Are Sirtuins 1 and 2 Relevant Players in Relapsing–Remitting Multiple Sclerosis?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Clinical Assessment
2.2.1. Clinical Parameters
- Symbol Digit Modalities Test (SDMT) [16] as a measure of cognitive function.
- Composite Autonomic Scoring Scale for Laboratory Quantification of Generalized Autonomic Failure (Low questionnaire)—to assess the symptoms of autonomic dysfunction [17].
- Hospital Anxiety and Depression Scale (Bjelland et al. 2002) to detect mood or anxiety disorders [18].
2.2.2. Biochemical Parameters
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kratz, E.M.; Sołkiewicz, K.; Kubis-Kubiak, A.; Piwowar, A. Sirtuins as Important Factors in Pathological States and the Role of Their Molecular Activity Modulators. Int. J. Mol. Sci. 2021, 22, 630. [Google Scholar] [CrossRef]
- Foolad, F.; Khodagholi, F.; Javan, M. Sirtuins in Multiple Sclerosis: The crossroad of neurodegeneration, autoimmunity and metabolism. Mult. Scler. Relat. Disord. 2019, 34, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Yamashita, T. Sirtuins in Neuroendocrine Regulation and Neurological Diseases. Front. Neurosci. 2018, 12, 778. [Google Scholar] [CrossRef]
- Elbaz, E.M.; Senousy, M.A.; El-Tanbouly, D.M.; Sayed, R.H. Neuroprotective effect of linagliptin against cuprizone-induced demyelination and behavioural dysfunction in mice: A pivotal role of AMPK/SIRT1 and JAK2/STAT3/NF-κB signalling pathway modulation. Toxicol. Appl. Pharmacol. 2018, 352, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Chojdak-Łukasiewicz, J.; Bizoń, A.; Waliszewska-Prosół, M.; Piwowar, A.; Budrewicz, S.; Pokryszko-Dragan, A. Role of Sirtuins in Physiology and Diseases of the Central Nervous System. Biomedicines 2022, 10, 2434. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis-a review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Lakin, L.; Davis, B.E.; Binns, C.C.; Currie, K.M.; Rensel, M.R. Comprehensive Approach to Management of Multiple Sclerosis: Addressing Invisible Symptoms—A Narrative Review. Neurol. Ther. 2021, 10, 75–98. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, J.; Tatomir, A.; Hewes, D.; Boodhoo, D.; Anselmo, F.; Rus, V.; Rus, H. Phosphorylated SIRT1 as a biomarker of relapse and response to treatment with glatiramer acetate in multiple sclerosis. Exp. Mol. Pathol. 2018, 105, 175–180. [Google Scholar] [CrossRef]
- Hewes, D.; Tatomir, A.; Kruszewski, A.M.; Rao, G.; Tegla, C.A.; Ciriello, J.; Nguyen, V.; Royal, W., III; Bever, C.; Rus, V.; et al. SIRT1 as a potential biomarker of response to treatment with glatiramer acetate in multiple sclerosis. Exp. Mol. Pathol. 2017, 102, 191–197. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Wilkinson, K.; Marcu, S.; Shapiro, C.M. Fatigue Severity Scale (FSS). In STOP, THAT and One Hundred Other Sleep Scales [Internet]; Shahid, A., Wilkinson, K., Marcu, S., Shapiro, C.M., Eds.; Springer: New York, NY, USA, 2011; pp. 167–168. Available online: https://link.springer.com/10.1007/978-1-4419-9893-4_35 (accessed on 31 December 2023).
