Effects of Prostaglandin E1 and Balloon Atrial Septostomy on Cerebral Blood Flow and Oxygenation in Newborns Diagnosed with Transposition of the Great Arteries
Abstract
1. Introduction
2. Materials and Methods
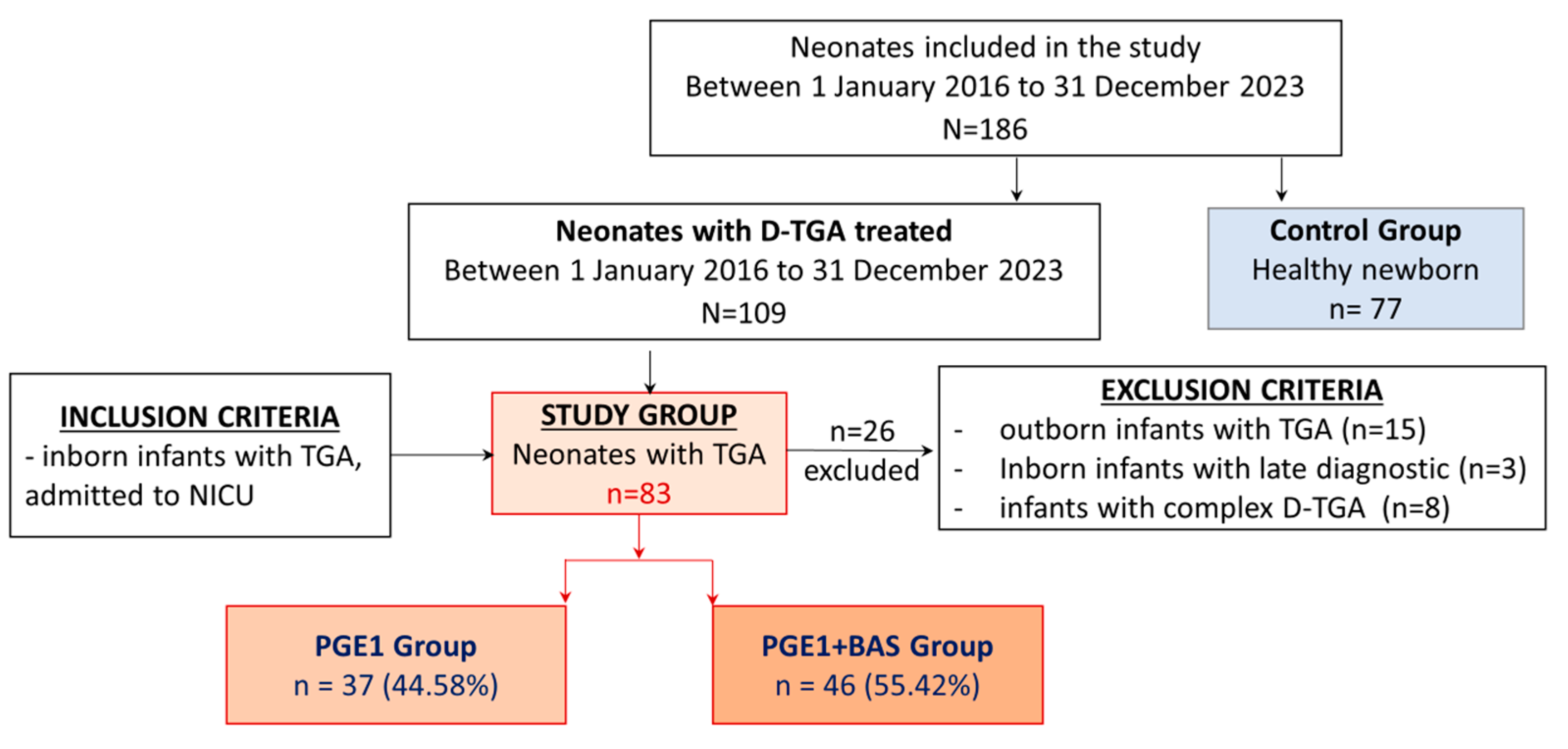
2.1. Study Groups
2.2. D-TGA Management and Study Intervention
2.3. Data Collection
2.4. Timing of Patient Evaluation
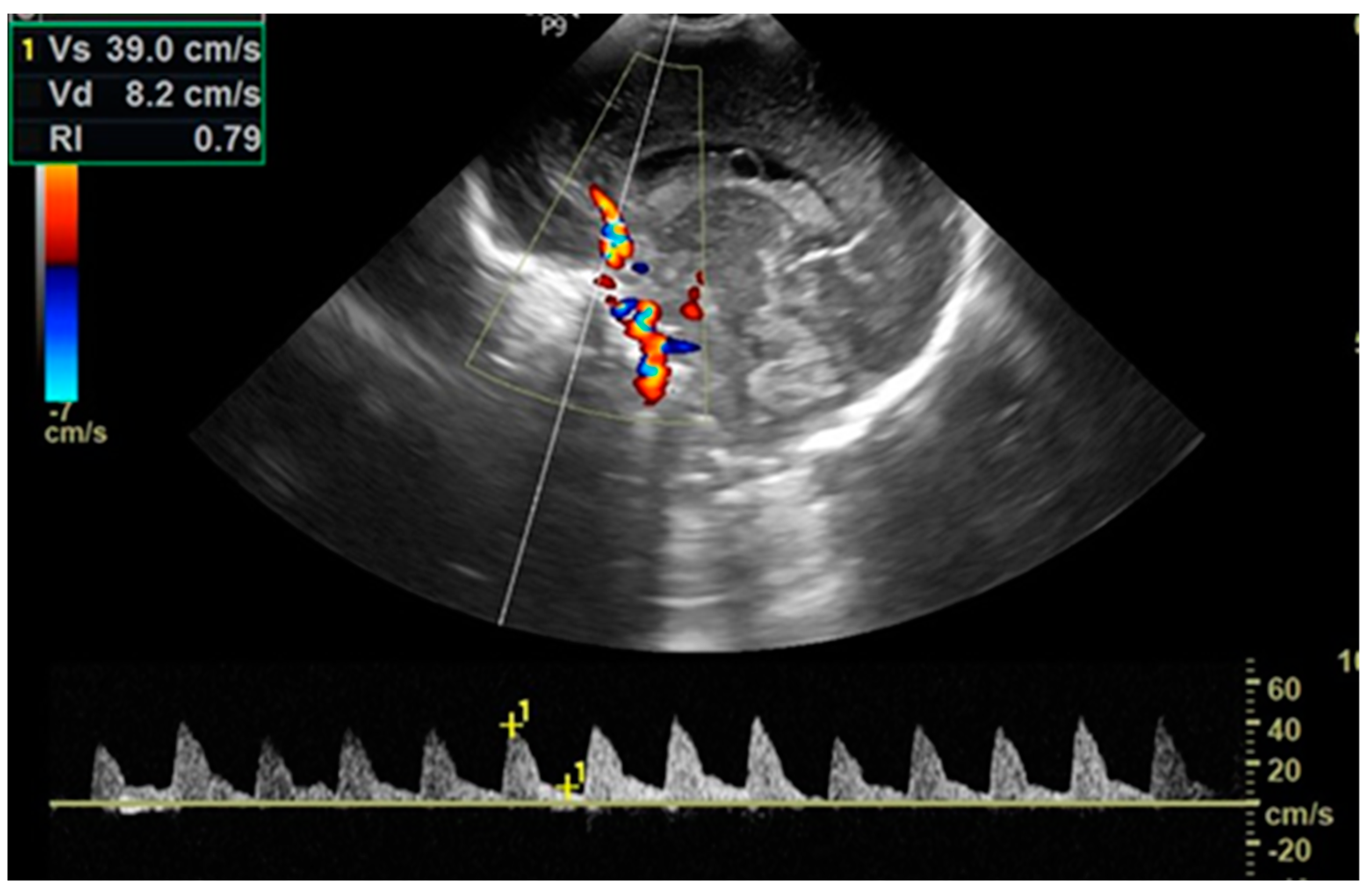
2.5. Cardiac Ultrasounds
2.6. Cerebral Ultrasounds
2.7. SpO2 Measurement
2.8. crSO2 and cFTOE Measurement
2.9. Blood Pressure Measurement
2.10. Blood Gases Measurement
2.11. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Clinical Characteristics, Interventions, and Heart Ultrasound Evaluation
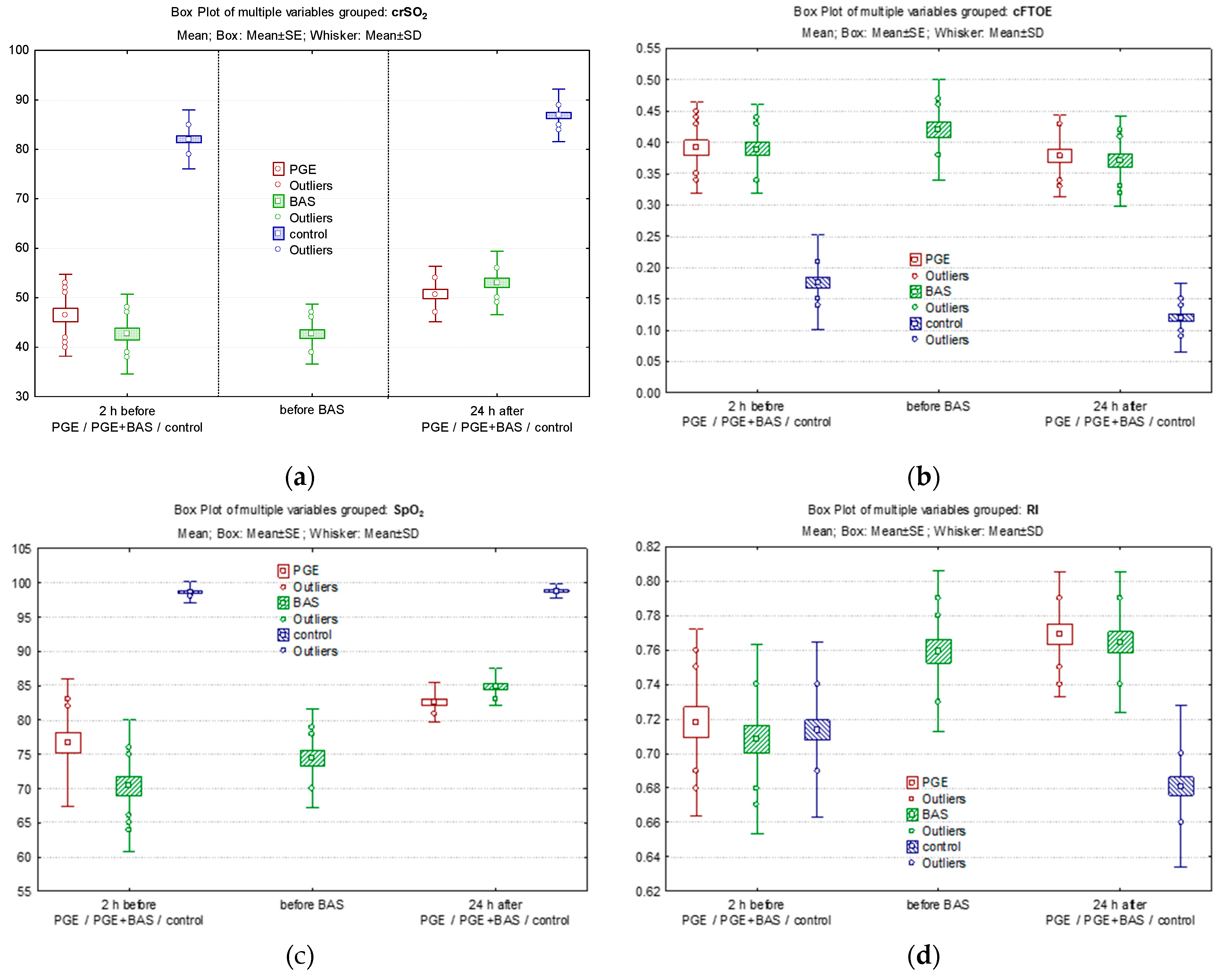
3.3. Effect of PGE1 and BAS on Peripheric and Cerebral Oxygenation
3.4. Effect of PGE1 and BAS
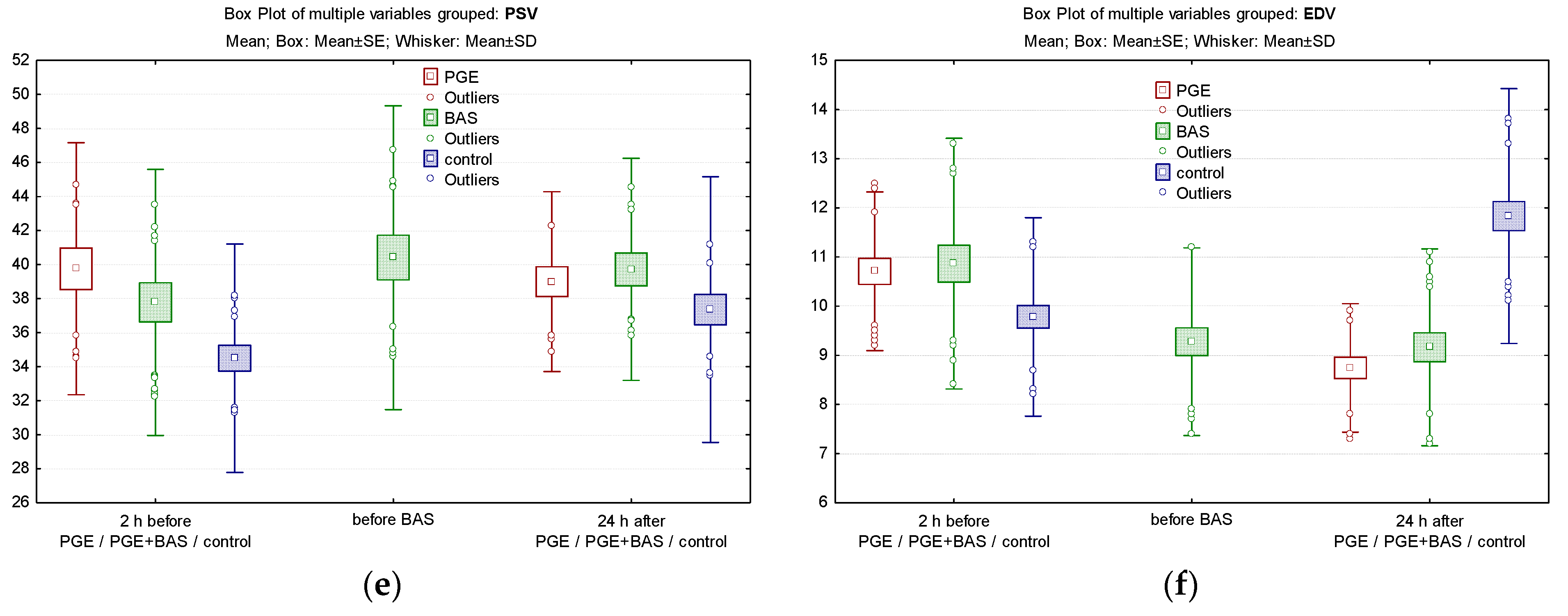
3.5. Comparison of Cerebral/Peripheric Oxygenation and Cerebral Velocities between Groups
4. Discussion
4.1. Cerebral Oxygenation
4.2. Cerebral Blood Flow
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, M.S.; Eidem, B.W.; Cetta, F.; Fogel, M.A.; Frommelt, P.C.; Ganame, J.; Han, B.K.; Kimball, T.R.; Johnson, R.K.; Mertens, L.; et al. Multimodality Imaging Guidelines of Patients with Transposition of the Great Arteries: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J. Am. Soc. Echocardiogr. 2016, 29, 571–621. [Google Scholar] [CrossRef] [PubMed]
- Bakker, M.K.; Bergman, J.E.H.; Krikov, S.; Amar, E.; Cocchi, G.; Cragan, J.; de Walle, H.E.K.; Gatt, M.; Groisman, B.; Liu, S.; et al. Prenatal diagnosis and prevalence of critical congenital heart defects: An international retrospective cohort study. BMJ Open 2019, 9, e028139. [Google Scholar] [CrossRef] [PubMed]
- Moscatelli, S.; Avesani, M.; Borrelli, N.; Sabatino, J.; Pergola, V.; Leo, I.; Montanaro, C.; Contini, F.V.; Gaudieri, G.; Ielapi, J.; et al. Complete Transposition of the Great Arteries in the Pediatric Field: A Multimodality Imaging Approach. Children 2024, 11, 626. [Google Scholar] [CrossRef]
- Talemal, L.; Donofrio, M.T. Hemodynamic consequences of a restrictive ductus arteriosus and foramen ovale in fetal transposition of the great arteries. J. Neonatal-Perinat. Med. 2016, 9, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Oboli, V.N.; Pizzolla, A.; Pattnaik, P. A Diagnostic Dilemma: Transposition of the Great Arteries. Cureus 2023, 15, e38931. [Google Scholar] [CrossRef] [PubMed]
- Alakhfash, A.A.; Alhawri, K.A.; Almesned, A.A.; Alqwaiee, A.M. Foramen ovale and ductus arteriosus hemodynamics in Dextro Transposition of Great Arteries (D-TGA) with intact ventricular septum, case report, and literature review. Prog. Pediatr. Cardiol. 2020, 57, 101228. [Google Scholar] [CrossRef]
- Martins, P.; Castela, E. Transposition of the great arteries. Orphanet. J. Rare Dis. 2008, 3, 27. [Google Scholar] [CrossRef]
- Haas, N.A.; Driscoll, D.J.; Rickert-Sperling, S. Clinical Presentation and Therapy of d-Transposition of the Great Arteries. Adv. Exp. Med. Biol. 2024, 1441, 663–670. [Google Scholar] [CrossRef]
