The Relationship between Smoking and Susceptibility to HIV Infection: A Two-Sample Mendelian Randomization Analysis
Abstract
1. Introduction
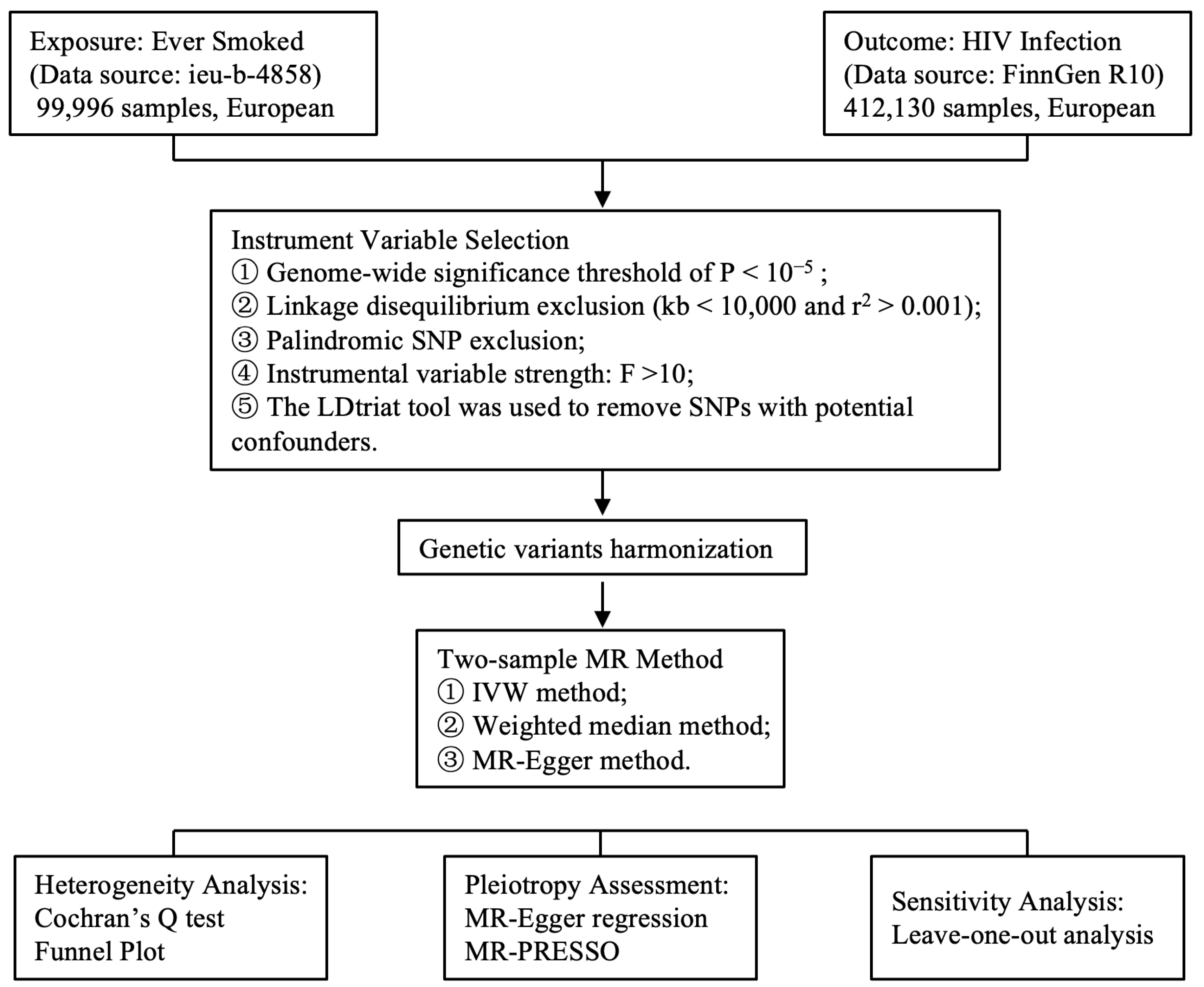
2. Materials and Methods
2.1. GWAS Summary Data Source
2.2. IVs Selection
