Preservation of Mitochondrial Function by SkQ1 in Skin Fibroblasts Derived from Patients with Leber’s Hereditary Optic Neuropathy Is Associated with the PINK1/PRKN-Mediated Mitophagy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells Construction and Culturing
2.2. SkQ1 Stock and Working Solutions
2.3. Cell Viability Detection
2.3.1. Cell Viability, Dosing, and Safety Ranges
2.3.2. Cell Viability with Long-Term SkQ1 Treatment
2.3.3. Cell Viability with H2O2 Treatment
2.4. Intramitochondrial Superoxide Detection
2.5. Mitochondrial Function Assay
2.5.1. Mitochondrial Membrane Potential Assay
2.5.2. Mitochondrial Permeability Transition Pore Assay
2.5.3. Mitochondrial Stress Test Assay
2.6. Network Pharmacology and Molecular Docking
2.7. Western Blot
2.8. Cellular Thermal Shift Assay
2.9. Statistical Analysis
3. Results
3.1. LHON-IFBs Presented Excessive Superoxide and Mitochondrial Dysfunction
3.2. Drug Safety of SkQ1
3.3. SkQ1 Scavenged Mitochondrial Superoxide
3.4. SkQ1 Protected Mitochondrial Function
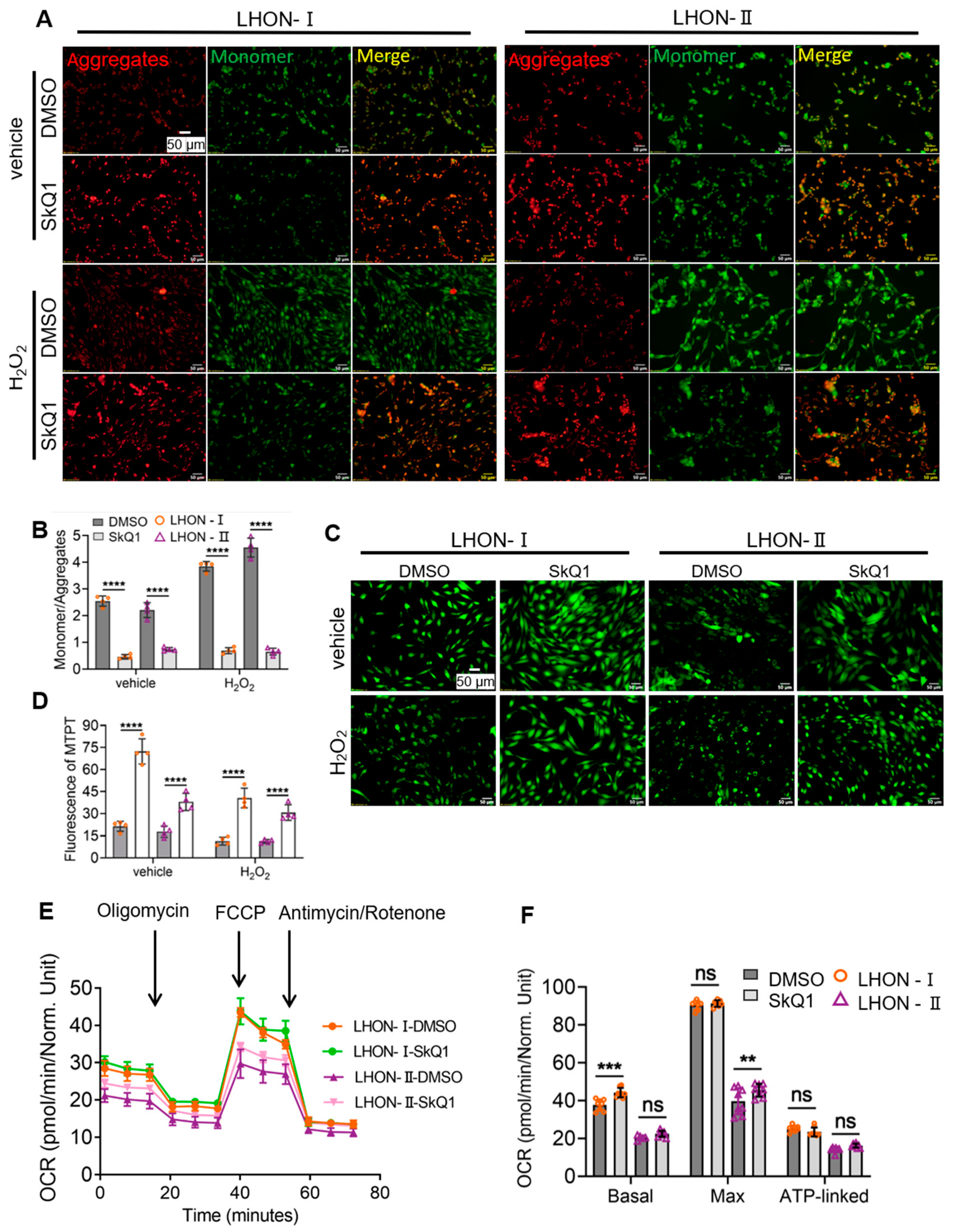
3.4.1. SkQ1 Promoted Mitochondrial Membrane Potential Stability
3.4.2. SkQ1 Reduced the Degree of MPTP Opening
3.4.3. SkQ1 Improved Mitochondrial Respiratory Function
3.5. Network Pharmacology and Molecular Docking Validation
3.5.1. Screening Potential Protein Targets of SkQ1
3.5.2. Constructing a PPI Network and Topological Analyses
3.5.3. GO and Enrichment Analysis and Molecular Docking Simulation
3.6. SkQ1 Interacted with PINK1
3.7. SkQ1 Inhibited PINK1/PRKN-Mediated Mitophagy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hage, R.; Vignal-Clermont, C. Leber Hereditary Optic Neuropathy: Review of Treatment and Management. Front. Neurol. 2021, 12, 651639. [Google Scholar] [CrossRef]
- Zuccarelli, M.; Vella-Szijj, J.; Serracino-Inglott, A.; Borg, J.-J. Treatment of Leber’s hereditary optic neuropathy: An overview of recent developments. Eur. J. Ophthalmol. 2020, 30, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wai-Man, P.; Chinnery, P.F. Leber Hereditary Optic Neuropathy; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Eds.; GeneReviews (®): Seattle, WA, USA, 1993. [Google Scholar]
- Howell, N. LHON and other optic nerve atrophies: The mitochondrial connection. Dev. Ophthalmol. 2003, 37, 94–108. [Google Scholar]
- Catalani, E.; Brunetti, K.; Del Quondam, S.; Cervia, D. Targeting Mitochondrial Dysfunction and Oxidative Stress to Prevent the Neurodegeneration of Retinal Ganglion Cells. Antioxidants 2023, 12, 2011. [Google Scholar] [CrossRef] [PubMed]
- Manickam, A.H.; Michael, M.J.; Ramasamy SManickam, A.H.; Michael, M.J.; Ramasamy, S. Mitochondrial genetics and therapeutic overview of Leber’s hereditary optic neuropathy. Indian J. Ophthalmol. 2017, 65, 1087–1092. [Google Scholar]
- Liu, Y.; Eastwood, J.D.; Alba, D.E.; Velmurugan, S.; Sun, N.; Porciatti, V.; Lee, R.K.; Hauswirth, W.W.; Guy, J.; Yu, H. Gene therapy restores mitochondrial function and protects retinal ganglion cells in optic neuropathy induced by a mito-targeted mutant ND1 gene. Gene Ther. 2022, 29, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Emmanuele, V.; Quinzii, C.M. Emerging therapies for mitochondrial diseases. Essays Biochem. 2018, 62, 467–481. [Google Scholar] [PubMed]
