Green Light Exposure Reduces Primary Hyperalgesia and Proinflammatory Cytokines in a Rodent Model of Knee Osteoarthritis: Shedding Light on Sex Differences
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
2.2. Unilateral Monoiodoacetate (MIA) Knee Injection
2.3. GLED Exposure
2.4. Knee Mechanical Threshold
2.5. Blood Serum Collection and Cytokine Quantification
2.6. Statistical Analysis
3. Results
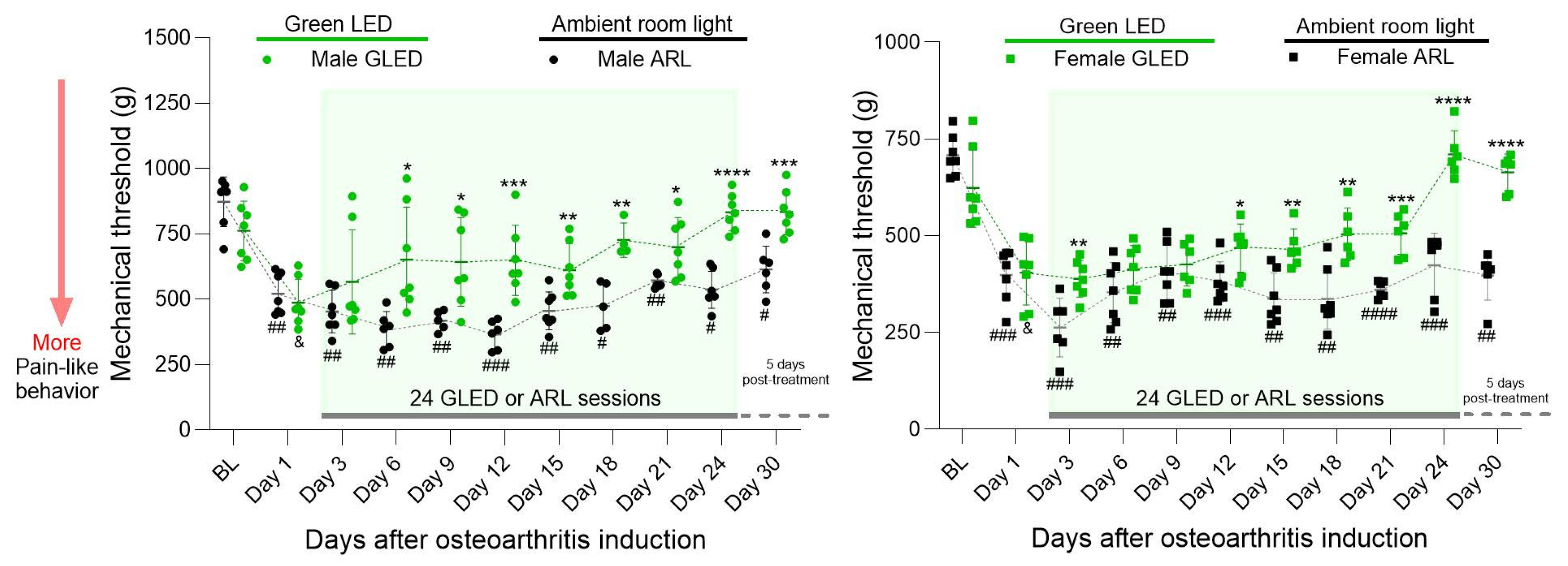
3.1. Green Light Reduces Primary Hyperalgesia in a Model of Chronic Knee Osteoarthritis in Males and Females, with Effects Lasting beyond Therapy Termination
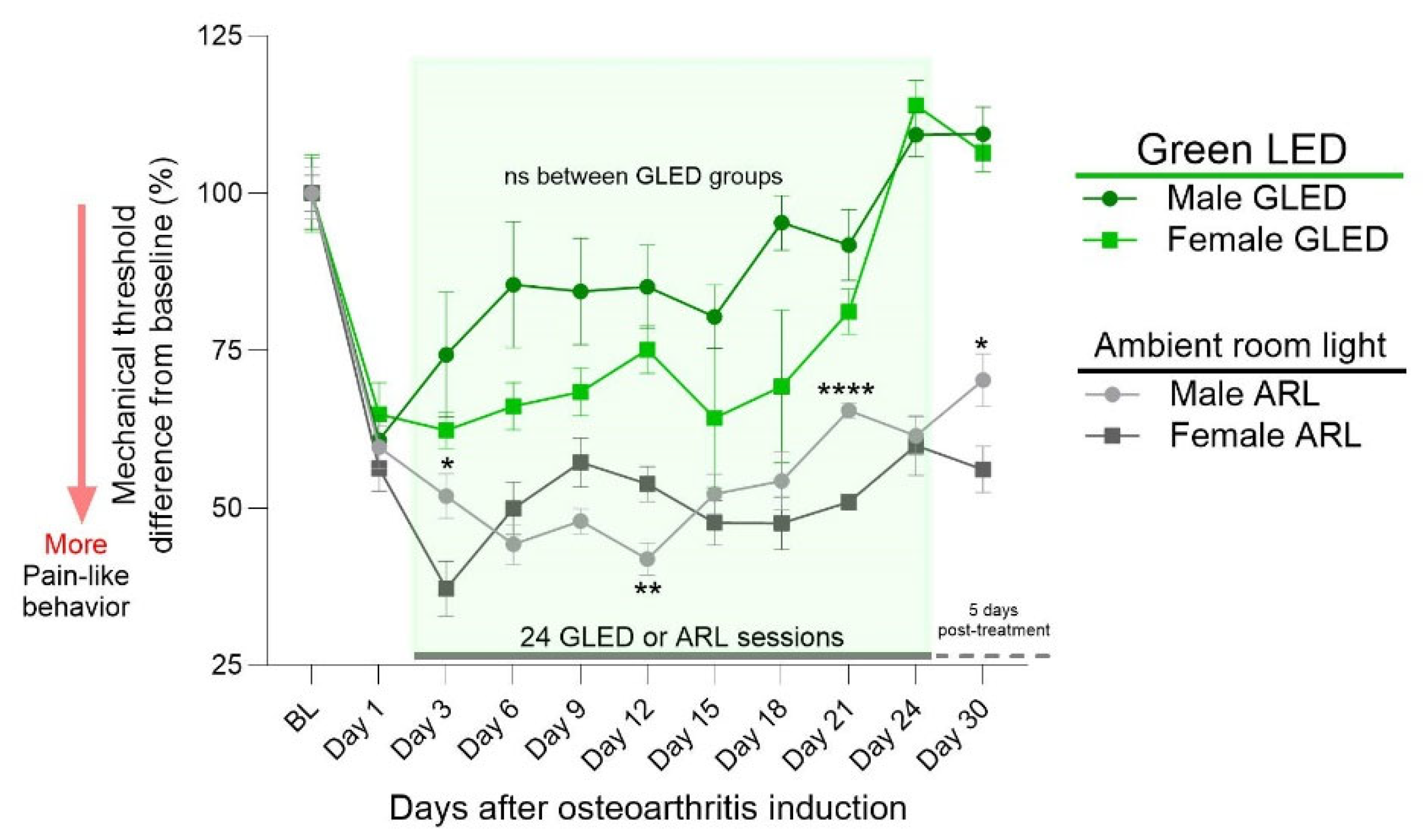
3.2. Sex Differences in Primary Hyperalgesia in a Model of Chronic Knee Osteoarthritis
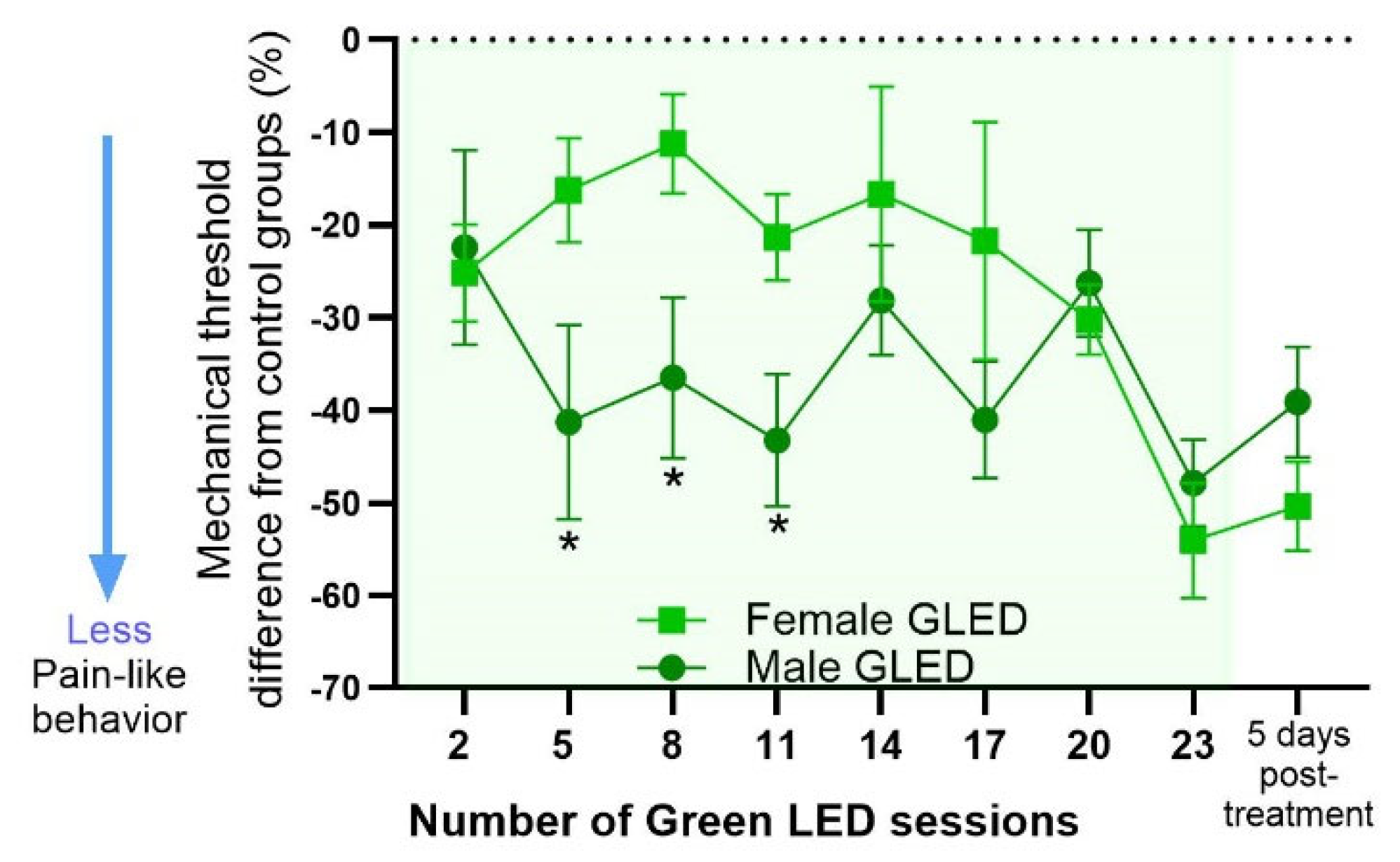
3.3. Magnitude of GLED Effects on Males and Females Relative to Their Sex-Matched Controls
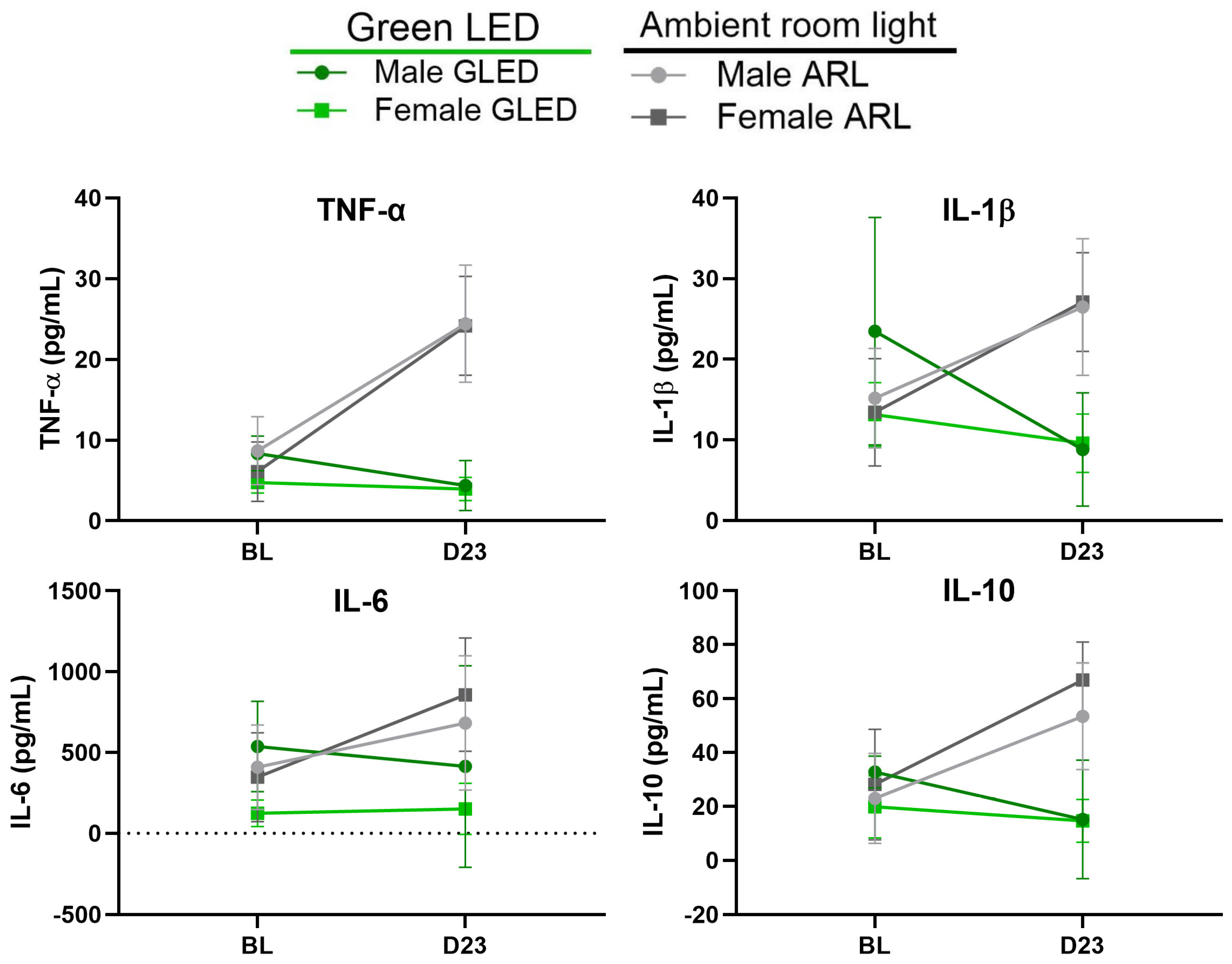
3.4. Green Light Reduces Serum Proinflammatory Cytokine Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rikard, S.M.; Strahan, A.E.; Schmit, K.M.; Guy, G.P., Jr. Chronic Pain Among Adults—United States, 2019–2021. MMWR 2023, 72, 379–385. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, E. Pharmacologic and Non-Pharmacologic Treatment of Osteoarthritis. Curr. Treat. Options Rheumatol. 2016, 2, 111–125. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Peloso, P.M.; Everett, S.V.; Rajagopalan, S.; Black, C.M.; Mavros, P.; Arden, N.K.; Phillips, C.J.; Rannou, F.; van de Laar, M.A.F.J.; et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: Evidence from a prospective multinational longitudinal study of osteoarthritis real-world therapies. Rheumatology 2014, 54, 270–277. [Google Scholar] [CrossRef] [PubMed]
- March, L.; Cross, M. Epidemiology and risk factors for osteoarthritis. In UpToDate; Hunter, D., Law, K., Eds.; UpToDate: Waltham, MA, USA, 2024. [Google Scholar]
- Perruccio, A.V.; Young, J.J.; Wilfong, J.M.; Power, J.D.; Canizares, M.; Badley, E.M. Osteoarthritis year in review 2023: Epidemiology & therapy. Osteoarthr. Cartil. 2024, 32, 159–165. [Google Scholar] [CrossRef]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef]
- Li, B.; Yang, Z.; Li, Y.; Zhang, J.; Li, C.; Lv, N. Exploration beyond osteoarthritis: The association and mechanism of its related comorbidities. Front. Endocrinol. 2024, 15, 1352671. [Google Scholar] [CrossRef]