- Séguéla, P.E.; Roubertie, F.; Kreitmann, B.; Mauriat, P.; Tafer, N.; Jalal, Z.; Thambo, J.B. Transposition of the great arteries: Rationale for tailored preoperative management. Arch. Cardiovasc. Dis. 2017, 110, 124–134. [Google Scholar] [CrossRef]
- Gottschalk, I.; Walter, A.; Menzel, T.; Weber, E.C.; Wendt, S.; Sreeram, N.; Gembruch, U.; Berg, C.; Abel, J.S. D-Transposition of the great arteries with restrictive foramen ovale in the fetus: The dilemma of predicting the need for postnatal urgent balloon atrial septostomy. Arch. Gynecol. Obstet. 2024, 309, 1353–1367. [Google Scholar] [CrossRef]
- Villafañe, J.; Lantin-Hermoso, M.R.; Bhatt, A.B.; Tweddell, J.S.; Geva, T.; Nathan, M.; Elliott, M.J.; Vetter, V.L.; Paridon, S.M.; Kochilas, L.; et al. D-transposition of the great arteries: The current era of the arterial switch operation. J. Am. Coll. Cardiol. 2014, 64, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.J.; Arulkumaran, S.; Tristão Pereira, C.; Cordero-Grande, L.; Hughes, E.J.; Teixeira, R.P.A.G.; Steinweg, J.K.; Victor, S.; Pushparajah, K.; Hajnal, J.V.; et al. Neuroimaging findings in newborns with congenital heart disease prior to surgery: An observational study. Arch. Dis. Child 2019, 104, 1042–1048. [Google Scholar] [CrossRef]
- Ortinau, C.M.; Smyser, C.D.; Arthur, L.; Gordon, E.E.; Heydarian, H.C.; Wolovits, J.; Nedrelow, J.; Marino, B.S.; Levy, V.Y. Optimizing Neurodevelopmental Outcomes in Neonates With Congenital Heart Disease. Pediatrics 2022, 150 (Suppl. S2), e2022056415L. [Google Scholar] [CrossRef]
- Rudolph, A.M. Impaired cerebral development in fetuses with congenital cardiovascular malformations: Is it the result of inadequate glucose supply? Pediatr. Res. 2016, 80, 172–177. [Google Scholar] [CrossRef]
- Peyvandi, S.; Chau, V.; Guo, T.; Xu, D.; Glass, H.C.; Synnes, A.; Poskitt, K.; Barkovich, A.J.; Miller, S.P.; McQuillen, P.S. Neonatal Brain Injury and Timing of Neurodevelopmental Assessment in Patients With Congenital Heart Disease. J. Am. Coll. Cardiol. 2018, 71, 1986–1996. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yan, Y.; Li, C.; Zheng, X.; Wang, G.; Jin, Z.; Shi, G.; He, X.; Tong, X.; Chen, H.; et al. Inattention and hyperactivity in children and adolescents with repaired D-transposition of the great arteries: Prevalence, perioperative risk factors, and clinical outcomes. Front. Cardiovasc. Med. 2022, 9, 937311. [Google Scholar] [CrossRef] [PubMed]
- El-Dib, M.; Soul, J.S. Monitoring and management of brain hemodynamics and oxygenation. Handb. Clin. Neurol. 2019, 162, 295–314. [Google Scholar] [CrossRef] [PubMed]
- Licht, D.J.; Wang, J.; Silvestre, D.W.; Nicolson, S.C.; Montenegro, L.M.; Wernovsky, G.; Tabbutt, S.; Durning, S.M.; Shera, D.M.; Gaynor, J.W.; et al. Preoperative cerebral blood flow is diminished in neonates with severe congenital heart defects. J. Thorac. Cardiovasc. Surg. 2004, 128, 841–849. [Google Scholar] [CrossRef]
- Durduran, T.; Zhou, C.; Buckley, E.M.; Kim, M.N.; Yu, G.; Choe, R.; Gaynor, J.W.; Spray, T.L.; Durning, S.M.; Mason, S.E.; et al. Optical measurement of cerebral hemodynamics and oxygen metabolism in neonates with congenital heart defects. J. Biomed. Opt. 2010, 15, 037004. [Google Scholar] [CrossRef]
