A Comparative Review of Tocosomes, Liposomes, and Nanoliposomes as Potent and Novel Nanonutraceutical Delivery Systems for Health and Biomedical Applications
Abstract
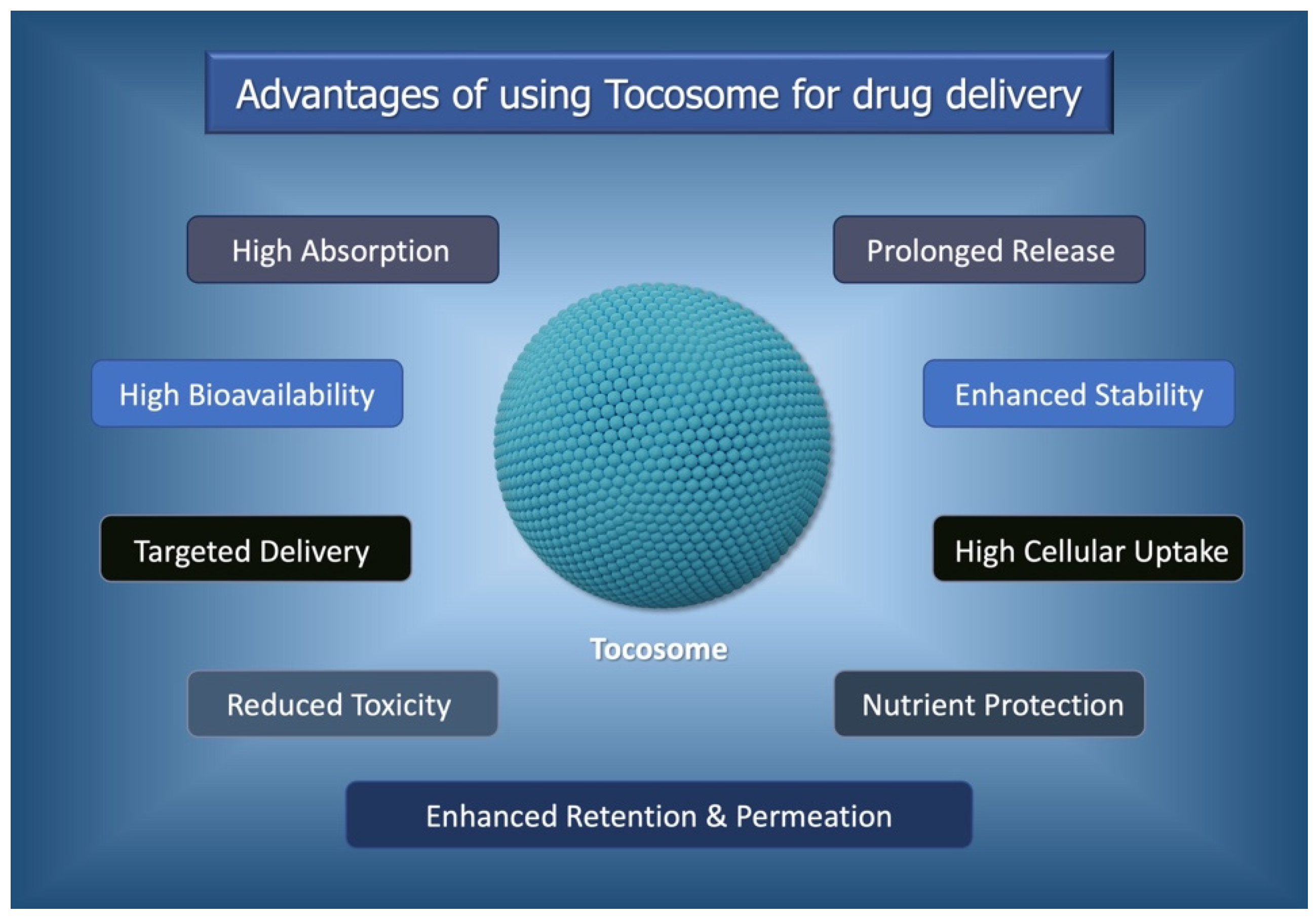
:1. Introduction
2. Main Physicochemical Properties
3. Preparation Methods
- In order to ensure optimal encapsulation efficiency and stability, high-quality TP, T2P, phospholipids, excipients, and bioactive compounds must be employed;
- To achieve desired characteristics and performance, formulation parameters (e.g., TP, T2P, lipid-to-drug ratio, ingredients’ composition) must be optimized;
- Appropriate sterilization and aseptic techniques must be applied if tocosomes are intended for human or animal use;
- (1)
- Biological and physicochemical properties of the material to be encapsulated.