- Marshall, D.A.; Liu, X.; Barnabe, C.; Yee, K.; Faris, P.D.; Barber, C.; Mosher, D.; Noseworthy, T.; Werle, J.; Lix, L. Existing comorbidities in people with osteoarthritis: A retrospective analysis of a population-based cohort in Alberta, Canada. BMJ Open 2019, 9, e033334. [Google Scholar] [CrossRef]
- Ide, J.; Shoaibi, A.; Wagner, K.; Weinstein, R.; Boyle, K.E.; Myers, A. Patterns of Comorbidities and Prescribing and Dispensing of Non-steroidal Anti-inflammatory Drugs (NSAIDs) Among Patients with Osteoarthritis in the USA: Real-World Study. Drugs Aging 2024, 41, 357–366. [Google Scholar] [CrossRef]
- GBD 2021 Osteoarthritis Collaborators. Global, regional, and national burden of osteoarthritis, 1990–2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Zhang, W.; Robertson, W.B.; Zhao, J.; Chen, W.; Xu, J. Emerging Trend in the Pharmacotherapy of Osteoarthritis. Front. Endocrinol. 2019, 10, 431. [Google Scholar] [CrossRef]
- Primorac, D.; Molnar, V.; Matišić, V.; Hudetz, D.; Jeleč, Ž.; Rod, E.; Čukelj, F.; Vidović, D.; Vrdoljak, T.; Dobričić, B.; et al. Comprehensive Review of Knee Osteoarthritis Pharmacological Treatment and the Latest Professional Societies’ Guidelines. Pharmaceuticals 2021, 14, 205. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A. “I wish it had a place to go”: A nominal group study of barriers to the effectiveness of non-surgical treatments for knee osteoarthritis inclusive of minority populations. Arthritis Res. Ther. 2021, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- DeRogatis, M.; Anis, H.K.; Sodhi, N.; Ehiorobo, J.O.; Chughtai, M.; Bhave, A.; Mont, M.A. Non-operative treatment options for knee osteoarthritis. Ann. Transl. Med. 2019, 7, S245. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.; Blanco, F.; Englund, M.; Karsdal, M.; Lohmander, L. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 2015, 23, 1233–1241. [Google Scholar] [CrossRef]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef]
- Sen, R.; Goyal, A.; Hurley, J.A. Osteoarthritis. In Seronegative Spondyloarthropathy; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2010, 7, 33–42. [Google Scholar] [CrossRef]
- Kobayashi, M.; Squires, G.R.; Mousa, A.; Tanzer, M.; Zukor, D.J.; Antoniou, J.; Feige, U.; Poole, A.R. Role of interleukin-1 and tumor necrosis factor α in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005, 52, 128–135. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M.; Plumb, D.A.; Dragomir, C.; Favero, M.; El Hachem, K.; Hashimoto, K.; Roach, H.I.; Olivotto, E.; Borzì, R.M.; et al. Roles of inflammatory and anabolic cytokines in cartilage metabolism: Signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur. Cells Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef]