- Stein, K.D.; Jacobsen, P.B.; Blanchard, C.M.; Thors, C. Further validation of the multidimensional fatigue symptom inventory-short form. J. Pain Symptom Manag. 2004, 27, 14–23. [Google Scholar] [CrossRef]
- Smith, A. Symbol Digit Modalities Test (SDMT); Manual (Revised); Western Psychological Services: Los Angele, CA, USA, 1982. [Google Scholar]
- Low, P.A. Composite Autonomic Scoring Scale for Laboratory Quantification of Generalized Autonomic Failure. Mayo Clin. Proc. 1993, 68, 748–752. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Chandramowlishwaran, P.; Vijay, A.; Abraham, D.; Li, G.; Mwangi, S.M.; Srinivasan, S. Role of Sirtuins in Modulating Neurodegeneration of the Enteric Nervous System and Central Nervous System. Front. Neurosci. 2020, 14, 614331. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.R.; Imai, S.I. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014, 33, 1321–1340. [Google Scholar] [CrossRef]
- Maxwell, M.M.; Tomkinson, E.M.; Nobles, J.; Wizeman, J.W.; Amore, A.M.; Quinti, L.; Chopra, V.; Hersch, S.M.; Kazantsev, A.G. The Sirtuin 2 microtubule deacetylase is an abundant neuronal protein that accumulates in the aging CNS. Hum. Mol. Genet. 2011, 20, 3986–3996. [Google Scholar] [CrossRef]
- Santos, L.; Escande, C.; Denicola, A. Potential Modulation of Sirtuins by Oxidative Stress. Oxidative Med. Cell. Longev. 2016, 2016, 9831825. [Google Scholar] [CrossRef]
- Nimmagadda, V.K.; Makar, T.K.; Chandrasekaran, K.; Sagi, A.R.; Ray, J.; Russell, J.W.; Bever, C.T. SIRT1 and NAD+ precursors: Therapeutic targets in multiple sclerosis a review. J. Neuroimmunol. 2017, 304, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lee, S.-M.; Shannon, S.; Gao, B.; Chen, W.; Chen, A.; Divekar, R.; McBurney, M.W.; Braley-Mullen, H.; Zaghouani, H.; et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J. Clin. Investig. 2009, 119, 3048–3058. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Cao, W.; Wei, X.; Chen, J.; Ying, W. SIRT2 Plays Significant Roles in Lipopolysaccharides-Induced Neuroinflammation and Brain Injury in Mice. Neurochem. Res. 2016, 41, 2490–2500. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.B.; Kuhlmann, K.; Shen, S.; Uecker, M.; Schardt, A.; Dimova, K.; Orfaniotou, F.; Dhaunchak, A.; Brinkmann, B.G.; Möbius, W.; et al. Proteolipid Protein Is Required for Transport of Sirtuin 2 into CNS Myelin. J. Neurosci. 2007, 27, 7717–7730. [Google Scholar] [CrossRef]