- Goff, D.A.; Buckley, E.M.; Durduran, T.; Wang, J.; Licht, D.J. Noninvasive cerebral perfusion imaging in high-risk neonates. Semin. Perinatol. 2010, 34, 46–56. [Google Scholar] [CrossRef]
- Jain, V.; Buckley, E.M.; Licht, D.J.; Lynch, J.M.; Schwab, P.J.; Naim, M.Y.; Lavin, N.A.; Nicolson, S.C.; Montenegro, L.M.; Yodh, A.G.; et al. Cerebral Oxygen Metabolism in Neonates with Congenital Heart Disease Quantified by MRI and Optics. J. Cereb. Blood Flow Metab. 2014, 34, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Jarmund, A.H.; Pedersen, S.A.; Torp, H.; Dudink, J.; Nyrnes, S.A. A Scoping Review of Cerebral Doppler Arterial Waveforms in Infants. Ultrasound Med. Biol. 2023, 49, 919–936. [Google Scholar] [CrossRef]
- De Silvestro, A.A.; Kellenberger, C.J.; Gosteli, M.; O’Gorman, R.; Knirsch, W. Postnatal cerebral hemodynamics in infants with severe congenital heart disease: A scoping review. Pediatr. Res. 2023, 94, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Yu, S.; Lowery, R.; Zampi, J.D. Timing of Balloon Atrial Septostomy in Patients with d-TGA and Association with Birth Location and Patient Outcomes. Pediatr. Cardiol. 2023, 44, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Nagata, H.; Glick, L.; Lougheed, J.; Grattan, M.; Mondal, T.; Thakur, V.; Schwartz, S.M.; Jaeggi, E. Prenatal Diagnosis of Transposition of the Great Arteries Reduces Postnatal Mortality: A Population-Based Study. Can. J. Cardiol. 2020, 36, 1592–1597. [Google Scholar] [CrossRef] [PubMed]
- Veal, C.; Hunt, R.; Tume, L.N. Do infants with transposition of the great arteries born outside a specialist centre have different outcomes? Cardiol. Young 2019, 29, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Dorobantu, D.M.; Espuny Pujol, F.; Kostolny, M.; Brown, K.L.; Franklin, R.C.; Crowe, S.; Pagel, C.; Stoica, S.C. Arterial Switch for Transposition of the Great Arteries: Treatment Timing, Late Outcomes, and Risk Factors. JACC Adv. 2023, 2, 100407. [Google Scholar] [CrossRef]
- Nevvazhay, T.; Chernogrivov, A.; Biryukov, E.; Biktasheva, L.; Karchevskaya, K.; Sulejmanov, S.; Kalinicheva, J.; Artemiev, N. Arterial switch in the first hours of life: No need for Rashkind septostomy? Eur. J. Cardiothorac. Surg. 2012, 42, 520–523. [Google Scholar] [CrossRef][Green Version]
- Sarris, G.E.; Balmer, C.; Bonou, P.; Comas, J.V.; da Cruz, E.; Di Chiara, L.; Di Donato, R.M.; Fragata, J.; Jokinen, T.E.; Kirvassilis, G.; et al. Clinical guidelines for the management of patients with transposition of the great arteries with intact ventricular septum: The Task Force on Transposition of the Great Arteries of the European Association for Cardio-Thoracic Surgery (EACTS) and the Association for European Paediatric and Congenital Cardiology (AEPC). Cardiol. Young 2017, 27, 530–569. [Google Scholar] [CrossRef]
- Gournay, V. The ductus arteriosus: Physiology, regulation, and functional and congenital anomalies. Arch. Cardiovasc. Dis. 2011, 104, 578–585. [Google Scholar] [CrossRef]
- Wheeler, C.R.; Sen, S.; Levy, P.T. The ductus arteriosus in neonates with critical congenital heart disease. J. Perinatol. 2022, 42, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Akkinapally, S.; Hundalani, S.G.; Kulkarni, M.; Fernandes, C.J.; Cabrera, A.G.; Shivanna, B.; Pammi, M. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst. Rev. 2018, 2, CD011417. [Google Scholar] [CrossRef]