- Sahel, J.A.; Newman, N.J.; Yu-Wai-Man, P.; Vignal-Clermont, C.; Carelli, V.; Biousse, V.; Moster, M.L.; Sergott, R.; Klopstock, T.; Sadun, A. Gene Therapies for the Treatment of Leber Hereditary Optic Neuropathy. Int. Ophthalmol. Clin. 2021, 61, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Bargiela, D.; Chinnery, P.F. Mitochondria in neuroinflammation - Multiple sclerosis (MS), leber hereditary optic neuropathy (LHON) and LHON-MS. Neurosci. Lett. 2019, 710, 132932. [Google Scholar] [CrossRef]
- Smith, R.A.; Porteous, C.M.; Gane, A.M.; Murphy, M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 5407–5412. [Google Scholar] [CrossRef]
- Titova, E.; Shagieva, G.; Ivanova, O.; Domnina, L.; Domninskaya, M.; Strelkova, O.; Khromova, N.; Kopnin, P.; Chernyak, B.; Skulachev, V.; et al. Mitochondria-targeted antioxidant SkQ1 suppresses fibrosarcoma and rhabdomyosarcoma tumour cell growth. Cell Cycle 2018, 17, 1797–1811. [Google Scholar] [CrossRef]
- Antonenko, Y.N.; Avetisyan, A.V.; Bakeeva, L.E.; Chernyak, B.V.; Chertkov, V.A.; Domnina, L.V.; Ivanova, O.Y.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; et al. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: Synthesis and in vitro studies. Biochemistry 2009, 73, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Nevinitsina, T.; Sheremet, N.; Andreeva, N.; Zhorzholadze, N.; Ronzina, I.; Lyamzaev, K.; Krylova, T.; Tsygankova, P.; Sku-lachev, M.; Karger, E.; et al. Effect of ophthalmic mitochondrial reactive oxygen species scavengerVisomitin® on visual acuity of patients diagnosed with Leber hereditary optic neuropathy: Findings of an observational clinical study. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4070-F0034. [Google Scholar]
- Gueven, N.; Nadikudi, M.; Daniel, A.; Chhetri, J. Targeting mitochondrial function to treat optic neuropathy. Mitochondrion 2017, 36, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.H.; Kreymerman, A.; Belle, K.; Ghiam, B.K.; Muscat, S.R.; Mahajan, V.B.; Enns, G.M.; Mercola, M.; Wood, E.H. The Present and Future of Mitochondrial-Based Therapeutics for Eye Disease. Transl. Vis. Sci. Technol. 2021, 10, 4. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Yao, S.; Zhou, Q.; Yang, M.; Li, Y.; Jin, X.; Guo, Q.; Yang, L.; Qin, F.; Lei, B. Multi-mtDNA Variants May Be a Factor Contributing to Mitochondrial Function Variety in the Skin-Derived Fibroblasts of Leber’s Hereditary Optic Neuropathy Patients. Front. Mol. Neurosci. 2022, 15, 920221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yao, S.; Yang, M.; Guo, Q.; Li, Y.; Li, L.; Lei, B. Superoxide dismutase 2 ameliorates mitochondrial dysfunction in skin fibroblasts of Leber’s hereditary optic neuropathy patients. Front. Neurosci. 2022, 16, 917348. [Google Scholar] [CrossRef]