- Beirowski, B.; Gustin, J.; Armour, S.M.; Yamamoto, H.; Viader, A.; North, B.J.; Orfaniotou, F.; Dhaunchak, A.; Brinkmann, B.G.; Möbius, W.; et al. Sir-two-homolog 2 (Sirt2) modulates peripheral myelination through polarity protein Par-3/atypical protein kinase C (aPKC) signaling. Proc. Natl. Acad. Sci. USA 2011, 108, E952–E961. Available online: https://pnas.org/doi/full/10.1073/pnas.1104969108 (accessed on 13 August 2023). [CrossRef]
- Jastorff, A.M.; Haegler, K.; Maccarrone, G.; Holsboer, F.; Weber, F.; Ziemssen, T.; Turck, C.W. Regulation of proteins mediating neurodegeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Proteom. Clin. Appl. 2009, 3, 1273–1287. [Google Scholar] [CrossRef]
- Shindler, K.S.; Ventura, E.; Rex, T.S.; Elliott, P.; Rostami, A. SIRT1 Activation Confers Neuroprotection in Experimental Optic Neuritis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3602. [Google Scholar] [CrossRef]
- Li, X. SIRT1 and energy metabolism. ABBS 2013, 45, 51–60. [Google Scholar] [CrossRef]
- Prozorovski, T.; Schulze-Topphoff, U.; Glumm, R.; Baumgart, J.; Schröter, F.; Ninnemann, O.; Siegert, E.; Bendix, I.; Brüstle, O.; Nitsch, R.; et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat. Cell Biol. 2008, 10, 385–394. [Google Scholar] [CrossRef]
- Lim, H.W.; Kang, S.G.; Ryu, J.K.; Schilling, B.; Fei, M.; Lee, I.S.; Kehasse, A.; Shirakawa, K.; Yokoyama, M.; Schnölzer, M.; et al. SIRT1 deacetylates RORγt and enhances Th17 cell generation. J. Exp. Med. 2015, 212, 607–617. [Google Scholar] [CrossRef]
- Martin, A.; Tegla, C.A.; Cudrici, C.D.; Kruszewski, A.M.; Azimzadeh, P.; Boodhoo, D.; Mekala, A.P.; Rus, V.; Rus, H. Role of SIRT1 in autoimmune demyelination and neurodegeneration. Immunol. Res. 2015, 61, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, I.; Kucharska-Lusina, A.; Miller, E.; Majsterek, I. Exploring the mRNA and Plasma Protein Levels of BDNF.; NT4, SIRT1, HSP27, and HSP70 in Multiple Sclerosis Patients and Healthy Controls. Int. J. Mol. Sci. 2023, 24, 16176. [Google Scholar] [CrossRef] [PubMed]