- Xin, Y.; Roh, K.; Cho, E.; Park, D.; Whang, W.; Jung, E. Isookanin Inhibits PGE2-Mediated Angiogenesis by Inducing Cell Arrest through Inhibiting the Phosphorylation of ERK1/2 and CREB in HMEC-1 Cells. Int. J. Mol. Sci. 2021, 22, 6466. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.-T.; Sun, W.-Y.; Li, X.-R.; Sun, J.-C.; Du, J.-J.; Wei, W. Emerging Roles of G Protein-Coupled Receptors in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2018, 19, 1366. [Google Scholar] [CrossRef]
- Gumułka, P.; Tarsa, M.; Dąbrowska, M.; Starek, M. Quantification of Grapiprant and Its Stability Testing under Changing Environmental Conditions. Biomedicines 2022, 10, 2821. [Google Scholar] [CrossRef]
- Socha, M.W.; Flis, W.; Pietrus, M.; Wartęga, M. Results of Induction of Labor with Prostaglandins E1 and E2 (The RIPE Study): A Real-World Data Analysis of Obstetrical Effectiveness and Clinical Outcomes of Pharmacological Induction of Labor with Vaginal Inserts. Pharmaceuticals 2023, 16, 982. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lin, X.; Meng, F.; Zhao, Y.; Wang, W.; Zhang, Y.; Chai, X.; Zhang, Y.; Yu, W.; Yang, J.; et al. A Novel Small Molecular Prostaglandin Receptor EP4 Antagonist, L001, Suppresses Pancreatic Cancer Metastasis. Molecules 2022, 27, 1209. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Tissot, C. Echocardiographic Evaluation of Transitional Circulation for the Neonatologists. Front. Pediatr. 2018, 6, 140. [Google Scholar] [CrossRef]
- Al-Kassmy, J.; Navarro-Castellanos, I.; Barlatay, F.G.; Miró, J.; Dahdah, N. Balloon Atrial Septostomy: Does the Balloon Size Matter? CJC Pediatr. Congenit. Heart Dis. 2022, 1, 253–259. [Google Scholar] [CrossRef]
- Hamzah, M.; Othman, H.F.; Peluso, A.M.; Sammour, I.; Aly, H. Prevalence and Outcomes of Balloon Atrial Septostomy in Neonates With Transposition of Great Arteries. Pediatr. Crit. Care Med. 2020, 21, 324–331. [Google Scholar] [CrossRef]
- Della Gatta, A.N.; Contro, E.; Lenzi, J.; Balducci, A.; Gargiulo, G.; Bodnar, T.; Palleri, D.; Bonetti, S.; Hasan, T.; Donti, A.; et al. Prenatal sonography of the foramen ovale predicts urgent balloon atrial septostomy in neonates with complete transposition of the great arteries. Am. J. Obstet. Gynecol. MFM 2021, 3, 100379. [Google Scholar] [CrossRef] [PubMed]
- Finan, E.; Mak, W.; Bismilla, Z.; McNamara, P.J. Early discontinuation of intravenous prostaglandin E1 after balloon atrial septostomy is associated with an increased risk of rebound hypoxemia. J. Perinatol. 2008, 28, 341–346. [Google Scholar] [CrossRef]