- Cerezo, L.A.; Navrátilová, A.; Kuklová, M.; Prokopcová, A.; Baloun, J.; Kropáčková, T.; Veigl, D.; Popelka, S.; Fulín, P.; Ballay, R.; et al. IL-40 is up-regulated in the synovial fluid and cartilage of osteoarthritis patients and contributes to the alteration of chondrocytes phenotype in vitro. Arthritis Res. Ther. 2024, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ungsudechachai, T.; Jittikoon, J.; Honsawek, S.; Udomsinprasert, W. Protective effect of clusterin against interleukin-1beta-induced apoptosis and inflammation in human knee osteoarthritis chondrocytes. Clin. Transl. Sci. 2024, 17, e13881. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.; Umoh, E.; Pessler, F.; Diaz-Torne, C.; Miles, T.; DiCarlo, E.; Potter, H.; Mandl, L.; Marx, R.; Rodeo, S.; et al. Local cytokine profiles in knee osteoarthritis: Elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthr. Cartil. 2009, 17, 1040–1048. [Google Scholar] [CrossRef]
- Yang, P.; Tan, J.; Yuan, Z.; Meng, G.; Bi, L.; Liu, J. Expression profile of cytokines and chemokines in osteoarthritis patients: Proinflammatory roles for CXCL8 and CXCL11 to chondrocytes. Int. Immunopharmacol. 2016, 40, 16–23. [Google Scholar] [CrossRef]
- Radojčić, M.R.; Thudium, C.S.; Henriksen, K.; Tan, K.; Karlsten, R.; Dudley, A.; Chessell, I.; Karsdal, M.A.; Bay-Jensen, A.-C.; Crema, M.D.; et al. Biomarker of extracellular matrix remodelling C1M and proinflammatory cytokine interleukin 6 are related to synovitis and pain in end-stage knee osteoarthritis patients. Pain 2017, 158, 1254–1263. [Google Scholar] [CrossRef]
- Shimura, Y.; Kurosawa, H.; Sugawara, Y.; Tsuchiya, M.; Sawa, M.; Kaneko, H.; Futami, I.; Liu, L.; Sadatsuki, R.; Hada, S.; et al. The factors associated with pain severity in patients with knee osteoarthritis vary according to the radiographic disease severity: A cross-sectional study. Osteoarthr. Cartil. 2013, 21, 1179–1184. [Google Scholar] [CrossRef]
- Imamura, M.; Ezquerro, F.; Marcon Alfieri, F.; Vilas Boas, L.; Tozetto-Mendoza, T.R.; Chen, J.; Ozcakar, L.; Arendt-Nielsen, L.; Rizzo Battistella, L. Serum Levels of Proinflammatory Cytokines in Painful Knee Osteoarthritis and Sensitization. Int. J. Inflamm. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Abbas, H.; Ambrosius, W.; Nicklas, B.J.; Davis, C.; Messier, S.P.; Pahor, M. Inflammatory markers and physical function among older adults with knee osteoarthritis. J. Rheumatol. 2004, 31, 2027–2031. [Google Scholar]
