 | 2.16 | 17.03 | 0.009 | 65,400 |
| Gingival | 1000 | 4200 | 0.63 | 0.53 | 3.817 | 0.0076 | 1091 |
| Alveolar Bone | 2060 | 1260 | 0.38 | 0.596 | 22.97 | 0.00369 | - |
| Crown (Zirconia) | 6080 | 450 | 2.80 | 0.10 | 20.43 | - | - |
| Abutment (Zirconia) | 6080 | 450 | 2.80 | 0.10 | 20.43 | - | - |
| Artificial tooth root (Ti-6Al-4V) | 4420 | 546 | 7.00 | 789,500 | ≈0 | - | - |
| Air | 1.205 | 1.006 | 0.0256 | 0 | 0 | - | - |
| Parameter | Case | Number | Remarks |
|---|---|---|---|
| Laser irradiation angle (θ) | 15 to 40° | 6 | Interval: 5° |
| Laser irradiation location (dl) | 0.1 to 1.3 mm | 5 | Interval: 0.3 mm |
| Laser Power (Pl) | 0.0 to 4.0 W | 101 | Interval: 0.04 W |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kim, H.; Kim, H.-S. Analysis of Peri-Implantitis Photothermal Therapy Effect According to Laser Irradiation Location and Angle: A Numerical Approach. Biomedicines 2024, 12, 1976. https://doi.org/10.3390/biomedicines12091976
Kim D, Kim H, Kim H-S. Analysis of Peri-Implantitis Photothermal Therapy Effect According to Laser Irradiation Location and Angle: A Numerical Approach. Biomedicines. 2024; 12(9):1976. https://doi.org/10.3390/biomedicines12091976
Chicago/Turabian StyleKim, Donghyuk, Hyunjung Kim, and Hee-Sun Kim. 2024. "Analysis of Peri-Implantitis Photothermal Therapy Effect According to Laser Irradiation Location and Angle: A Numerical Approach" Biomedicines 12, no. 9: 1976. https://doi.org/10.3390/biomedicines12091976
APA StyleKim, D., Kim, H., & Kim, H.-S. (2024). Analysis of Peri-Implantitis Photothermal Therapy Effect According to Laser Irradiation Location and Angle: A Numerical Approach. Biomedicines, 12(9), 1976. https://doi.org/10.3390/biomedicines12091976