- Kubiliute, A.; Gedvilaite, G.; Vilkeviciute, A.; Kriauciuniene, L.; Bruzaite, A.; Zaliuniene, D.; Liutkeviciene, R. The role of SIRT1 level and SIRT1 gene polymorphisms in optic neuritis patients with multiple sclerosis. Orphanet J. Rare Dis. 2023, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, G.; Cornelius, C.; Cavallaro, M.; Salinaro, A.T.; Cambria, M.; Pennisi, M.; Bella, R.; Milone, P.; Ventimiglia, B.; Migliore, M.; et al. Redox regulation of cellular stress response in multiple sclerosis. Biochem. Pharmacol. 2011, 82, 1490–1499. [Google Scholar] [CrossRef]
- Tegla, C.A.; Azimzadeh, P.; Andrian-Albescu, M.; Martin, A.; Cudrici, C.D.; Trippe, R.; Sugarman, A.; Chen, H.; Boodhoo, D.; Vlaicu, S.I.; et al. SIRT1 is decreased during relapses in patients with multiple sclerosis. Exp. Mol. Pathol. 2014, 96, 139–148. [Google Scholar] [CrossRef]
- Lovato, L.; Cianti, R.; Gini, B.; Marconi, S.; Bianchi, L.; Armini, A.; Anghileri, E.; Locatelli, F.; Paoletti, F.; Franciotta, D.; et al. Transketolase and 2′,3′-Cyclic-nucleotide 3′-Phosphodiesterase Type I Isoforms Are Specifically Recognized by IgG Autoantibodies in Multiple Sclerosis Patients. Mol. Cell. Proteom. 2008, 7, 2337–2349. [Google Scholar] [CrossRef]
- Gammoh, O.; AlQudah, A.; Al Rob, O.A.; Hmedat, A.; Kifaieh, A.; Weshah, F.; Ennab, W.; Qnais, E. Modulation of salivary ICAM-1 and SIRT1 by disease modifying drugs in undepressed relapsing-remitting multiple sclerosis patients. Mult. Scler. Relat. Disord. 2022, 68, 104257. [Google Scholar] [CrossRef]
- Ryan, A.S.; Li, G. Sex differences in muscle SIRT1 and SIRT3 and exercise + weight loss effects on muscle sirtuins. Exp. Biol. Med. 2023, 248, 302–308. [Google Scholar] [CrossRef]
- Shimabukuro, M. SIRT1 and Gender Differences in Atherosclerotic Cardiovascular Disease. J. Atheroscler. Thromb. 2020, 27, 8–10. [Google Scholar] [CrossRef]
