Rapid Detection of Acinetobacter baumannii Suspension and Biofilm Nanomotion and Antibiotic Resistance Estimation
Abstract
:1. Introduction
2. Materials and Methods
2.1. A. baumannii 173-p1 Culturing
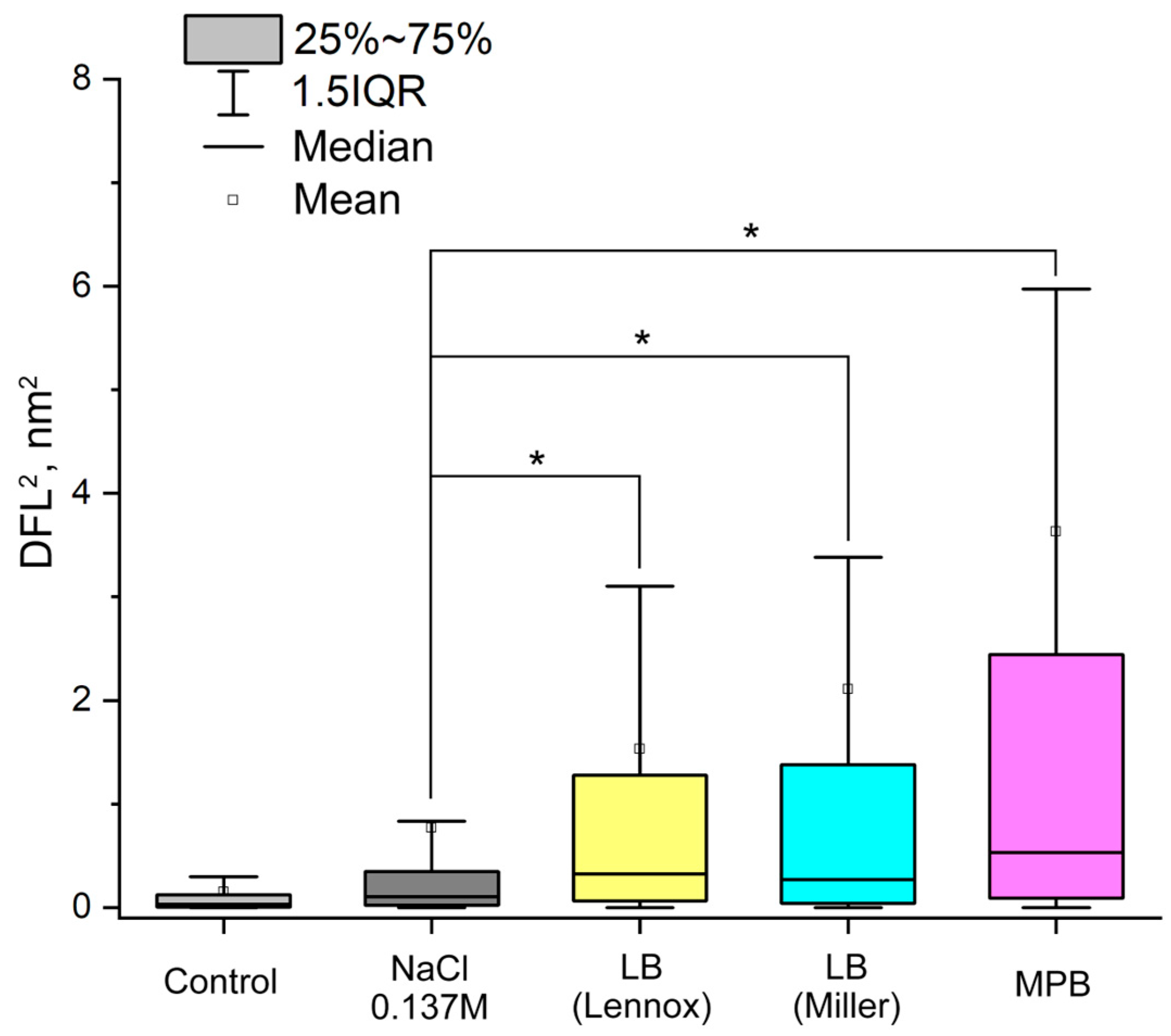
2.2. Detection of A. baumannii 173-p1 Nanomotion in Different Media
2.3. A. baumannii 173-p1 Biofilm Culturing and Measurement of its Nanomotion
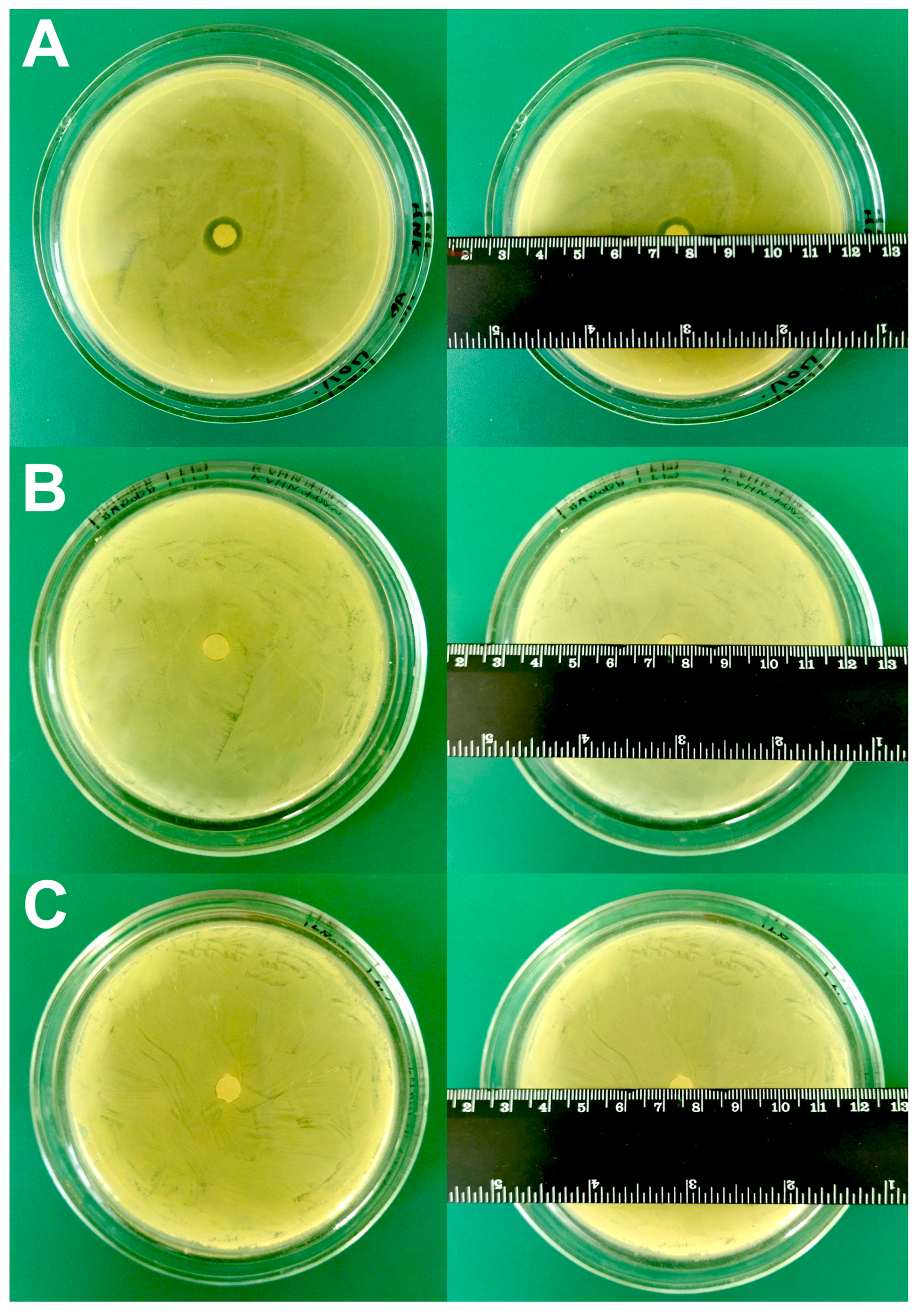
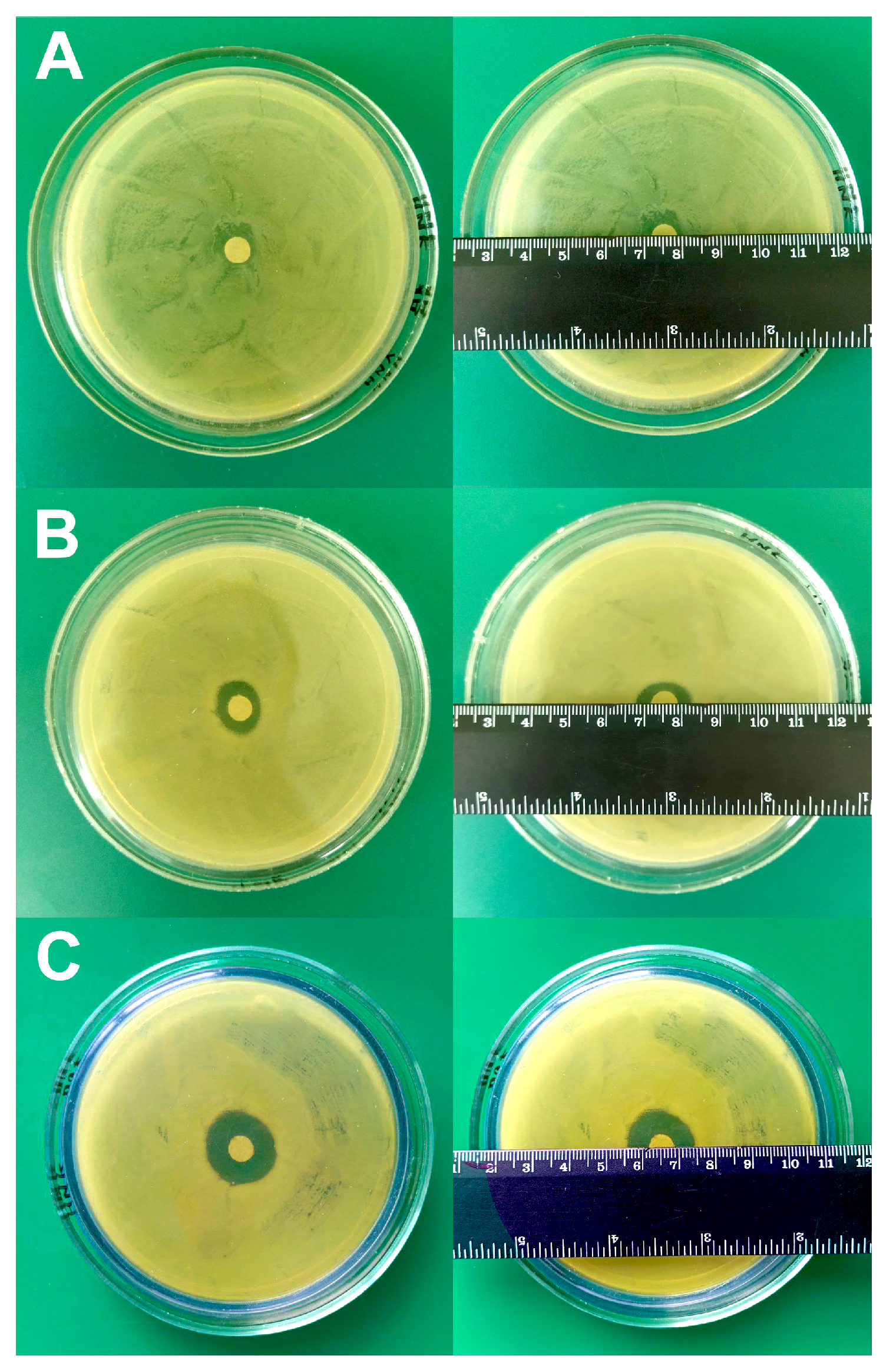
2.4. Disk Diffusion Test for Study Sensitivity/Resistance of A. baumannii 173-p1 to Antibiotics
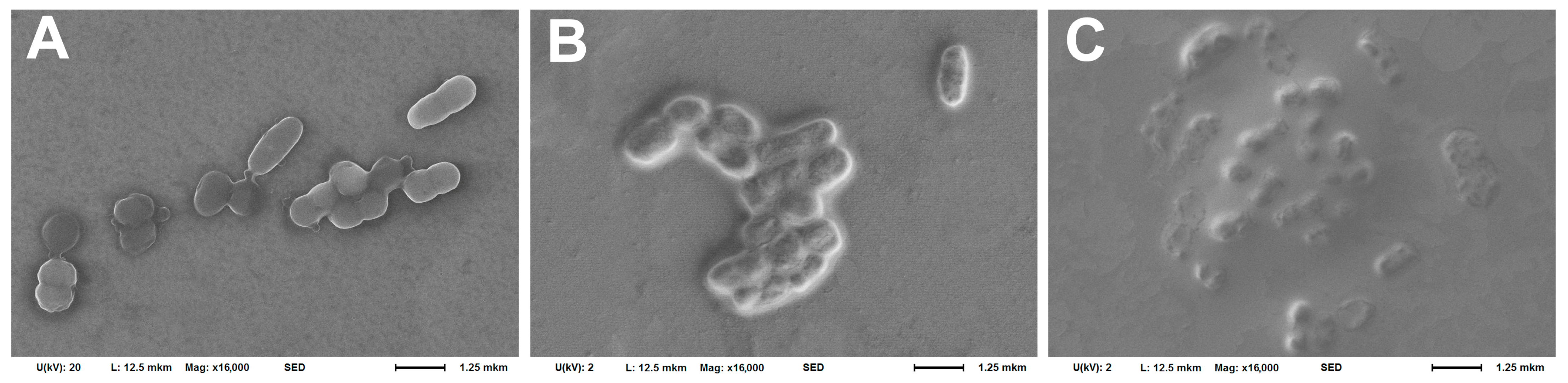
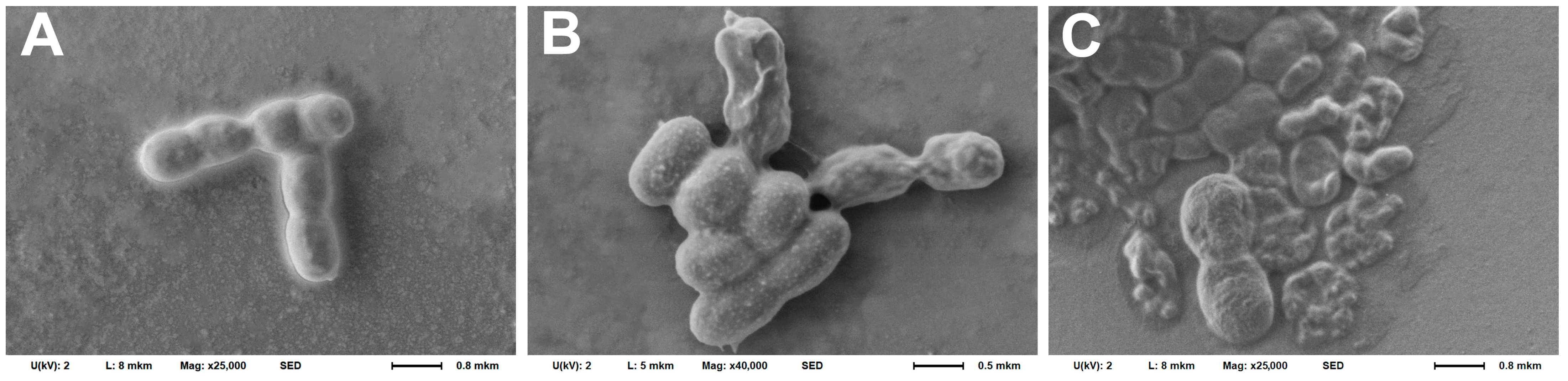
2.5. Scanning Electron Microscopy
2.6. Express Test for Determining the Sensitivity/Resistance of A. baumannii 173-p1 to Antibiotics
2.7. Statistical Analysis
3. Results
3.1. Selection of the Optimal Fixing Agent for Fixing Bacteria on a Cantilever
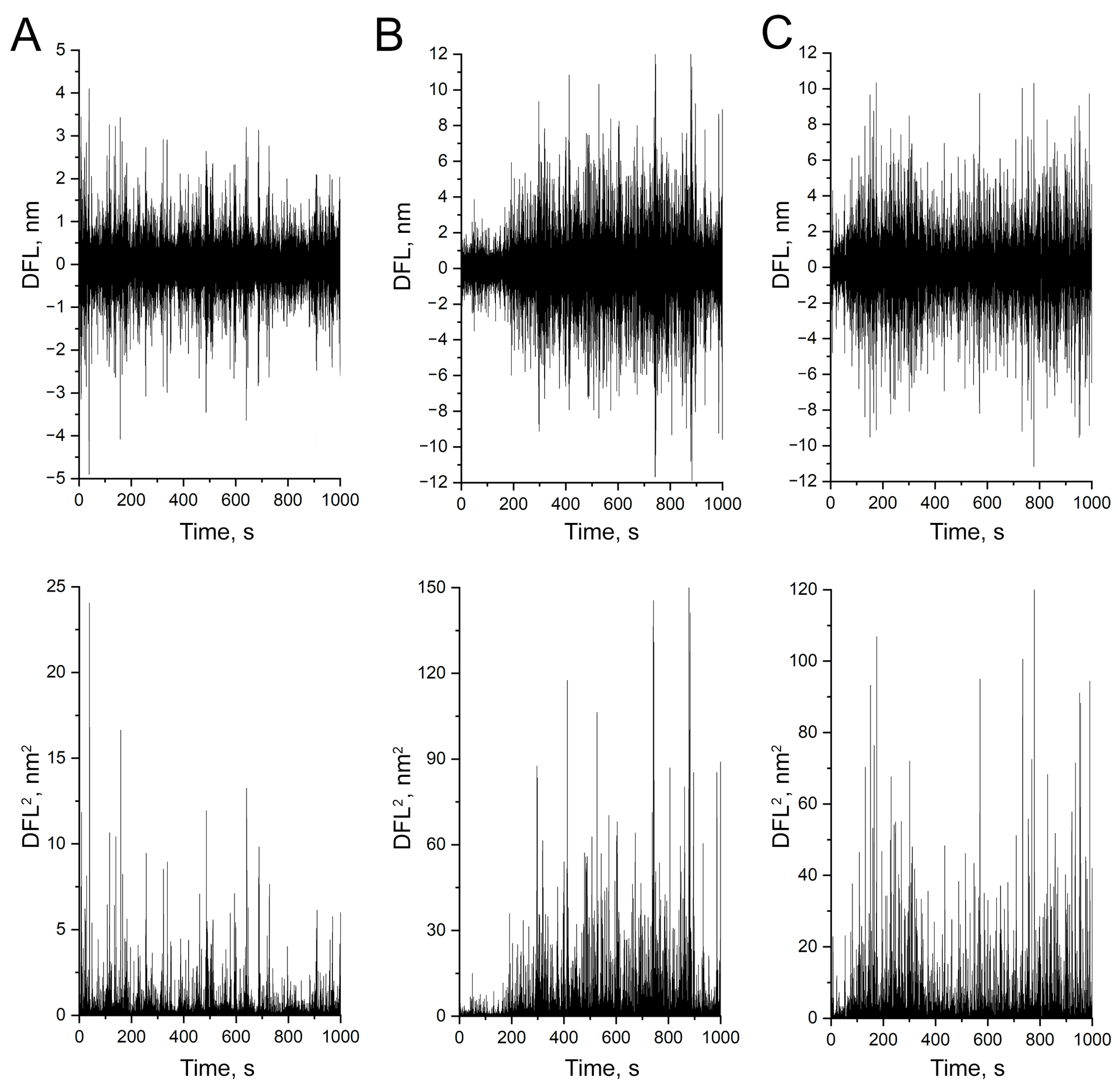
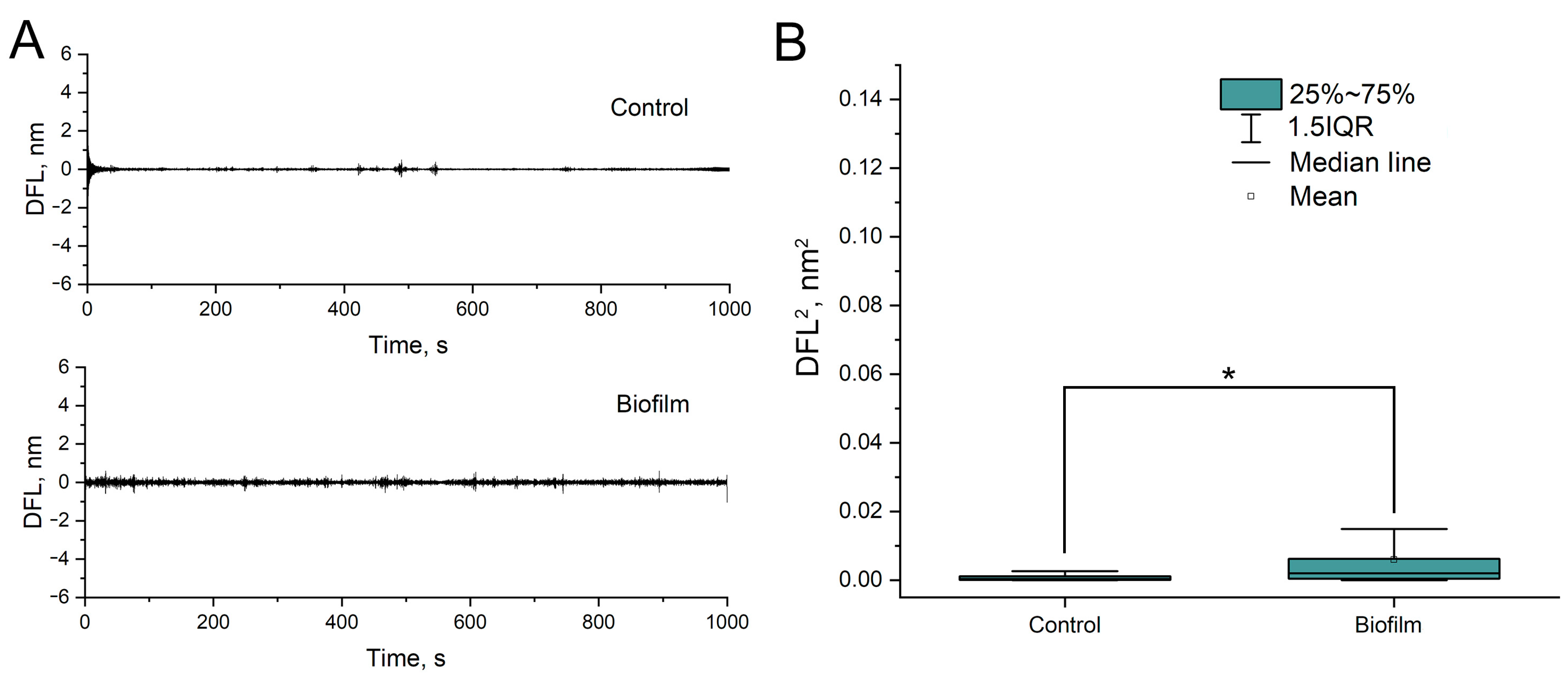