- Orita, S.; Koshi, T.; Mitsuka, T.; Miyagi, M.; Inoue, G.; Arai, G.; Ishikawa, T.; Hanaoka, E.; Yamashita, K.; Yamashita, M.; et al. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet. Disord. 2011, 12, 144. [Google Scholar] [CrossRef]
- Zhang, R.-X.; Ren, K.; Dubner, R. Osteoarthritis pain mechanisms: Basic studies in animal models. Osteoarthr. Cartil. 2013, 21, 1308–1315. [Google Scholar] [CrossRef]
- Longo, U.G.; Papalia, R.; De Salvatore, S.; Picozzi, R.; Sarubbi, A.; Denaro, V. Induced Models of Osteoarthritis in Animal Models: A Systematic Review. Biology 2023, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Udo, M.; Muneta, T.; Tsuji, K.; Ozeki, N.; Nakagawa, Y.; Ohara, T.; Saito, R.; Yanagisawa, K.; Koga, H.; Sekiya, I. Monoiodoacetic acid induces arthritis and synovitis in rats in a dose- and time-dependent manner: Proposed model-specific scoring systems. Osteoarthr. Cartil. 2016, 24, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Pomonis, J.D.; Boulet, J.M.; Gottshall, S.L.; Phillips, S.; Sellers, R.; Bunton, T.; Walker, K. Development and pharmacological characterization of a rat model of osteoarthritis pain. Pain 2005, 114, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Bove, S.; Calcaterra, S.; Brooker, R.; Huber, C.; Guzman, R.; Juneau, P.; Schrier, D.; Kilgore, K. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr. Cartil. 2003, 11, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Alves-Simões, M. Rodent models of knee osteoarthritis for pain research. Osteoarthr. Cartil. 2022, 30, 802–814. [Google Scholar] [CrossRef]
- Orita, S.; Ishikawa, T.; Miyagi, M.; Ochiai, N.; Inoue, G.; Eguchi, Y.; Kamoda, H.; Arai, G.; Toyone, T.; Aoki, Y.; et al. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet. Disord. 2011, 12, 134. [Google Scholar] [CrossRef]
- O’brien, M.S.; McDougall, J.J. Targeting Proteinase Activated Receptor-4 Reduces Mechanonociception During the Acute Inflammatory Phase but not the Chronic Neuropathic Phase of Osteoarthritis in Rats. Front. Pharmacol. 2021, 12, 756632. [Google Scholar] [CrossRef]
- Park, M.H.; Jung, J.C.; Hill, S.; Cartwright, E.; Dohnalek, M.H.; Yu, M.; Jun, H.J.; Han, S.B.; Hong, J.T.; Son, D.J. FlexPro MD®, a Combination of Krill Oil, Astaxanthin and Hyaluronic Acid, Reduces Pain Behavior and Inhibits Inflammatory Response in Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Nutrients 2020, 12, 956. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- da Costa, B.R.; Pereira, T.V.; Saadat, P.; Rudnicki, M.; Iskander, S.M.; Bodmer, N.S.; Bobos, P.; Gao, L.; Kiyomoto, H.D.; Montezuma, T.; et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: Network meta-analysis. BMJ 2021, 375, n2321. [Google Scholar] [CrossRef] [PubMed]