- Gonçalinho, G.H.F.; Kuwabara, K.L.; Faria, N.F.d.O.; Goes, M.F.d.S.; Roggerio, A.; Avakian, S.D.; Strunz, C.M.C.; Mansur, A.d.P. Sirtuin 1 and Vascular Function in Healthy Women and Men: A Randomized Clinical Trial Comparing the Effects of Energy Restriction and Resveratrol. Nutrients 2023, 15, 2949. [Google Scholar] [CrossRef]
- Opstad, T.B.; Sundfør, T.; Tonstad, S.; Seljeflot, I. Effect of intermittent and continuous caloric restriction on Sirtuin1 concentration depends on sex and body mass index. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Ikeda, Y.; Miyauchi, T.; Uchikado, Y.; Akasaki, Y.; Ohishi, M. Estrogen-SIRT1 Axis Plays a Pivotal Role in Protecting Arteries Against Menopause-Induced Senescence and Atherosclerosis. J. Atheroscler. Thromb. 2020, 27, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Yang, S.J. Aging-Related Correlation between Serum Sirtuin 1 Activities and Basal Metabolic Rate in Women, but not in Men. Clin. Nutr. Res. 2017, 6, 18. [Google Scholar] [CrossRef]
- Lopes, C.R.; Silva, J.S.; Santos, J.; Rodrigues, M.S.; Madeira, D.; Oliveira, A.; Moreira-De-Sá, A.; Lourenço, V.S.; Gonçalves, F.Q.; Silva, H.B.; et al. Downregulation of Sirtuin 1 Does Not Account for the Impaired Long-Term Potentiation in the Prefrontal Cortex of Female APPswe/PS1dE9 Mice Modelling Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 6968. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, Y.; Wang, X.X.; Truong, D.; Wu, Y.C. The Critical Role of SIRT1 in Parkinson’s Disease: Mechanism and Therapeutic Considerations. Aging Dis. 2020, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- Cartas-Cejudo, P.; Lachén-Montes, M.; Ferrer, I.; Fernández-Irigoyen, J.; Santamaría, E. Sex-divergent effects on the NAD+-dependent deacetylase sirtuin signaling across the olfactory–entorhinal–amygdaloid axis in Alzheimer’s and Parkinson’s diseases. Biol. Sex Differ. 2023, 14, 5. [Google Scholar] [CrossRef]
- Batoee, S.; Etminaniesfahani, M.; Mazdeh, M.; Soltanian, A.; Nouri, F. Evaluation of Rosuvastatin Therapy on SIRT1 Gene Expression in Patients with Multiple Sclerosis: An Uncontrolled Clinical Trial. Curr. Ther. Res. 2023, 99, 100718. [Google Scholar] [CrossRef]