3.2. Evidence That Nanomotion Is Driven by Bacterial Metabolic Activity
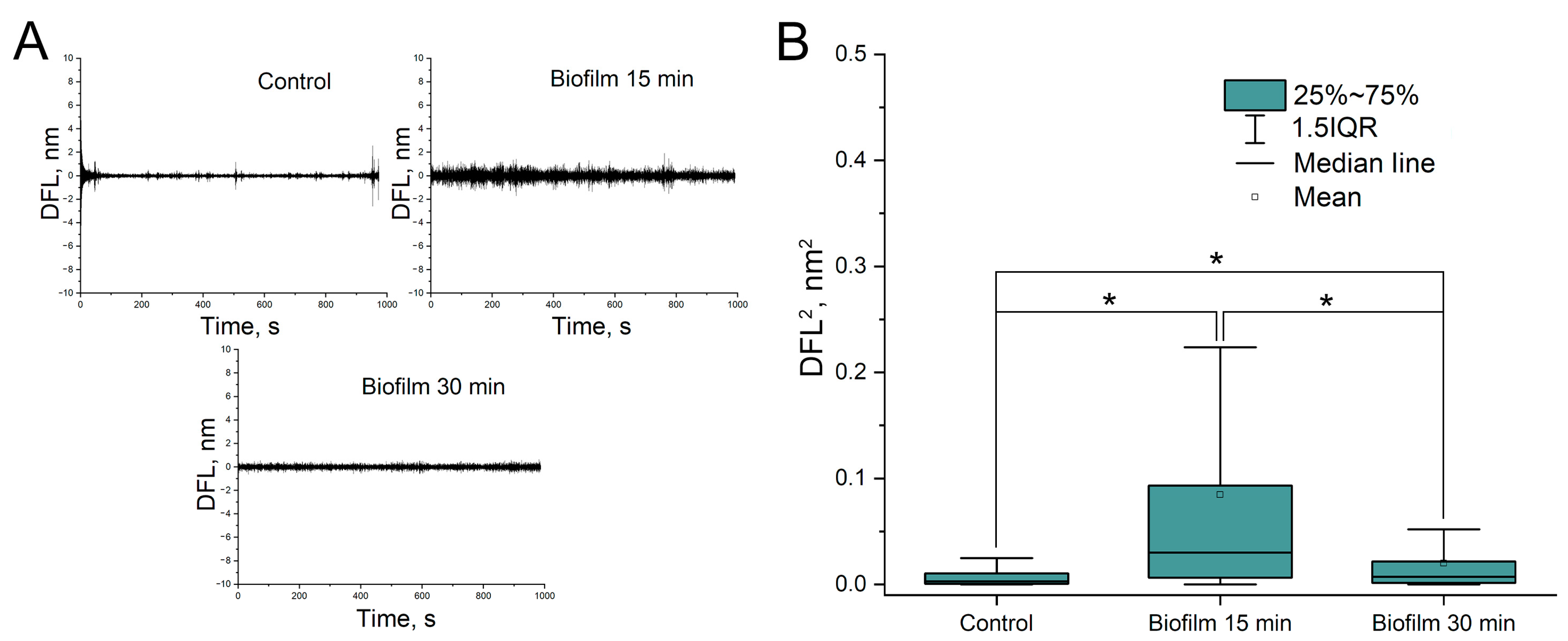
3.3. Study of Bacterial Nanomotion in Biofilms
3.4. Detection of Nanomotion Depended on the Density of the Biofilm
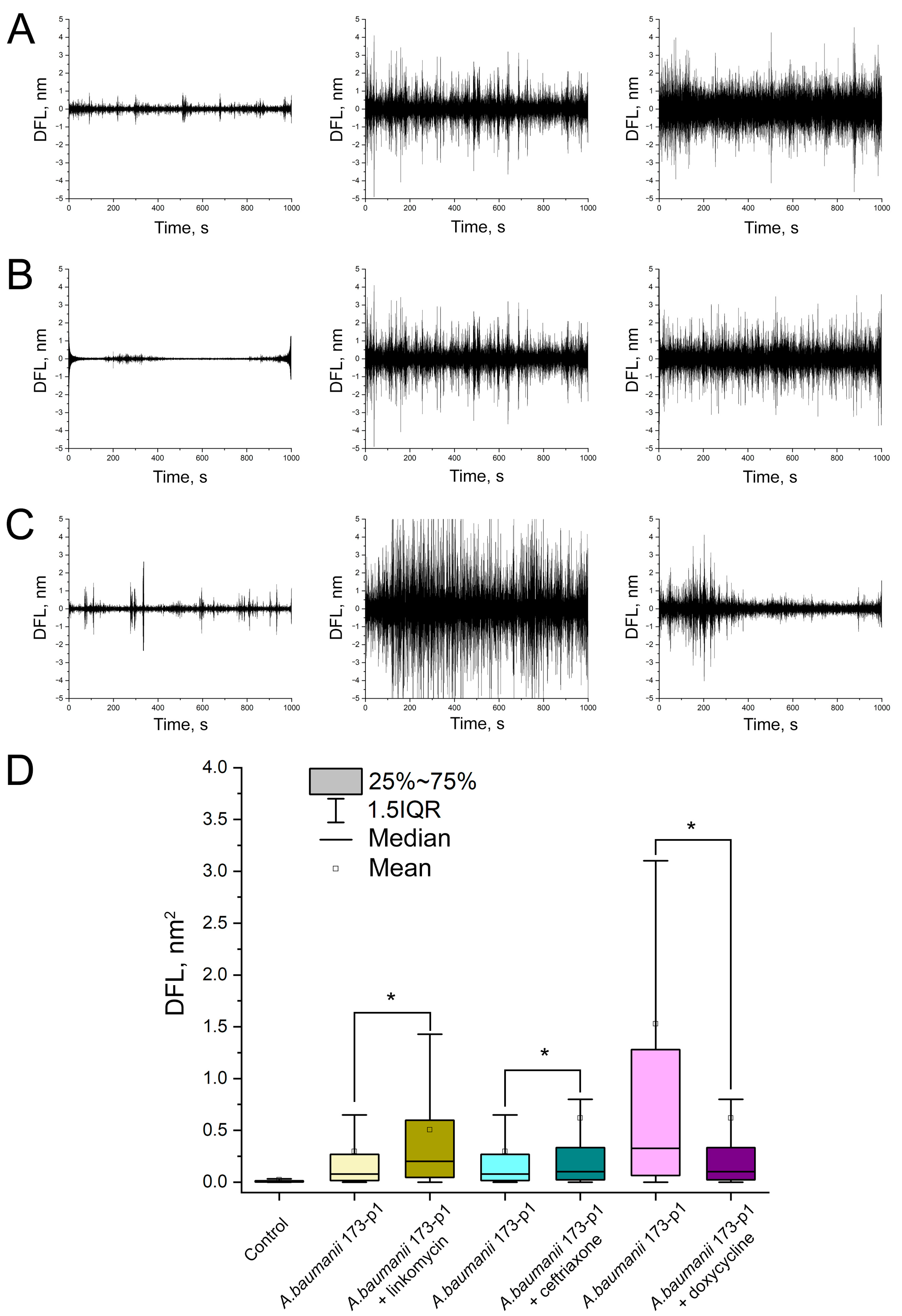
3.5. Testing of A. baumannii 173-p1 Sensitivity/Resistance to Antibiotics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chebotar, I.V.; Lazareva, A.V.; Masalov, Y.K.; Mikhailovich, V.M.; Mayansky, N.A. Acinetobacter: Microbiological, pathogenetic and resistance properties. Vestn. RAMS 2014, 69, 39–50. (In Russian) [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Bacher, J.M.; Metzgar, D.; de Crecy-Lagard, V. Rapid evolution of diminished transformability in Acinetobacter baylyi. J. Bacteriol. 2006, 188, 8534–8542. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Bogomolova, N.S.; Bolshakov, L.V.; Kuznetsova, S.M. The problem of treating purulent-inflammatory complications caused by Acinetobacter. Anesthesiol. Reanimatol. 2014, 1, 26–32. (In Russian) [Google Scholar]