- (2)
- Ideal level of encapsulation or entrapment efficiency.
- (3)
- The route of administration of drug or other bioactive molecules/compounds.
- (4)
- The shelf life and stability of the end product.
- (5)
- Characteristics of the medium or solvent(s) in which the lipid vesicles, drug molecules, and other excipients of the formulation are dispersed.
- (6)
- Required size, charge, polydispersity index, and release profile of the formulations.
- (7)
- Safety profile, potential toxicity, and effective concentration of the encapsulated material in the vesicles.
- (8)
- Number of steps and vessels required in the manufacturing protocol.
- (9)
4. Targeting Strategies
5. Applications in Food and Nutraceutical Industries
- (1)
- Protection: Encapsulation shields the sensitive nutraceutical compounds from degradation due to light, oxygen, moisture, and other environmental factors. This protection helps to maintain the stability and potency of the compounds during storage and transportation.
- (2)
- Controlled release: Encapsulation allows for the controlled release of nutraceuticals in the body. By regulating the rate of release, encapsulation can optimize the absorption and bioavailability of these compounds, ensuring maximum effectiveness.
- (3)
- Masking taste and odor: Some nutraceutical compounds may have an unpleasant taste or odor. Encapsulation can mask these sensory properties, making the products more palatable and consumer-friendly.
- (4)
- Enhanced solubility: Encapsulation techniques can improve the solubility of poorly soluble nutraceutical compounds, enhancing their absorption and bioavailability in the body.
- (5)
- Targeted delivery: Encapsulation can facilitate targeted nutraceutical delivery to particular sites inside the body, such as the gastrointestinal tract or bloodstream. This targeted approach can enhance the therapeutic effects of the compounds while minimizing side-effects.
- (6)
6. Biomedical Applications
7. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Latrobdiba, Z.M.; Fulyani, F.; Anjani, G. Liposome optimization for oral delivery of nutraceuticals in food: A review. Food Res. 2023, 7, 233–246. [Google Scholar] [CrossRef]
- Kumar, A.; Pramanik, J.; Prajapati, B. Food and Nutraceuticals. In Lipid-Based Drug Delivery Systems; Jenny Stanford Publishing: Singapore, 2024; pp. 651–679. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Karim, N.; Xu, Y.; Xie, J.; Cui, H.; Mozafari, M.R.; Chen, W. Potential micro-/nano-encapsulation systems for improving stability and bioavailability of anthocyanins: An updated review. Crit. Rev. Food Sci. Nutr. 2023, 63, 3362–3385. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.B.; Sung, B.; Yadav, V.R.; Kannappan, R.; Chaturvedi, M.M.; Aggarwal, B.B. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem. Pharmacol. 2010, 80, 1833–1843. [Google Scholar] [CrossRef]
- Lee, M.H.; Do Kim, H.; Jang, Y.J. Delivery systems designed to enhance stability and suitability of lipophilic bioactive compounds in food processing: A review. Food Chem. 2024, 437, 137910. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Javanmard, R.; Raji, M. Tocosome: Novel drug delivery system containing phospholipids and tocopheryl phosphates. Int. J. Pharm. 2017, 528, 381–382. [Google Scholar] [CrossRef]