- Michán, S.; Li, Y.; Chou, M.M.-H.; Parrella, E.; Ge, H.; Long, J.M.; Allard, J.S.; Lewis, K.; Miller, M.; Xu, W.; et al. SIRT1 Is Essential for Normal Cognitive Function and Synaptic Plasticity. J. Neurosci. 2010, 30, 9695–9707. [Google Scholar] [CrossRef]
- Toorie, A.M.; Nillni, E.A. Minireview: Central Sirt1 Regulates Energy Balance via the Melanocortin System and Alternate Pathways. Mol. Endocrinol. 2014, 28, 1423–1434. [Google Scholar] [CrossRef]
- Chang, H.C.; Guarente, L. SIRT1 Mediates Central Circadian Control in the SCN by a Mechanism that Decays with Aging. Cell 2013, 153, 1448–1460. [Google Scholar] [CrossRef]
- Lu, G.; Li, J.; Zhang, H.; Zhao, X.; Yan, L.J.; Yang, X. Role and Possible Mechanisms of Sirt1 in Depression. Oxidative Med. Cell. Longev. 2018, 2018, 8596903. [Google Scholar] [CrossRef] [PubMed]
- Libert, S.; Pointer, K.; Bell, E.L.; Das, A.; Cohen, D.E.; Asara, J.M.; Kapur, K.; Bergmann, S.; Preisig, M.; Otowa, T.; et al. SIRT1 Activates MAO-A in the Brain to Mediate Anxiety and Exploratory Drive. Cell 2011, 147, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.J.; Zhang, C. Down-Regulation of SIRT1 Gene Expression in Major Depressive Disorder. Am. J. Psychiatry 2016, 173, 1046. [Google Scholar] [CrossRef]
- Kovanen, L.; Donner, K.; Partonen, T. SIRT1 Polymorphisms Associate with Seasonal Weight Variation, Depressive Disorders, and Diastolic Blood Pressure in the General Population. PLoS ONE 2015, 10, e0141001. [Google Scholar] [CrossRef]
- Wongchitrat, P.; Pakpian, N.; Kitidee, K.; Phopin, K.; Dharmasaroja, P.A.; Govitrapong, P. Alterations in the Expression of Amyloid Precursor Protein Cleaving Enzymes mRNA in Alzheimer Peripheral Blood. Curr. Alzheimer Res. 2018, 16, 29–38. [Google Scholar] [CrossRef] [PubMed]
| Study Group n = 115 | Control Group n = 39 | p-Value | |
|---|---|---|---|
| Age [years] | 43 ± 9.91 | 42.5 ± 10.65 | 0.76 |
| Sex [F/M] | 78/37 | 27/12 | |
| BMI | 24.5 ± 4.25 | 25.98 ± 5.09 | 0.09 |
| AIS | 6.71 ± 4.35 | 6.00 ± 4.01 | 0.41 |
| ISI | 8.45 ± 6.44 | 6.62 ± 5.71 | 0.16 |
| Low questionnaire | 5.23 ± 4.54 | 2.86 ± 2.33 | 0.03 |
| HADS—depression | 8.09 ± 4.30 | 6.59 ± 4.29 | 0.10 |
| HADS—anxiety | 7.75 ± 4.24 | 7.10 ± 3.70 | 0.44 |
| SDMT | 38.71 ± 12.43 | 55.14 ± 8.88 | <0.05 |