- Gregori, D.; Giacovelli, G.; Minto, C.; Barbetta, B.; Gualtieri, F.; Azzolina, D.; Vaghi, P.; Rovati, L.C. Association of Pharmacological Treatments With Long-term Pain Control in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. JAMA 2018, 320, 2564–2579. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, H.; Chen, D.; Xu, K.; Li, Z.; Wu, H.; Geng, X.; Wei, X.; Wu, J.; Cui, W.; et al. Looking for a Beam of Light to Heal Chronic Pain. J. Pain Res. 2024, ume 17, 1091–1105. [Google Scholar] [CrossRef]
- Martin, L.; Porreca, F.; Mata, E.I.; Salloum, M.; Goel, V.; Gunnala, P.; Killgore, W.D.S.; Jain, S.; Jones-MacFarland, F.N.; Khanna, R.; et al. Green Light Exposure Improves Pain and Quality of Life in Fibromyalgia Patients: A Preliminary One-Way Crossover Clinical Trial. Pain Med. 2021, 22, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.F.; Patwardhan, A.M.; Jain, S.V.; Salloum, M.M.; Freeman, J.; Khanna, R.; Gannala, P.; Goel, V.; Jones-MacFarland, F.N.; Killgore, W.D.; et al. Evaluation of green light exposure on headache frequency and quality of life in migraine patients: A preliminary one-way cross-over clinical trial. Cephalalgia 2020, 41, 135–147. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Patwardhan, A.; Gilbraith, K.B.; Moutal, A.; Yang, X.; Chew, L.A.; Largent-Milnes, T.; Malan, T.P.; Vanderah, T.W.; Porreca, F.; et al. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain 2017, 158, 347–360. [Google Scholar] [CrossRef]
- Martin, L.F.; Moutal, A.; Cheng, K.; Washington, S.M.; Calligaro, H.; Goel, V.; Kranz, T.; Largent-Milnes, T.M.; Khanna, R.; Patwardhan, A.; et al. Green Light Antinociceptive and Reversal of Thermal and Mechanical Hypersensitivity Effects Rely on Endogenous Opioid System Stimulation. J. Pain 2021, 22, 1646–1656. [Google Scholar] [CrossRef]
- Tang, Y.-L.; Liu, A.-L.; Lv, S.-S.; Zhou, Z.-R.; Cao, H.; Weng, S.-J.; Zhang, Y.-Q. Green light analgesia in mice is mediated by visual activation of enkephalinergic neurons in the ventrolateral geniculate nucleus. Sci. Transl. Med. 2022, 14, eabq6474. [Google Scholar] [CrossRef]