- Gilg, S.; Acosta, S.; Loomba, R.S.; Rizk, C.; Stapleton, G.E.; Faraoni, D.; Savorgnan, F. Association between balloon atrial septostomy and prostaglandin E1 therapy until repair of transposition of the great arteries in neonates. Pediatr. Investig. 2024, 8, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Pichler, G.; Binder, C.; Avian, A.; Beckenbach, E.; Schmölzer, G.M.; Urlesberger, B. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J. Pediatr. 2013, 163, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Urlesberger, B.; Grossauer, K.; Pocivalnik, M.; Avian, A.; Muller, W.; Pichler, G. Regional oxygen saturation of the brain and peripheral tissue during birth transition of term infants. J. Pediatr. 2010, 157, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Baek, J.S.; Kim, J.A.; Cha, S.G.; Yu, J.J. Cerebral and Somatic Oxygen Saturation in Neonates with Congenital Heart Disease before Surgery. J. Clin. Med. 2021, 10, 2455. [Google Scholar] [CrossRef]
- Kurth, C.D.; Steven, J.L.; Montenegro, L.M.; Watzman, H.M.; Gaynor, J.W.; Spray, T.L.; Nicolson, S.C. Cerebral oxygen saturation before congenital heart surgery. Ann. Thorac. Surg. 2001, 72, 187–192. [Google Scholar] [CrossRef]
- Lim, J.M.; Kingdom, T.; Saini, B.; Chau, V.; Post, M.; Blaser, S.; Macgowan, C.; Miller, S.P.; Seed, M. Cerebral oxygen delivery is reduced in newborns with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2016, 152, 1095–1103. [Google Scholar] [CrossRef]
- Nagaraj, U.D.; Evangelou, I.E.; Donofrio, M.T.; Vezina, L.G.; McCarter, R.; du Plessis, A.J.; Limperopoulos, C. Impaired Global and Regional Cerebral Perfusion in Newborns with Complex Congenital Heart Disease. J. Pediatr. 2015, 167, 1018–1024. [Google Scholar] [CrossRef]
- Licht, D.J.; Shera, D.M.; Clancy, R.R.; Wernovsky, G.; Montenegro, L.M.; Nicolson, S.C.; Zimmerman, R.A.; Spray, T.L.; Gaynor, J.W.; Vossough, A. Brain maturation is delayed in infants with complex congenital heart defects. J. Thorac. Cardiovasc. Surg. 2009, 137, 529–536, discussion 536–537. [Google Scholar] [CrossRef]
- Mir, M.; Moore, S.S.; Wutthigate, P.; Simoneau, J.; Villegas Martinez, D.; Shemie, S.D.; Brossard-Racine, M.; Dancea, A.; Bertolizio, G.; Altit, G. Newborns with a Congenital Heart Defect and Diastolic Steal Have an Altered Cerebral Arterial Doppler Profile. J. Pediatr. 2023, 257, 113369. [Google Scholar] [CrossRef] [PubMed]
- Jenks, C.L.; Hernandez, A.; Stavinoha, P.L.; Morris, M.C.; Tian, F.; Liu, H.; Garg, P.; Forbess, J.M.; Koch, J. Elevated cranial ultrasound resistive indices are associated with improved neurodevelopmental outcomes one year after pediatric cardiac surgery: A single center pilot study. Heart Lung 2017, 46, 251–257. [Google Scholar] [CrossRef] [PubMed]
| Time | PGE1 Group | PGE1 + BAS Group | Control Group |
|---|---|---|---|
| 1. | Echocardiogram | Echocardiogram | - |
| CDU (PSV, EDV, and RI) | CDU (PSV, EDV, and RI) | CDU (PSV, EDV, and RI) | |
| crSO2 and cFTOE | crSO2 and cFTOE | crSO2 and cFTOE | |
| Pre-ductal SpO2 | Pre-ductal SpO2 | Pre-ductal SpO2 | |
| BP (systolic/diastolic/MAP) | BP (systolic/diastolic/MAP) | BP (systolic/diastolic/MAP) | |
| FiO2 | FiO2 | FiO2 | |
| pH, pO2, and lactate | pH, pO2, and lactate | pH, pO2, and lactate | |
| 2. | - | Echocardiogram | - |
| - | CDU (PSV, EDV, and RI) | - | |
| - | crSO2 and cFTOE | - | |
| - | Pre-ductal SpO2 | - | |
| - | BP (systolic/diastolic/MAP) | - | |
| 3. | Echocardiogram | Echocardiogram | |
| CDU (PSV, EDV, and RI) | CDU (PSV, EDV, and RI) | CDU (PSV, EDV, and RI) | |
| crSO2 and cFTOE | crSO2 and cFTOE | crSO2 and cFTOE | |