| FSS | 35.97 ± 14.70 | 23.60 ± 12.13 | <0.05 |
| MFSI | 40.84 ± 19.27 | 22.83 ± 14.96 | <0.05 |
| Study Group | Control Group | p-Value | |
|---|---|---|---|
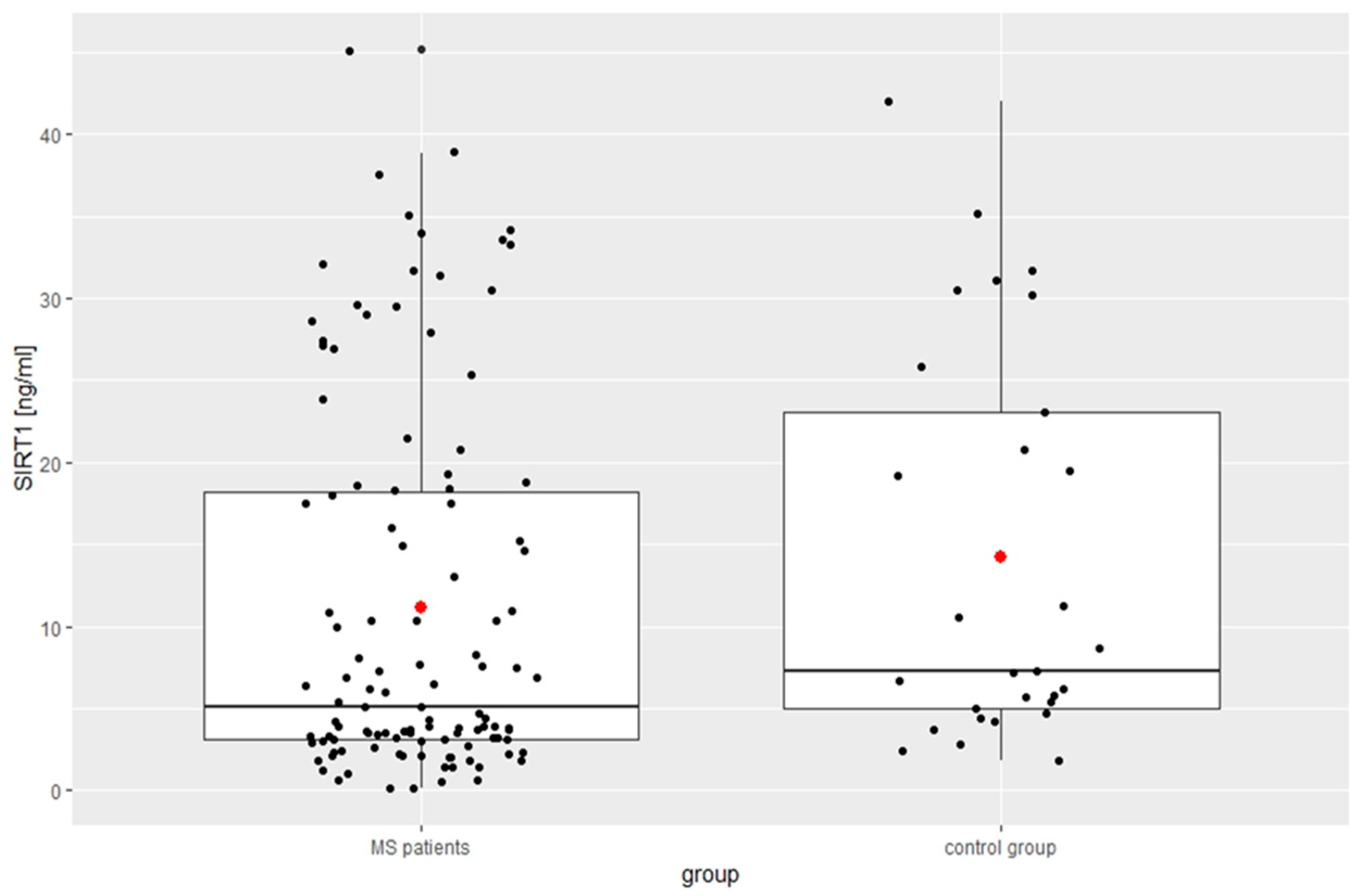
| SIRT1 [ng/mL] | 11.14 ± 11.39 | 14.23 ± 12.00 | 0.04 |
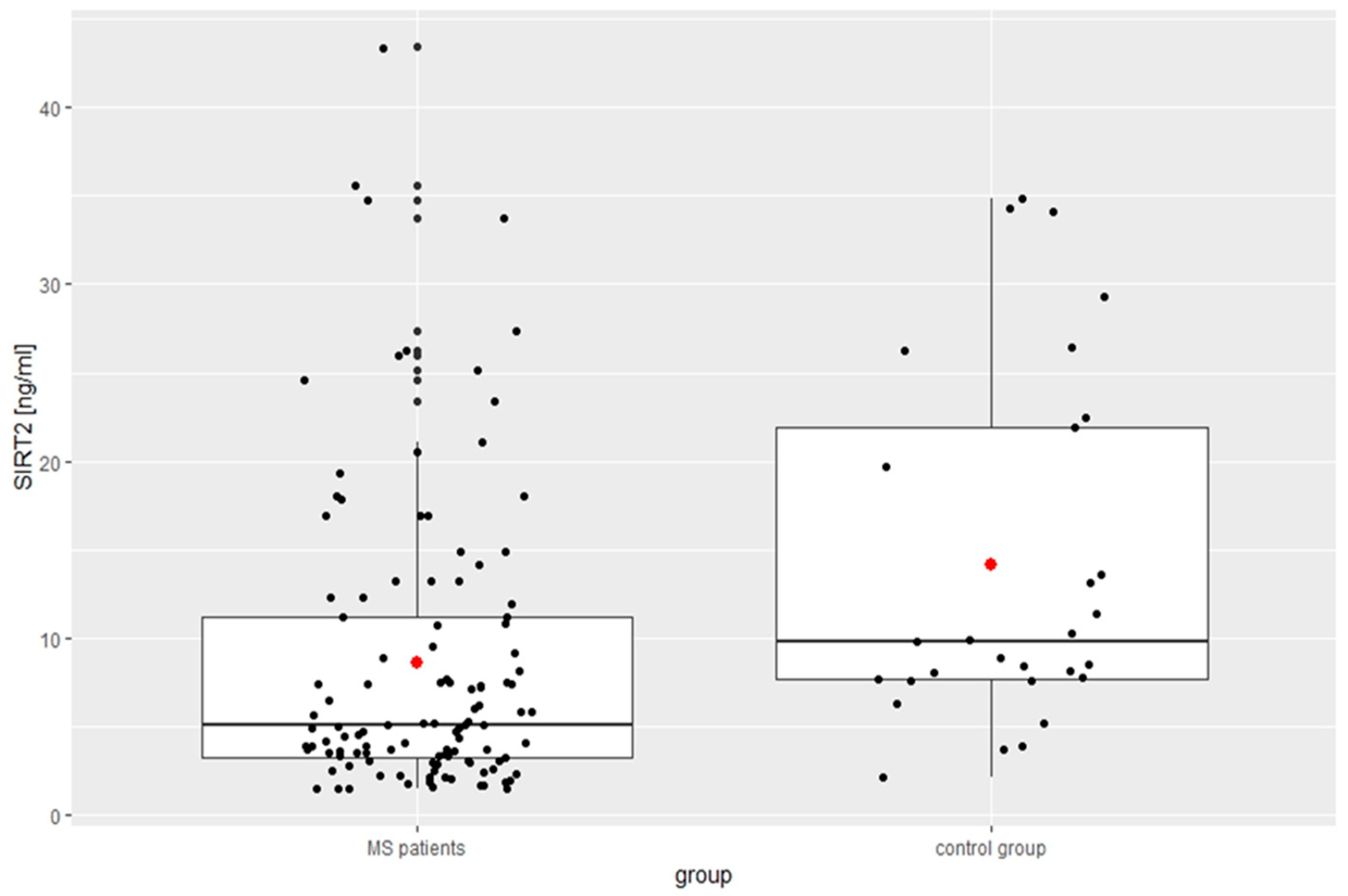
| SIRT2 [ng/mL] | 8.62 ± 8.39 | 14.20 ± 10.03 | <0.01 |
| SIRT 1 [ng/mL] | SIRT 2 [ng/mL] | |||
|---|---|---|---|---|
| R | p | R | p | |
| Disease duration (years) | −0.055 | 0.878 | −0.003 | 0.626 |
| EDSS | −0.253 | 0.018 | −0.143 | 0.016 |
| BMI | 0.151 | 0.137 | −0.147 | 0.218 |
| AIS | −0.130 | 0.220 | 0.143 | 0.094 |
| ISI | −0.068 | 0.456 | −0.086 | 0.708 |
| Low questionnaire | −0.090 | 0.324 | −0.011 | 0.121 |
| HADS—depression | 0.061 | 0.814 | −0.140 | 0.473 |
| HADS—anxiety | −0.076 | 0.422 | 0.018 | 0.451 |
| SDMT | 0.004 | 0.559 | −0.050 | 0.590 |
| FSS | −0.103 | 0.175 | −0.020 | 0.518 |
| MFSI | −0.149 | 0.059 | −0.109 | 0.208 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojdak-Łukasiewicz, J.; Bizoń, A.; Kołtuniuk, A.; Waliszewska-Prosół, M.; Budrewicz, S.; Piwowar, A.; Pokryszko-Dragan, A. Are Sirtuins 1 and 2 Relevant Players in Relapsing–Remitting Multiple Sclerosis? Biomedicines 2024, 12, 2027. https://doi.org/10.3390/biomedicines12092027
Chojdak-Łukasiewicz J, Bizoń A, Kołtuniuk A, Waliszewska-Prosół M, Budrewicz S, Piwowar A, Pokryszko-Dragan A. Are Sirtuins 1 and 2 Relevant Players in Relapsing–Remitting Multiple Sclerosis? Biomedicines. 2024; 12(9):2027. https://doi.org/10.3390/biomedicines12092027
Chicago/Turabian StyleChojdak-Łukasiewicz, Justyna, Anna Bizoń, Aleksandra Kołtuniuk, Marta Waliszewska-Prosół, Sławomir Budrewicz, Agnieszka Piwowar, and Anna Pokryszko-Dragan. 2024. "Are Sirtuins 1 and 2 Relevant Players in Relapsing–Remitting Multiple Sclerosis?" Biomedicines 12, no. 9: 2027. https://doi.org/10.3390/biomedicines12092027
APA StyleChojdak-Łukasiewicz, J., Bizoń, A., Kołtuniuk, A., Waliszewska-Prosół, M., Budrewicz, S., Piwowar, A., & Pokryszko-Dragan, A. (2024). Are Sirtuins 1 and 2 Relevant Players in Relapsing–Remitting Multiple Sclerosis? Biomedicines, 12(9), 2027. https://doi.org/10.3390/biomedicines12092027