- Petersen, K.; Riddle, M.S.; Danko, J.R.; Blazes, D.L.; Hayden, R. Trauma-Related infections in battlefield casualties from Iraq. Ann. Surg. 2007, 245, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.; O’Donoghue, M.; Feeney, A.; Sleator, R.D. Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Rodriguez-Bano, J.; Marti, S.; Soto, S.; Fernandez-Cuenca, F.; Cisneros, J.M.; Pachon, J.; Pascual, A.; Martínez-Martínez, L.; McQueary, C.; Actis, L.A.; et al. Biofilm formation in Acinetobacter baumannii: Associated features and clinical implications. Clin. Microbiol. Infect. 2008, 14, 276–278. [Google Scholar] [CrossRef]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology 2003, 149, 3473–3484. [Google Scholar] [CrossRef]
- Cerqueira, G.M.; Peleg, A.Y. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life 2011, 63, 1055–1060. [Google Scholar] [CrossRef]
- Choi, A.H.; Slamti, L.; Avci, F.Y.; Pier, G.B.; Maira-Litran, T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J. Bacteriol. 2009, 191, 5953–5963. [Google Scholar] [CrossRef]
- Pleskova, S.N.; Fomichev, O.I.; Kryukov, R.N.; Sudakova, I.S. Nanovibrations of an atomic force microscope cantilever as a system for detecting antibiotic resistance of bacteria in real time. Biophysics 2021, 66, 1116–1122. [Google Scholar] [CrossRef]
- Pleskova, S.N.; Lazarenko, E.V.; Sudakova, I.S.; Kryukov, R.N.; Bezrukov, N.A. A new method for rapid detection of antibiotic resistance. Appl. Biochem. Microbiol. 2023, 59, 74–80. [Google Scholar] [CrossRef]
- Longo, G.; Alonso-Sarduy, L.; Rio, L.M.; Bizzini, A.; Trampuz, A.; Notz, J.; Dietler, G.; Kasas, S. Rapid detection of bacterial resistance to antibiotics using AFM cantilevers as nanomechanical sensors. Nat. Nanotechnol. 