- Rasti, B.; Jinap, S.; Mozafari, M.R.; Abd-Manap, M.Y. Optimization on preparation condition of polyunsaturated fatty acids nanoliposome prepared by Mozafari method. J. Liposome Res. 2014, 24, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yurdugul, S.E.; Mozafari, M.R. Recent advances in micro-and nanoencapsulation of food ingredients. Cell Mol. Biol. Lett. 2004, 9, 64–65. [Google Scholar]
- Mor, S.; Nain, N.; Das, A.; Kumari, A.; Swarup, V. Scope of Nanoencapsulation for Delivery of Functional Food Ingredients. In Food Process Engineering and Technology: Safety, Packaging, Nanotechnologies and Human Health; Springer Nature: Singapore, 2024; pp. 303–317. [Google Scholar] [CrossRef]
- Rostami, M.; Beheshtizadeh, N.; Mazaheri, E.L.; Mirzaei, G.; Andishmand, H.; Mafi, A.; Esfandiari, Z.; Safavizadeh, V.; Assadpour, E.; Sani, M.A.; et al. Resveratrol-loaded nanocarriers: Characteristics, sources, health effects, recent delivery systems, and their food and biomedical applications. Food Biosci. 2024, 61, 104845. [Google Scholar] [CrossRef]
- Esfahani, F.N.; Karimi, S.; Jalilian, Z.; Alavi, M.; Aziz, B.; Gorgich, E.A.; Mozafari, M.R.; Taghavi, E.; Ataei, S. Functionalized and theranostic lipidic and tocosomal drug delivery systems: Potentials and limitations in cancer photodynamic therapy. Adv. Pharm. Bull. 2024. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Jetwa, P.; Kumar, U.; Awasthi, S.; Agrawal, A. Review on Usage of Nano Encapsulated Nutraceuticals and Dietary Foods in Food Healthcare. Pharm. Biosci. J. 2024, 12, 1–7. [Google Scholar]
- Rao, T.J.; Kesharwani, R.K.; Keservani, R.K.; Sharma, A.K. (Eds.) Formulations, Regulations, and Challenges of Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar] [CrossRef]
- Tang, C.H.; Chen, H.L.; Dong, J.R. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) as food-grade nanovehicles for hydrophobic nutraceuticals or bioactives. Appl. Sci. 2023, 13, 1726. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, X.; Tan, J.; Mei, C.; Cai, X.; Kong, F. Lipid-based nanoparticles to address the limitations of GBM therapy by overcoming the blood-brain barrier, targeting glioblastoma stem cells, and counteracting the immunosuppressive tumor microenvironment. Biomed. Pharmacother. 2024, 171, 116113. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, W. Elastic properties of lipid bilayers: Theory and possible experiments. Z. Für Naturforschung C 1973, 28, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Kalantari, M.; Raji, M.; Fekri, H.S.; Saber, R.; Asnani, G.P.; Mortazavi, S.M.; Mozafari, M.R.; Rasti, B.; Taheriazam, A. Probing nanoliposomes using single particle analytical techniques: Effect of excipients, solvents, phase transition and zeta potential. Heliyon 2018, 4, e01088. [Google Scholar] [CrossRef]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, H.; Mozafari, M.R.; Bumrungpert, A.; Parsaei, H.; Taheri, S.V.; Mardani, P.; Dehkharghani, F.M.; Pudza, M.Y.; Alavi, M. Prospects and challenges of synergistic effect of fluorescent carbon dots, liposomes and nanoliposomes for theragnostic applications. Photodiagnosis Photodyn. Ther. 2023, 42, 103614. [Google Scholar] [CrossRef] [PubMed]
- Chrai, S.S.; Murari, R.; Ahmad, I. Liposomes (a review). Part two: Drug delivery systems. BioPharm 2002, 15. Available online: https://www.biopharminternational.com/view/liposomes-review-part-two-drug-delivery-systems (accessed on 24 July 2024).