| Pre-ductal SpO2 | Pre-ductal SpO2 | Pre-ductal SpO2 | |
| BP (systolic/diastolic/MAP) | BP (systolic/diastolic/MAP) | BP (systolic/diastolic/MAP) | |
| FiO2 | FiO2 | FiO2 | |
| pH, pO2, and lactate | pH, pO2, and lactate | pH, pO2, and lactate |
| Demographic Variables | Study Group | Control Group n = 77 | p-Value PGE1 vs. PGE1 + BAS | p-Value PGE1 vs. Control | p-Value PGE1 + BAS vs. Control | |
|---|---|---|---|---|---|---|
| PGE1 Group n = 37 | PGE1 + BAS Group n = 46 | |||||
| GA §, weeks, mean (SD) | 39 (1.5) | 39 (1.3) | 39.4 (1.1) | 0.9970 | 0.3691 | 0.3301 |
| BW §, g, mean (SD) | 3292.4 (528.5) | 3302 (442.8) | 3380.1 (467.9) | 0.9245 | 0.3580 | 0.3817 |
| HC §, cm, mean (SD) | 34 (1.4) | 33.8 (1.3) | 34.5 (1.0) | 0.8619 | 0.1085 | 0.0151 * |
| Antenatal care ‡, n (%) | 30 (81.1) | 42 (91.3) | 61 (79.2) | 0.1721 | 0.8167 | 0.0788 |
| Prenatal diagnosis ‡, n (%) | 26 (70.3) | 29 (63.04) | - | 0.7869 | - | - |
| In utero transfer ‡, n (%) | 19 (51.4) | 18 (39.1) | - | 0.5377 | - | - |
| Cesarian delivery ‡, n (%) | 18 (48.7) | 27 (58.7) | 27 (35.1) | 0.3611 | 0.1647 | 0.0106 * |
| Apgar 1 min §, median (IQR) | 8 (8–9) | 8 (8–8) | 10 (9–10) | 0.9912 | <0.001 * | <0.001 * |
| Apgar 5 min ‡ median (IQR) | 8 (8–9) | 8 (8–9) | 10 (10–10) | 0.9991 | <0.001 * | <0.001 * |
| Age at surgery (days) §, mean (SD) | 14.8 (10.1) | 14.1 (6.6) | - | 0.6865 | - | - |
| Deaths ‡, n (%) | 1 (2.7) | 8 (17.4) | - | 0.0240 * | - | - |
| Pre-surgery death ‡, n (%) | 0 (0) | 3 (6.5) | - | 0.1627 | - | - |
| Post-surgery death ‡, n (%) | 1 (2.7) | 5 (10.8) | - | 0.0715 | - | - |
| PGE1 Group (n = 37) | PGE1 + BAS Group (n = 46) | p-Value | |
|---|---|---|---|
| 1. Clinical characteristics | |||
| Tachypnea ‡, n (%) | 24 (64.9) | 38 (82.6) | 0.0645 |
| Cyanosis ‡, n (%) | 37 (100) | 46 (100) | 0.9999 |
| Cardiac murmur (grade) §, median (IQR) | 3 (2–3) | 3 (2–3) | 0.4134 |
| Need for MV §, mean (SD) | 11 (29.7) | 23 (50) | 0.0619 |
| Need for inotrope §, mean (SD) | 14 (37.8) | 18 (39.1) | 0.9042 |
| 2. Interventions | |||
| PGE1 doses (μg/kg/min) §, mean (SD) | |||
| Initial §, mean (SD) | 0.042 (0.024) | 0.050 (0.025) | 0.1440 |
| Before BAS §, mean (SD) | - | 0.054 (0.019) | - |
| At 24 h of life §, mean (SD) | 0.040 (0.016) | 0.031 (0.015) | 0.0136 * |
| Time to BAS (hours after birth) §, mean (SD) | - | 8.71 (5.05) | - |
| 3. Heart ultrasound evaluation | |||
| PDA | |||
| Size at birth (mm) §, mean (SD) | 4.08 (1.31) | 3.74 (1.00) | 0.1929 |
| Left–Right flow ‡, n (%) | 22 (59.5) | 21 (45.7) | 0.2108 |
| Right–Left flow ‡, n (%) | 1 (2.70) | 1 (2.71) | 0.8759 |
| Bidirectional flow ‡, n (%) | 16 (43.24) | 24 (52.17) | 0.4183 |
| ASD/PFO | |||
| Size ASD at birth (mm) §, median (IQR) | 4.8 (4.0–5.2) | 3 (2.5–3.3) | <0.001 * |
| Size after BAS (mm) §, median (IQR) | - | 4.65 (4.1–5.2) | - |
| Restrictive < 4 mm ‡, at birth n (%) | 0 (0) | 46 (100) | <0.001 * |
| Gradient (mmHg) §, median (IQR) | 5 (3–6.6) | 8.7 (7.7–11) | <0.001 * |
| VSD (mm) §, mean (SD) | 1.57 (2.59) | 0.63 (1.99) | 0.0393 * |
| Number of cardiac ultrasound examinations §, median (IQR) | 7 (6–9) | 7 (6–8) | 0.7382 |