- Martin, L.F.; Cheng, K.; Washington, S.M.; Denton, M.; Goel, V.; Khandekar, M.; Largent-Milnes, T.M.; Patwardhan, A.; Ibrahim, M.M. Green Light Exposure Elicits Anti-inflammation, Endogenous Opioid Release and Dampens Synaptic Potentiation to Relieve Post-surgical Pain. J. Pain 2022, 24, 509–529. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Tan, B.; Du, Y.; Yang, L.; Hu, T.-T.; Ding, Y.-L.; Qiu, X.-Y.; Moutal, A.; Khanna, R.; Yu, J.; et al. Glutamatergic and GABAergic neurons in the vLGN mediate the nociceptive effects of green and red light on neuropathic pain. Neurobiol. Dis. 2023, 183, 106164. [Google Scholar] [CrossRef]
- Cao, P.; Zhang, M.; Ni, Z.; Song, X.-J.; Yang, C.-L.; Mao, Y.; Zhou, W.; Dong, W.-Y.; Peng, X.; Zheng, C.; et al. Green light induces antinociception via visual-somatosensory circuits. Cell Rep. 2023, 42, 112290. [Google Scholar] [CrossRef]
- Perruccio, A.V.; Badley, E.M.; Power, J.D.; Canizares, M.; Kapoor, M.; Rockel, J.; Chandran, V.; Gandhi, R.; Mahomed, N.M.; Davey, J.R.; et al. Sex differences in the relationship between individual systemic markers of inflammation and pain in knee osteoarthritis. Osteoarthr. Cartil. Open 2019, 1, 100004. [Google Scholar] [CrossRef] [PubMed]
- E Lenert, M.; Avona, A.; Garner, K.M.; Barron, L.R.; Burton, M.D. Sensory Neurons, Neuroimmunity, and Pain Modulation by Sex Hormones. Endocrinology 2021, 162, bqab109. [Google Scholar] [CrossRef] [PubMed]
- Stratton, H.; Lee, G.; Dolatyari, M.; Ghetti, A.; Cotta, T.; Mitchell, S.; Yue, X.; Ibrahim, M.; Dumaire, N.; Salih, L.; et al. Nociceptors are functionally male or female: From mouse to monkey to man. Brain 2024, awae179. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.S.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.-S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, Y.; Sheets, P.L. Mouse models of surgical and neuropathic pain produce distinct functional alterations to prodynorphin expressing neurons in the prelimbic cortex. Neurobiol. Pain 2023, 13, 100121. [Google Scholar] [CrossRef]
- Cardenas, A.; Papadogiannis, A.; Dimitrov, E. The role of medial prefrontal cortex projections to locus ceruleus in mediating the sex differences in behavior in mice with inflammatory pain. FASEB J. 2021, 35, e21747. [Google Scholar] [CrossRef] [PubMed]
- Ro, J.Y.; Zhang, Y.; Tricou, C.; Yang, D.; da Silva, J.T.; Zhang, R. Age and Sex Differences in Acute and Osteoarthritis-Like Pain Responses in Rats. J. Gerontol. Ser. A 2020, 75, 1465–1472. [Google Scholar] [CrossRef]