- Giordani, S.; Marassi, V.; Zattoni, A.; Roda, B.; Reschiglian, P. Liposomes characterization for market approval as pharmaceutical products: Analytical methods, guidelines and standardized protocols. J. Pharm. Biomed. Anal. 2023, 236, 115751. [Google Scholar] [CrossRef]
- Naghib, S.M.; Mohammad-Jafari, K. Microfluidics-mediated Liposomal Nanoparticles for Cancer Therapy: Recent Developments on Advanced Devices and Technologies. Curr. Top. Med. Chem. 2024, 24, 1185–1211. [Google Scholar] [CrossRef]
- Orthoefer, F.T.; List, G.R. Phospholipids/lecithin: A class of nutraceutical lipids. In Nutraceutical and Specialty Lipids and their Co-Products; CRC Press: Boca Raton, FL, USA, 2006; pp. 523–544. [Google Scholar] [CrossRef]
- Rasti, B.; Erfanian, A.; Selamat, J. Novel nanoliposomal encapsulated omega-3 fatty acids and their applications in food. Food Chem. 2017, 230, 690–696. [Google Scholar] [CrossRef]
- Sohrabi, B. Amphiphiles. In Self-Assembly of Materials and Their Applications; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Nkanga, C.I.; Bapolisi, A.M.; Okafor, N.I.; Krause, R.W. General Perception of Liposomes: Formation. Manufacturing and Applications, Liposomes-Advances and Perspectives; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Khosravi-Darani, K. Liposome vesicle cannot be formed in non-aqueous phase. Food Chem. 2024, 430, 136824. [Google Scholar] [CrossRef] [PubMed]
- Nemati, S.; Mottaghi, M.; Karami, P.; Mirjalali, H. Development of solid lipid nanoparticles-loaded drugs in parasitic diseases. Discov. Nano 2024, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Gupta, G.; Alsayari, A.; Wahab, S.; Sahebkar, A.; Kesharwani, P. Advancements in liposomal formulations: A comprehensive exploration of industrial production techniques. Int. J. Pharm. 2024, 658, 124212. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Jafari, K.; Naghib, S.M. 3D Printing of Microfluidic-assisted Liposomes Production for Drug Delivery and Nanobiomedicine: A Review. Curr. Med. Chem. 2024. [Google Scholar] [CrossRef]
- Peng, T.; Xu, W.; Li, Q.; Ding, Y.; Huang, Y. Pharmaceutical liposomal delivery—Specific considerations of innovation and challenges. Biomater. Sci. 2023, 11, 62–75. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yamauchi, H.; Hirota, S. A polyol dilution method for mass production of liposomes. J. Liposome Res. 1994, 4, 71–91. [Google Scholar] [CrossRef]
- Talsma, H.; Van Steenbergen, M.J.; Borchert, J.C.; Crommelin, D.J. A novel technique for the one-step preparation of liposomes and nonionic surfactant vesicles without the use of organic solvents. Liposome formation in a continuous gas stream: The ‘Bubble’ method. J. Pharm. Sci. 1994, 83, 276–280. [Google Scholar] [CrossRef]
- Akram, N.; Afzaal, M.; Saeed, F.; Shah, Y.A.; Faisal, Z.; Asghar, A.; Ateeq, H.; Nayik, G.A.; Wani, S.H.; Hussain, M.; et al. Liposomes: A promising delivery system for active ingredients in food and nutrition. Int. J. Food Prop. 2023, 26, 2476–2492. [Google Scholar] [CrossRef]
- Majumdar, S.; Mahanti, B.; Kar, A.K.; Parya, H.; Ghosh, A.; Kar, B. Nanoliposome: As a smart nanocarrier in transdermal drug delivery system. Intell. Pharm. 2024, in press. [Google Scholar] [CrossRef]
- Maleki, G.; Bahrami, Z.; Woltering, E.J.; Khorasani, S.; Mozafari, M.R. A review of patents on “Mozafari method” as a green technology for manufacturing bioactive carriers. Biointerface Res. Appl. Chem. 2023, 13, 1–15. [Google Scholar] [CrossRef]
- Swain, B.; Koilpillai, J.; Narayanasamy, D. Systematic review on recent advancements and liposomal technologies to develop stable liposome. Curr. Trends Biotechnol. Pharm. 2023, 17, 735–748. [Google Scholar]