- Zhou, L.; Chan, J.C.Y.; Chupin, S.; Gueguen, N.; Desquiret-Dumas, V.; Koh, S.K.; Li, J.; Gao, Y.; Deng, L.; Verma, C.; et al. Increased Protein S-Glutathionylation in Leber’s Hereditary Optic Neuropathy (LHON). Int. J. Mol. Sci. 2020, 21, 3027. [Google Scholar] [CrossRef]
- Piotrowska-Nowak, A.; Krawczyński, M.R.; Kosior-Jarecka, E.; Ambroziak, A.M.; Korwin, M.; Ołdak, M.; Tońska, K.; Bartnik, E. Mitochondrial genome variation in male LHON patients with the m.11778G > A mutation. Metab. Brain Dis. 2020, 35, 1317–1327. [Google Scholar] [CrossRef]
- Fink, B.D.; Herlein, J.A.; Yorek, M.A.; Fenner, A.M.; Kerns, R.J.; Sivitz, W.I. Bioenergetic Effects of Mitochondrial-Targeted Coenzyme Q Analogs in Endothelial Cells. J. Pharmacol. Exp. Ther. 2012, 342, 709–719. [Google Scholar] [CrossRef]
- Zeviani, M.; Carelli, V. Mitochondrial Retinopathies. Int. J. Mol. Sci. 2021, 23, 210. [Google Scholar] [CrossRef]
- Chinnery, P.F.; Hudson, G. Mitochondrial genetics. Br. Med. Bull. 2013, 106, 135–159. [Google Scholar] [CrossRef] [PubMed]
- Jankauskaitė, E.; Bartnik, E.; Kodroń, A. Investigating Leber’s hereditary optic neuropathy: Cell models and future perspectives. Mitochondrion 2017, 32, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bahr, T.; Welburn, K.; Donnelly, J.; Bai, Y. Emerging model systems and treatment approaches for Leber’s hereditary optic neuropathy: Challenges and opportunities. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Tian, R. Mitochondrial dysfunction in pathophysiology of heart failure. J. Clin. Investig. 2018, 128, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P.; Anisimov, V.N.; Antonenko, Y.N.; Bakeeva, L.E.; Chernyak, B.V.; Erichev, V.P.; Filenko, O.F.; Kalinina, N.I.; Kapelko, V.I.; Kolosova, N.G. An attempt to prevent senescence: A mitochondrial approach. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1787, 437–461. [Google Scholar] [CrossRef]
- Jia, B.; Ye, J.; Gan, L.; Li, R.; Zhang, M.; Sun, D.; Weng, L.; Xiong, Y.; Xu, J.; Zhang, P.; et al. Mitochondrial antioxidant SkQ1 decreases inflammation following hemorrhagic shock by protecting myocardial mitochondria. Front. Physiol. 2022, 13, 1047909. [Google Scholar] [CrossRef]
- Wei, Y.; Troger, A.; Spahiu, V.; Perekhvatova, N.; Skulachev, M.; Petrov, A.; Chernyak, B.; Asbell, P. The Role of SKQ1 (Visomitin) in Inflammation and Wound Healing of the Ocular Surface. Ophthalmol. Ther. 2018, 8, 63–73. [Google Scholar] [CrossRef]
- Ježek, J.; Engstová, H.; Ježek, P. Antioxidant mechanism of mitochondria-targeted plastoquinone SkQ1 is suppressed in aglycemic HepG2 cells dependent on oxidative phosphorylation. Biochim. Biophys. Acta (BBA) Bioenerg. 2017, 1858, 750–762. [Google Scholar] [CrossRef]
- Jin, D.; Zhang, J.; Zhang, Y.; An, X.; Zhao, S.; Duan, L.; Zhang, Y.; Zhen, Z.; Lian, F.; Tong, X. Network pharmacology-based and molecular docking prediction of the active ingredients and mechanism of ZaoRenDiHuang capsules for application in insomnia treatment. Comput. Biol. Med. 2021, 135, 104562. [Google Scholar] [CrossRef]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-J.; Chen, Y.-N.; Tsao, Y.-T.; Cheng, C.-M.; Wu, W.-C.; Chen, H.-C. The Pathomechanism, Antioxidant Biomarkers, and Treatment of Oxidative Stress-Related Eye Diseases. Int. J. Mol. Sci. 2022, 23, 1255. [Google Scholar] [CrossRef]