| Clinical Variables | Study Group | Control Group n = 77 | p-Value PGE1 vs. BAS | p-Value PGE1 vs. Control | p-Value PGE1 + BAS vs. Control | |
|---|---|---|---|---|---|---|
| PGE1 Group n = 37 | PGE1 + BAS Group n = 46 | |||||
| crSO2 §, (%) median (IQR) | ||||||
| within 2 h of life (before PGE1) | 47 (41–55) | 43 (39–47) | 83 (78–86) | 0.0412 * | <0.001 * | <0.001 * |
| before BAS | - | 42 (39–46) | - | |||
| 24 h of life | 50 (47–54) | 51 (48–59) | 87 (84–91) | 0.2096 | <0.001 * | <0.001 * |
| cFTOE §, median (IQR) | ||||||
| within 2 h of life (before PGE1), mean (SD) | 0.38 (0.34–0.4) | 0.39 (0.33–0.45) | 0.16 (0.12–0.21) | 0.8938 | <0.001 * | <0.001 * |
| before BAS | 0.45 (0.35–0.48) | |||||
| 24 h of life | 0.38 (0.33–0.43) | 0.38 (0.31–0.42) | 0.11 (0.08–0.15) | 0.8240 | <0.001 * | <0.001 * |
| SpO2 pre-ductal (%) §, median (IQR) | ||||||
| within 2 h of life (before PGE1) | 80 (75–83) | 72 (65–78) | 99 (98–100) | 0.00012 * | 0.00002 * | 0.00002 * |
| before BAS | 77 (72–80) | |||||
| 24 h of life | 82 (80–85) | 84.5 (83–87) | 99 (98–100) | 0.00003 * | 0.00002 * | 0.00002 * |
| RI §, mean (SD) | ||||||
| within 2 h of life (before PGE1) | 0.718 (0.054) | 0.708 (0.054) | 0.713 (0.05) | 0.3990 | 0.6812 | 0.5761 |
| before BAS | 0.759 (0.046) | |||||
| 24 h of life | 0.769 (0.036) | 0.764 (0.041) | 0.681 (0.046) | 0.6272 | 0.00002 * | 0.00002 * |
| PSV §, (cm/s), mean (SD) | ||||||
| within 2 h of life (before PGE1) | 39.75 (7.41) | 37.78 (7.82) | 34.49 (6.71) | 0.4177 | 0.0007 * | 0.03831 |
| before BAS | 40.41 (8.92) | |||||
| 24 h of life | 38.99 (5.29) | 39.72 (6.53) | 37.35 (7.80) | 0.8958 | 0.5651 | 0.2313 |
| EDV §, (cm/s), mean (SD) | ||||||
| within 2 h of life (before PGE1) | 10.71 (1.61) | 10.86 (2.54) | 9.78 (2.01) | 0.7171 | 0.0292 * | 0.0153 * |
| before BAS | 9.27 (1.91) | |||||
| 24 h of life | 8.74 (1.31) | 9.16 (2.00) | 11.83 (2.39) | 0.687 | 0.00001 * | 0.00002 * |
| pH §, mean (SD) | ||||||
| within 2 h of life (before PGE1) | 7.29 (0.09) | 7.29 (0.07) | 7.34 (0.05) | 0.6967 | 0.0043 * | 0.0054 * |
| before BAS | 7.31 (0.11) | |||||
| 24 h of life | 7.35 (0.08) | 7.35 (0.06) | 7.38 (0.03) | 0.872 | 0.0211 * | 0.03638 * |
| pO2 § (mmHg), median (IQR) | ||||||
| within 2 h of life (before PGE1) | 31 (28–34) | 26 (21–29) | 31 (28–36) | 0.0431 | 0.8072 | 0.2038 |
| before BAS | 25.60 (7.06) | |||||
| 24 h of life | 39 (35–41) | 38 (29–46) | 56 (51–61) | 0.9879 | <0.001 * | <0.001 * |
| Lactate § (mmol/L), median (IQR) | ||||||
| within 2 h of life (before PGE1) | 3.2 (2.4–4.1) | 3.05 (2–4.7) | 2.9 (1.9–3.7) | 0.0499 | 0.5567 | 0.1385 |
| before BAS | 4.60 (2.30) | |||||
| 24 h of life | 2.2 (1.9–3.1) | 2.15 (1.5–4.5) | 2.1 (1.8–2.4) | 0.9818 | 0.2498 | 0.3138 |
| Hgb § (g/dL), median (IQR) | ||||||
| within 2 h of life (before PGE1) | 16.3 (15.6–16.7) | 15.9 (15.3–17.2) | 17.7 (16.7–18.3) | 0.9902 | <0.0001 * | <0.0001 * |
| Mean BP (MAP) §, (mmHg), mean (SD) | ||||||
| within 2 h of life (before PGE1) | 43.73 (9.18) | 49.32 (7.83) | 47.47 (7.12) | 0.0012 * | 0.0189 * | 0.2429 |
| before BAS | 45.41 (5.67) | |||||
| 24 h of life | 48.59 (6.76) | 50.67 (6.34) | 52.22 (7.14) | 0.1338 | 0.0242 * | 0.2648 |
| Systolic BP §, (mmHg), mean (SD) | ||||||