- Chan, H.W.; Chow, S.; Zhang, X.; Kwok, P.C.; Chow, S.F. Role of particle size in translational research of nanomedicines for successful drug delivery: Discrepancies and inadequacies. J. Pharm. Sci. 2023, 112, 2371–2384. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, Z.; Mozafari, M.R.; Aminnezhad, S.; Taghavi, E. Insight into heating method and Mozafari method as green processing techniques for the synthesis of micro-and nano-drug carriers. Green Process. Synth. 2024, 13, 20230136. [Google Scholar] [CrossRef]
- Shim, G.; Youn, Y.S. Precise subcellular targeting approaches for organelle-related disorders. Adv. Drug Deliv. Rev. 2024, 212, 115411. [Google Scholar] [CrossRef]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A review of manufacturing techniques and targeting strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Agiba, A.M.; Arreola-Ramírez, J.L.; Carbajal, V.; Segura-Medina, P. Light-responsive and dual-targeting liposomes: From mechanisms to targeting strategies. Molecules 2024, 29, 636. [Google Scholar] [CrossRef]
- Hu, R.; Lan, J.; Zhang, D.; Shen, W. Nanotherapeutics for prostate cancer treatment: A comprehensive review. Biomaterials 2024, 305, 122469. [Google Scholar] [CrossRef]
- Singh, R.K. Liposomes—An Updated overview. Int. J. Pharma Prof. Res. (IJPPR) 2024, 15, 119–127. [Google Scholar]
- Li, Z.Z.; Zhong, N.N.; Cao, L.M.; Cai, Z.M.; Xiao, Y.; Wang, G.R.; Liu, B.; Xu, C.; Bu, L.L. Nanoparticles Targeting Lymph Nodes for Cancer Immunotherapy: Strategies and Influencing Factors (Small 19/2024). Small 2024, 20, 2470149. [Google Scholar] [CrossRef]
- Khan, F.; Singh, P.; Joshi, A.S.; Tabassum, N.; Jeong, G.J.; Bamunuarachchi, N.I.; Mijakovic, I.; Kim, Y.M. Multiple potential strategies for the application of nisin and derivatives. Crit. Rev. Microbiol. 2023, 49, 628–657. [Google Scholar] [CrossRef] [PubMed]
- Colas, J.C.; Shi, W.; Rao, V.M.; Omri, A.; Mozafari, M.R.; Singh, H. Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron 2007, 38, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Puttasiddaiah, R.; Lakshminarayana, R.; Somashekar, N.L.; Gupta, V.K.; Inbaraj, B.S.; Usmani, Z.; Raghavendra, V.B.; Sridhar, K.; Sharma, M. Advances in nanofabrication technology for nutraceuticals: New insights and future trends. Bioengineering 2022, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.M.; El-Sayed, H.S.; Youssef, A.M. Recent developments in encapsulation techniques for innovative and high-quality dairy products: Demands and challenges. Bioact. Carbohydr. Diet. Fibre 2024, 31, 100406. [Google Scholar] [CrossRef]
- Hashemi, B.; Assadpour, E.; Jafari, S.M. Bigels as novel carriers of bioactive compounds: Applications and research trends. Food Hydrocoll. 2024, 147, 109427. [Google Scholar] [CrossRef]
- Patel, S.; Patel, R.; Dharamsi, A. A review: Proliposomes as a stable novel drug delivery. World J. Pharm. Res. 2019, 8, 638–651. [Google Scholar] [CrossRef]
- Rudzińska, M.; Grygier, A.; Knight, G.; Kmiecik, D. Liposomes as Carriers of Bioactive Compounds in Human Nutrition. Foods 2024, 13, 1814. [Google Scholar] [CrossRef]
- Abbasi, A.; Hashemi, M.; Kafil, H.S.; Astamal, M.A.; Lahouty, M.; Tajani, A.G.; Hosseini, H.; Nasirifar, S.Z. A Critical Review on the Bioavailability Promotion of the Food Bioactive Compounds: Nano Lipid Carriers Perspective. Pharm. Sci. 2024, 30, 282–303. [Google Scholar] [CrossRef]