- Agarkov, A.A.; Popova, T.N.; Boltysheva, Y.G. Influence of 10-(6-plastoquinonyl) decyltriphenylphosphonium on free-radical homeostasis in the heart and blood serum of rats with streptozotocin-induced hyperglycemia. World J. Diabetes 2019, 10, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.M.; Melov, S. SOD2 in mitochondrial dysfunction and neurodegeneration. Free. Radic. Biol. Med. 2013, 62, 4–12. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide Dismutases: Role in Redox Signaling, Vascular Function, and Diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Wei, S.; Jo, Y.; Zhang, Y.; Park, W.; Gariani, K.; Oh, C.-M.; Kim, H.H.; Ha, K.-T.; Park, K.S.; et al. Mitochondria-associated programmed cell death as a therapeutic target for age-related disease. Exp. Mol. Med. 2023, 55, 1595–1619. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Zhang, S.; Zhang, T.; Liu, Y.; Zhang, L. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics 2023, 13, 736–766. [Google Scholar] [CrossRef]
- Vives-Bauza, C.; Zhou, C.; Huang, Y.; Cui, M.; De Vries, R.L.; Kim, J.; May, J.; Tocilescu, M.A.; Liu, W.; Ko, H.S.; et al. PINK1-dependent recruitment of PRKN to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA 2009, 107, 378–383. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, X.; Yu, L.; Yang, J.; Calvo, R.; Patnaik, S.; Hu, X.; Gao, Q.; Yang, M.; Lawas, M.; et al. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 2016, 7, 12109. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.M.; Lazarou, M.; Wang, C.; Kane, L.A.; Narendra, D.P.; Youle, R.J. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 2010, 191, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Blasiak, J.; Liton, P.; Boulton, M.; Klionsky, D.J.; Sinha, D. Autophagy in age-related macular degeneration. Autophagy 2023, 19, 388–400. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Li, Y.; Yao, S.; Jin, X.; Yang, M.; Guo, Q.; Qiu, R.; Lei, B. Preservation of Mitochondrial Function by SkQ1 in Skin Fibroblasts Derived from Patients with Leber’s Hereditary Optic Neuropathy Is Associated with the PINK1/PRKN-Mediated Mitophagy. Biomedicines 2024, 12, 2020. https://doi.org/10.3390/biomedicines12092020
Xu J, Li Y, Yao S, Jin X, Yang M, Guo Q, Qiu R, Lei B. Preservation of Mitochondrial Function by SkQ1 in Skin Fibroblasts Derived from Patients with Leber’s Hereditary Optic Neuropathy Is Associated with the PINK1/PRKN-Mediated Mitophagy. Biomedicines. 2024; 12(9):2020. https://doi.org/10.3390/biomedicines12092020
Chicago/Turabian StyleXu, Jin, Yan Li, Shun Yao, Xiuxiu Jin, Mingzhu Yang, Qingge Guo, Ruiqi Qiu, and Bo Lei. 2024. "Preservation of Mitochondrial Function by SkQ1 in Skin Fibroblasts Derived from Patients with Leber’s Hereditary Optic Neuropathy Is Associated with the PINK1/PRKN-Mediated Mitophagy" Biomedicines 12, no. 9: 2020. https://doi.org/10.3390/biomedicines12092020