2013, 8, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Willaert, R.G.; Vanden Boer, P.; Malovichko, A.; Alioscha-Perez, M.; Radotić, K.; Bartolić, D.; Kalauzi, A.; Villalba, M.I.; Sanglard, D.; Dietler, G.; et al. Single yeast cell nanomotion correlate with cellular activity. Sci. Adv. 2020, 6, eaba3139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liao, C.; Zou, M.; Villalba, M.I.; Xiong, C.; Zhao, C.; Venturelli, L.; Liu, D.; Kohler, A.C.; Sekatskii, S.K.; et al. An Optical Fiber-Based Nanomotion Sensor for Rapid Antibiotic and Antifungal Susceptibility Tests. Nano Lett. 2024, 24, 2980–2988. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.I.; Rossetti, E.; Bonvallat, A.; Yvanoff, C.; Radonicic, V.; Willaert, R.G.; Kasas, S. Simple optical nanomotion method for single-bacterium viability and antibiotic response testing. Proc. Natl. Acad. Sci. USA 2023, 120, e2221284120. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.; Villalba, M.I.; Horii Huber, A.S.; Kalauzi, A.; Bartolić, D.; Radotić, K.; Willaert, R.G.; MacFabe, D.F.; Kasas, S. Mitochondrial nanomotion measured by optical microscopy. Front. Microbiol. 2023, 14, 1133773. [Google Scholar] [CrossRef]
- Starodubtseva, M.N.; Shkliarava, N.M.; Chelnokova, I.A.; Villalba, M.I.; Krylov, A.Y.; Nadyrov, E.A.; Kasas, S. Mechanical Properties and Nanomotion of BT-20 and ZR-75 Breast Cancer Cells Studied by Atomic Force Microscopy and Optical Nanomotion Detection Method. Cells 2023, 12, 2362. [Google Scholar] [CrossRef]
- Pleskova, S.N.; Lazarenko, E.V.; Bezrukov, N.A.; Bobyk, S.Z.; Boryakov, A.V.; Kriukov, R.N. Differences in bacteria nanomotion profiles and neutrophil nanomotion during phagocytosis. Front. Microbiol. 2023, 14, 1113353. [Google Scholar] [CrossRef]
- Kohler, A.C.; Venturelli, L.; Longo, G.; Dietler, G.; Kasas, S. Nanomotion detection based on atomic force microscopy cantilevers. Cell Surf. 2019, 5, 100021. [Google Scholar] [CrossRef] [PubMed]