- Sarabandi, K.; Rafiee, Z.; Khodaei, D.; Jafari, S.M. Encapsulation of food ingredients by nanoliposomes. In Lipid-Based Nanostructures for Food Encapsulation Purposes; Academic Press: Cambridge, MA, USA, 2019; pp. 347–404. [Google Scholar] [CrossRef]
- Law, B.A.; King, J.S. Use of liposomes for proteinase addition to Cheddar cheese. J. Dairy Res. 1985, 52, 183–188. [Google Scholar] [CrossRef]
- Amalraj, A.; Jude, S.; Sukumaran, N.P.; Gopi, S. Nanomaterials in nutraceutical and phytonutrient industries. In Industrial Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 441–474. [Google Scholar] [CrossRef]
- Peng, X.; Ma, Y.; Yan, C.; Wei, X.; Zhang, L.; Jiang, H.; Ma, Y.; Zhang, S.; Xing, M.; Gao, Y. Mechanism, Formulation, and Efficacy Evaluation of Natural Products for Skin Pigmentation Treatment. Pharmaceutics 2024, 16, 1022. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, D.; Soundarya, R.; Prashantkumar, C.S. Various facets of nanotechnology in food processing. Int. J. Funct. Nutr. 2024, 5, 1–9. [Google Scholar] [CrossRef]
- Noore, S.; Pathania, S.; Fuciños, P.; O’Donnell, C.P.; Tiwari, B.K. Nanoencapsulation of Bioactive Compounds. In Nanocarriers for Controlled Release and Target Delivery of Bioactive Compounds; Springer Nature: Cham, Switzerland, 2024; pp. 49–61. [Google Scholar]
- Chen, Q.; Yang, Z.; Liu, H.; Man, J.; Oladejo, A.O.; Ibrahim, S.; Wang, S.; Hao, B. Novel Drug Delivery Systems: An Important Direction for Drug Innovation Research and Development. Pharmaceutics 2024, 16, 674. [Google Scholar] [CrossRef]
- Ceylan, Z.; Meral, R. A practiced nanobiotechnology approach with the scope of nutrition, food safety, dietetics, gastronomy, and sustainability for humans by fish meat and fish products preservation—A review. Ann. Anim. Sci. 2023, 23, 725–734. [Google Scholar] [CrossRef]
- Sahin, O.I.; Dundar, A.N.; Uzuner, K.; Parlak, M.E.; Dagdelen, A.F.; Saricaoglu, F.T. Lyophilized nano-liposomal system for red onion (Allium cepa L.) peel anthocyanin: Characterization, bioaccessibility and release kinetics. Food Biosci. 2023, 53, 102702. [Google Scholar] [CrossRef]
- Girija, S.; Wilson, J. Lipids and Liposomes Delivery of Nutritional Components. In Handbook of Nutraceuticals: Science, Technology and Engineering; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–36. [Google Scholar]
- Mozafari, M.R. Tocosome without the essential ingredient? How to avoid a mistake to be repeated again by scientists in industry and academia. J. Pharm. Sci. 2023, 112, 2004. [Google Scholar] [CrossRef]
- Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Vesicular delivery systems: Applications and future trends in food technology. In Liposomal Encapsulation in Food Science and Technology; Academic Press: Cambridge, MA, USA, 2023; pp. 15–38. [Google Scholar]
- Weng, Y.; Li, Y.; Chen, X.; Song, H.; Zhao, C.X. Encapsulation of enzymes in food industry using spray drying: Recent advances and process scale-ups. Crit. Rev. Food Sci. Nutr. 2023, 1–8. [Google Scholar] [CrossRef]
- Singh, R.P.; Gangadharappa, H.V.; Mruthunjaya, K. Phospholipids: Unique carriers for drug delivery systems. J. Drug Deliv. Sci. Technol. 2017, 39, 166–179. [Google Scholar] [CrossRef]
- Li, Q.; Yuan, L.; Gao, S.; Wang, Z.; Tang, Y.; Chen, C.; Zhao, C.; Fu, X. Self-assembled nanodrug delivery systems for anti-cancer drugs from traditional Chinese medicine. Biomater. Sci. 2024, 12, 1662–1692. [Google Scholar] [CrossRef]
- Zhou, Z.; Shi, P.; Wang, C.; Sun, Y.; Gao, C. Recent updates in nanoscale delivery systems of platinum (IV) antitumor prodrugs. Coord. Chem. Rev. 2024, 508, 215774. [Google Scholar] [CrossRef]
- Azzi, A. Tocopheryl phosphate, a novel natural form of vitamin E: In vitro and in vivo studies. FASEB J. 2006, 20, LB79–LB80. [Google Scholar] [CrossRef]