| within 2 h of life (before PGE1) | 65.41 (11.44) | 65.54 (7.19) | 67.40 (9.15) | 0.9413 | 0.3187 | 0.3214 |
| before BAS | 63.87 (9.01) | |||||
| 24 h of life | 65.41 (11.44) | 69.78 (7.98) | 73.10 (7.86) | 0.0147 * | 0.00003 * | 0.0642 |
| Diastolic BP §, (mmHg), mean (SD) | ||||||
| within 2 h of life (before PGE1) | 29.70 (10.07) | 33.67 (7.05) | 36.96 (7.32) | 0.0140 * | 0.00004 * | 0.0283 * |
| before BAS | 29.93 (6.32) | |||||
| 24 h of life | 31 (26–34) | 33 (29–37) | 39 (34–47) | 0.5780 | <0.0001 * | <0.0001 * |
| FiO2 (%) §, median (IQR) | ||||||
| within 2 h of life (before PGE1) | 30 (21–40) | 40 (30–60) | 21 (21–21) | 0.1006 | <0.0001 * | <0.0001 * |
| before BAS | 40 (30–60) | |||||
| 24 h of life | 21 (21–30) | 23 (21–30) | 21 (21–21) | 0.9981 | 0.0008 * | <0.0001 * |
| Clinical Variables | PGE1 Group n = 37 | PGE1, BAS + Group n = 46 | |||||
|---|---|---|---|---|---|---|---|
| 2 h of Life | 24 h of Life (after PGE) | p-Value | 2 h of Life | Before BAS | 24 h of Life (after BAS) | p-Value | |
| crSO2 § | 47 (41–55) | 50 (47–54) | 0.00029 * | 43 (39–47) | 42 (39–46) | 51 (48–59) | <0.0001 * |
| cFTOE § | 0.38 (0.34–0.4) | 0.38 (0.33–0.43) | 0.1717 * | 0.39 (0.33–0.45) | 0.45 (0.35–0.48) | 0.38 (0.31–0.42) | 0.0002 * |
| SpO2 preductal § | 80 (75–83) | 82 (80–85) | 0.0053 * | 72 (65–78) | 77 (72–80) | 84.5 (83–87) | <0.0001 * |
| RI § | 0.718 (0.054) | 0.769 (0.036) | 0.000002 * | 0.708 (0.054) | 0.759 (0.046) | 0.764 (0.041) | 0.00002 * |
| PSV § | 39.75 (7.41) | 38.99 (5.29) | 0.2512 | 37.666 (7.82) | 40.41 (8.92) | 39.72 (6.53) | 0.49415 |
| EDV § | 10.71 (1.61) | 8.74 (1.31) | <0.0001 * | 10.86 (2.54) | 9.27 (1.91) | 9.16 (2.00) | 0.00004 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucerea, M.; Ognean, M.-L.; Pinzariu, A.-C.; Simon, M.; Suciu, L.M.; Ghiga, D.-V.; Moldovan, E.; Moscalu, M. Effects of Prostaglandin E1 and Balloon Atrial Septostomy on Cerebral Blood Flow and Oxygenation in Newborns Diagnosed with Transposition of the Great Arteries. Biomedicines 2024, 12, 2018. https://doi.org/10.3390/biomedicines12092018
Cucerea M, Ognean M-L, Pinzariu A-C, Simon M, Suciu LM, Ghiga D-V, Moldovan E, Moscalu M. Effects of Prostaglandin E1 and Balloon Atrial Septostomy on Cerebral Blood Flow and Oxygenation in Newborns Diagnosed with Transposition of the Great Arteries. Biomedicines. 2024; 12(9):2018. https://doi.org/10.3390/biomedicines12092018
Chicago/Turabian StyleCucerea, Manuela, Maria-Livia Ognean, Alin-Constantin Pinzariu, Marta Simon, Laura Mihaela Suciu, Dana-Valentina Ghiga, Elena Moldovan, and Mihaela Moscalu. 2024. "Effects of Prostaglandin E1 and Balloon Atrial Septostomy on Cerebral Blood Flow and Oxygenation in Newborns Diagnosed with Transposition of the Great Arteries" Biomedicines 12, no. 9: 2018. https://doi.org/10.3390/biomedicines12092018
APA StyleCucerea, M., Ognean, M.-L., Pinzariu, A.-C., Simon, M., Suciu, L. M., Ghiga, D.-V., Moldovan, E., & Moscalu, M. (2024). Effects of Prostaglandin E1 and Balloon Atrial Septostomy on Cerebral Blood Flow and Oxygenation in Newborns Diagnosed with Transposition of the Great Arteries. Biomedicines, 12(9), 2018. https://doi.org/10.3390/biomedicines12092018