- Villalba, M.I.; Venturelli, L.; Willaert, R.; Vela, M.E.; Yantorno, O.; Dietler, G.; Longo, G.; Kasas, S. Nanomotion spectroscopy as a new approach to characterize bacterial virulence. Microorganisms 2021, 9, 1545. [Google Scholar] [CrossRef] [PubMed]
- McQueary, C.N.; Actis, L.A. Acinetobacter baumannii biofilms: Variations among strains and correlations with other cell properties. J. Microbiol. 2011, 49, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, L.; Kohler, A.C.; Stupar, P.; Villalba, M.I.; Kalauzi, A.; Radotic, K.; Bertacchi, M.; Dinarelli, S.; Girasole, M.; Pešić, M.; et al. A perspective view on the nanomotion detection of living organisms and its features. J. Mol. Recognit. 2020, 33, e2849. [Google Scholar] [CrossRef]


| Criterion | Disk Diffusion Method | Oscillation Method |
|---|---|---|
| Availability of an official standard | EUCAST | - |
| Current price | Lower | Higher |
| Reproducibility | + | + |
| Sensitivity | Low | High |
| Time of analysis | 48 h | Less than 1 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleskova, S.N.; Bezrukov, N.A.; Nikolaeva, E.D.; Boryakov, A.V.; Kuzina, O.V. Rapid Detection of Acinetobacter baumannii Suspension and Biofilm Nanomotion and Antibiotic Resistance Estimation. Biomedicines 2024, 12, 2034. https://doi.org/10.3390/biomedicines12092034
Pleskova SN, Bezrukov NA, Nikolaeva ED, Boryakov AV, Kuzina OV. Rapid Detection of Acinetobacter baumannii Suspension and Biofilm Nanomotion and Antibiotic Resistance Estimation. Biomedicines. 2024; 12(9):2034. https://doi.org/10.3390/biomedicines12092034
Chicago/Turabian StylePleskova, Svetlana N., Nikolay A. Bezrukov, Ekaterina D. Nikolaeva, Alexey V. Boryakov, and Olga V. Kuzina. 2024. "Rapid Detection of Acinetobacter baumannii Suspension and Biofilm Nanomotion and Antibiotic Resistance Estimation" Biomedicines 12, no. 9: 2034. https://doi.org/10.3390/biomedicines12092034