- Gianello, R.; Libinaki, R.; Azzi, A.; Gavin, P.D.; Negis, Y.; Zingg, J.M.; West, S.M.; Ogru, E. α-Tocopheryl phosphate: A novel, natural form of vitamin E. Free Radic. Biol. Med. 2005, 39, 970–976. [Google Scholar] [CrossRef]
- Noore, S.; Pathania, S.; Fuciños, P.; O’Donnell, C.P.; Tiwari, B.K. Nanocarriers for Controlled Release and Target Delivery of Bioactive Compounds; Springer Nature: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Shahgholian, N. Encapsulation and Delivery of Nutraceuticals and Bioactive Compounds by Nanoliposomes and Tocosomes as Promising Nanocarriers. Handb. Nutraceuticals Nat. Prod. Biol. Med. Nutr. Prop. Appl. 2022, 1, 403–439. [Google Scholar] [CrossRef]
- Nishio, K.; Ishida, N.; Saito, Y.; Ogawa-Akazawa, Y.; Shichiri, M.; Yoshida, Y.; Hagihara, Y.; Noguchi, N.; Chirico, J.; Atkinson, J.; et al. α-Tocopheryl phosphate: Uptake, hydrolysis, and antioxidant action in cultured cells and mouse. Free. Radic. Biol. Med. 2011, 50, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Mohammadabadi, M.R.; El-Tamimy, M.; Gianello, R.; Mozafari, M.R. Supramolecular assemblies of zwitterionic nanoliposome-polynucleotide complexes as gene transfer vectors: Nanolipoplex formulation and in vitro characterisation. J. Liposome Res. 2009, 19, 105–115. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Mehrarya, M.; Gharehchelou, B.; Kabarkouhi, Z.; Ataei, S.; Esfahani, F.N.; Wintrasiri, M.N.; Mozafari, M.R. Functionalized nanostructured bioactive carriers: Nanoliposomes, quantum dots, tocosome, and theranostic approach. Curr. Drug Deliv. 2022, 19, 1001–1011. [Google Scholar] [CrossRef]
- Bolhassani, A. Lipid-based delivery systems in development of genetic and subunit vaccines. Mol. Biotechnol. 2023, 65, 669–698. [Google Scholar] [CrossRef] [PubMed]
- Raoufi, E.; Bahramimeimandi, B.; Salehi-Shadkami, M.; Chaosri, P.; Mozafari, M.R. Methodical design of viral vaccines based on avant-Garde nanocarriers: A multi-domain narrative review. Biomedicines 2021, 9, 520. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Khalili-Tanha, G.; Fiuji, H.; Gharib, M.; Moghbeli, M.; Khalili-Tanha, N.; Rahmani, F.; Shakour, N.; Maftooh, M.; Hassanian, S.M.; Asgharzadeh, F.; et al. Dual targeting of TGF-β and PD-L1 inhibits tumor growth in TGF-β/PD-L1-driven colorectal carcinoma. Life Sci. 2023, 328, 121865. [Google Scholar] [CrossRef] [PubMed]


| No. | Product | Manufacturer | Webpage |
|---|---|---|---|
| 1 | Liposomal Vitamin K2 + D3 | California Gold Nutrition | www.californiagoldnutrition.com |
| 2 | Nanoliposomal Vitamin C | NOW Foods | www.nowfoods.com |
| 3 | Vesicular Phospholipid Complex | Body Bio | https://bodybio.com |
| 4 | Liposomal Creatine | Codeage | www.codeage.com |
| 5 | Liposomal Glutathione | Codeage | www.codeage.com |
| 6 | Liposomal Carnosine | Dr. Mercola | www.mercolamarket.com |
| 7 | Pro-Liposomal Apigenin | MCS Formulas | www.mcsformulas.com |
| 8 | Liposomal Curcumin | Lipolife | https://lipolife.co.uk |
| 9 | Liposomal Zinc | Vinco | https://vinco.com.au |
| 10 | Liposomal Resveratrol | Renue By Science | https://renuebyscience.com |
| 11 | Liposomal Vitamin C + Zinc | PlantaCorp | https://plantacorp.com |
| 12 | Liposomal Quercetin | PlantaCorp | https://plantacorp.com |
| Item | Vesicle Type | Abbreviation | Number of Lamellae | Size Range * |
|---|---|---|---|---|
| 1 | Small unilamellar vesicle | SUV | One bilayer | 20–100 nm |
| 2 | Double-bilayer vesicle | DBV | Two bilayers | 200–500 nm |
| 3 | Oligolamellar vesicle | OLV | Between two to several bilayers | 500–700 nm |