- Sannajust, S.; Imbert, I.; Eaton, V.; Henderson, T.; Liaw, L.; May, M.; Barbe, M.F.; King, T. Females have greater susceptibility to develop ongoing pain and central sensitization in a rat model of temporomandibular joint pain. Pain 2019, 160, 2036–2049. [Google Scholar] [CrossRef]
- Nwosu, L.; Mapp, P.; Chapman, V.; Walsh, D. Relationship between structural pathology and pain behaviour in a model of osteoarthritis (OA). Osteoarthr. Cartil. 2016, 24, 1910–1917. [Google Scholar] [CrossRef]
- Lockwood, S.; Lopes, D.; McMahon, S.; Dickenson, A. Characterisation of peripheral and central components of the rat monoiodoacetate model of Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 712–722. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.I. Osteoarthritis of the Hip and Knee: Sex and Gender Differences. Orthop. Clin. 2006, 37, 559–568. [Google Scholar] [CrossRef]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Tschon, M.; Contartese, D.; Pagani, S.; Borsari, V.; Fini, M. Gender and Sex Are Key Determinants in Osteoarthritis Not Only Confounding Variables. A Systematic Review of Clinical Data. J. Clin. Med. 2021, 10, 3178. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.A.; Nilges, J.M.; Oo, W.M. Sex differences in osteoarthritis prevalence, pain perception, physical function and therapeutics. Osteoarthr. Cartil. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, A.C.; Clark, A.K.; Gentry, C.; Hobbs, C.; Malcangio, M. Pain-like behaviour and spinal changes in the monosodium iodoacetate model of osteoarthritis in C57Bl/6 mice. Eur. J. Pain 2012, 17, 514–526. [Google Scholar] [CrossRef]
- Da Silva, J.T.; Tricou, C.; Zhang, Y.; Tofighbakhsh, A.; Seminowicz, D.A.; Ro, J.Y. Pain modulatory network is influenced by sex and age in a healthy state and during osteoarthritis progression in rats. Aging Cell 2021, 20, e13292. [Google Scholar] [CrossRef]
- Da Silva, J.T.; Tricou, C.; Zhang, Y.; Seminowicz, D.A.; Ro, J.Y. Brain networks and endogenous pain inhibition are modulated by age and sex in healthy rats. Pain 2020, 161, 1371–1380. [Google Scholar] [CrossRef]
- Yamada, E.F.; Bobinski, F.; Martins, D.F.; Palandi, J.; Folmer, V.; da Silva, M.D. Photobiomodulation therapy in knee osteoarthritis reduces oxidative stress and inflammatory cytokines in rats. J. Biophotonics 2019, 13, e201900204. [Google Scholar] [CrossRef]