| 4 | Large unilamellar vesicle | LUV | One bilayer | 0.1 to 1.0 μm |
| 5 | Multilamellar vesicle | MLV | More than 10 | 0.5 to several μm |
| 6 | Large multilamellar vesicle | LMV | More than 10 | 1.0 to several μm |
| 7 | Giant unilamellar vesicle | GUV | One bilayer | >2.0 μm |
| 8 | Multivesicular vesicle | MVV | Several vesicles encapsulated inside a giant vesicle with one bilayer | >5.0 μm |
| Item | Bioactive Compound | Health Benefits | Source | Molecular Weight (g/mol) |
|---|---|---|---|---|
| 1 | Astaxanthin | Protects against UV damage, boosts immune system, reduces inflammation | Algae, yeast, shrimp, and different fish | 596.841 |
| 2 | Berberine | Treatment of different types of cancer | Barks, twigs, leaves, stems, roots, and rhizomes of various medicinal plant species | 336.3612 |
| 3 | Curcumin | Antibacterial, antioxidant, and anti-inflammatory effects | Turmeric | 368.38 |
| 4 | Hesperidin | Therapeutic potential in heart disease, blood vessel disorders, metabolic disorders, and neurodegenerative diseases | Citrus fruits | 610.1898 |
| 5 | Kaempferol | Antioxidant and anticarcinogenic effects | Apples, peaches, tomatoes, grapes, green tea, lettuce, broccoli | 286.23 |
| 6 | Luteolin | Anticarcinogenic effects | Green pepper, oregano, carrots, broccoli | 286.24 |
| 7 | Quercetin | Promotes cardiovascular health and improves blood flow | Grapes, onions, lemon tea, citrus fruits, etc. | 302.236 |
| 8 | Resveratrol | Protects against Alzheimer’s disease, cardiovascular diseases, cancer, liver ailments, obesity, diabetes, etc. | The skin of peanuts, grapes, raspberries, mulberries, and blueberries | 228.25 |
| 9 | Rutin | Treats conditions associated with poor blood flow, chronic pain, and high cholesterol | Buckwheat, Japanese pagoda tree, and Eucalyptus | 610.517 |
| 10 | Tocopherol Phosphates * | Anti-inflammatory, atherosclerotic-preventing, and cardioprotective effects | Green vegetables, dairy products, cereals, different seeds and nuts | TP: 510.69 T2P: 923.54 |
| 11 | Omega-3 fatty acids | Can fight inflammation and fight autoimmune diseases, promotes brain health during pregnancy, can fight depression and anxiety | Fish oil, flaxseed, krill oil, squid oil | 909.39 |
| 12 | Ubiquinone (Coenzyme Q10) | Possesses health benefits for cardiovascular diseases, energy-boosting, reduces the symptoms of mitochondrial disorders | Oily fish (such as salmon and tuna), organ meats (such as liver), and whole grains | 863.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atrooz, O.; Kerdari, E.; Mozafari, M.R.; Reihani, N.; Asadi, A.; Torkaman, S.; Alavi, M.; Taghavi, E. A Comparative Review of Tocosomes, Liposomes, and Nanoliposomes as Potent and Novel Nanonutraceutical Delivery Systems for Health and Biomedical Applications. Biomedicines 2024, 12, 2002. https://doi.org/10.3390/biomedicines12092002
Atrooz O, Kerdari E, Mozafari MR, Reihani N, Asadi A, Torkaman S, Alavi M, Taghavi E. A Comparative Review of Tocosomes, Liposomes, and Nanoliposomes as Potent and Novel Nanonutraceutical Delivery Systems for Health and Biomedical Applications. Biomedicines. 2024; 12(9):2002. https://doi.org/10.3390/biomedicines12092002
Chicago/Turabian StyleAtrooz, Omar, Elham Kerdari, M. R. Mozafari, Nasim Reihani, Ali Asadi, Sarabanou Torkaman, Mehran Alavi, and Elham Taghavi. 2024. "A Comparative Review of Tocosomes, Liposomes, and Nanoliposomes as Potent and Novel Nanonutraceutical Delivery Systems for Health and Biomedical Applications" Biomedicines 12, no. 9: 2002. https://doi.org/10.3390/biomedicines12092002