- Yu, Y.; Kim, S.-M.; Park, K.; Kim, H.J.; Kim, J.G.; Kim, S.E. Therapeutic Nanodiamonds Containing Icariin Ameliorate the Progression of Osteoarthritis in Rats. Int. J. Mol. Sci. 2023, 24, 15977. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Lee, H.H.; Kim, J.; Choi, E.-O.; Hwang-Bo, H.; Kim, H.J.; Kim, M.Y.; Ahn, K.I.; Kim, G.-Y.; Lee, K.W.; et al. Mori Folium water extract alleviates articular cartilage damages and inflammatory responses in monosodium iodoacetate-induced osteoarthritis rats. Mol. Med. Rep. 2017, 16, 3841–3848. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Ren, J.; Gong, S.; Qiao, F.; He, J. CTRP9 protects against MIA-induced inflammation and knee cartilage damage by deactivating the MAPK/NF-kappaB pathway in rats with osteoarthritis. Open Life Sci. 2020, 15, 971–980. [Google Scholar] [CrossRef]
- Kim, H.L.; Lee, H.J.; Lee, D.-R.; Choi, B.-K.; Yang, S.H. Herbal Composition LI73014F2 Alleviates Articular Cartilage Damage and Inflammatory Response in Monosodium Iodoacetate-Induced Osteoarthritis in Rats. Molecules 2020, 25, 5467. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.; Rogers, V.E.; Henriksen, V.T.; Trawick, R.H.; Momberger, N.G.; Rasmussen, G.L. Circulating IL-10 is compromised in patients predisposed to developing and in patients with severe knee osteoarthritis. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Palada, V.; Ahmed, A.S.; Freyhult, E.; Hugo, A.; Kultima, K.; Svensson, C.I.; Kosek, E. Elevated inflammatory proteins in cerebrospinal fluid from patients with painful knee osteoarthritis are associated with reduced symptom severity. J. Neuroimmunol. 2020, 349, 577391. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura, L.; do Espírito-Santo, R.F.; Keaser, M.; Zhang, Y.; Ro, J.Y.; Da Silva, J.T. Green Light Exposure Reduces Primary Hyperalgesia and Proinflammatory Cytokines in a Rodent Model of Knee Osteoarthritis: Shedding Light on Sex Differences. Biomedicines 2024, 12, 2005. https://doi.org/10.3390/biomedicines12092005
Ventura L, do Espírito-Santo RF, Keaser M, Zhang Y, Ro JY, Da Silva JT. Green Light Exposure Reduces Primary Hyperalgesia and Proinflammatory Cytokines in a Rodent Model of Knee Osteoarthritis: Shedding Light on Sex Differences. Biomedicines. 2024; 12(9):2005. https://doi.org/10.3390/biomedicines12092005
Chicago/Turabian StyleVentura, Laura, Renan F. do Espírito-Santo, Michael Keaser, Youping Zhang, Jin Y. Ro, and Joyce T. Da Silva. 2024. "Green Light Exposure Reduces Primary Hyperalgesia and Proinflammatory Cytokines in a Rodent Model of Knee Osteoarthritis: Shedding Light on Sex Differences" Biomedicines 12, no. 9: 2005. https://doi.org/10.3390/biomedicines12092005