2.3. MR Analysis
2.4. Sensitive Analysis
2.5. Molecular Pathways Connecting Smoking and HIV Infection
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Genetic Instruments
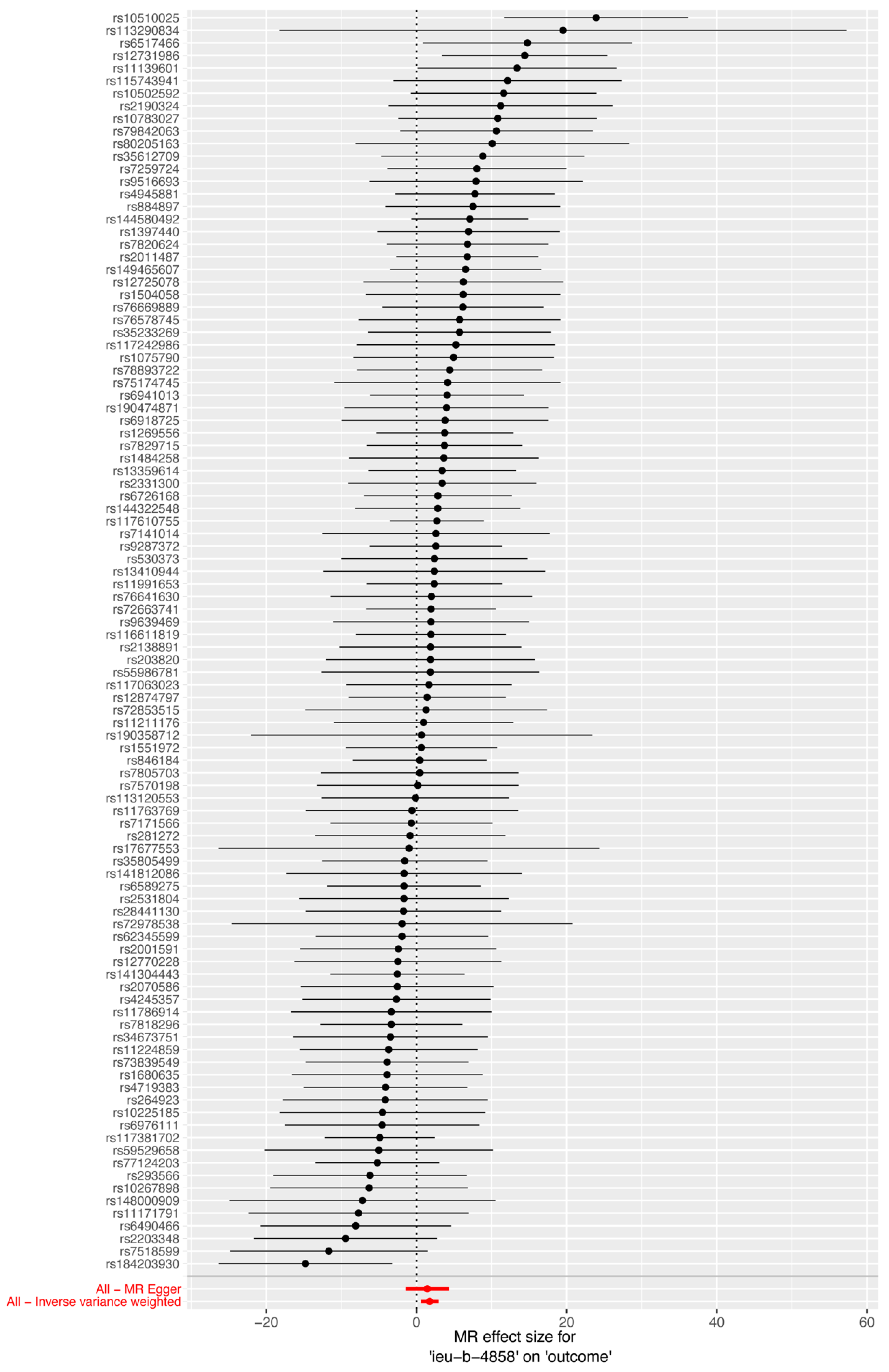
3.2. Effect of Smoking on HIV Infection
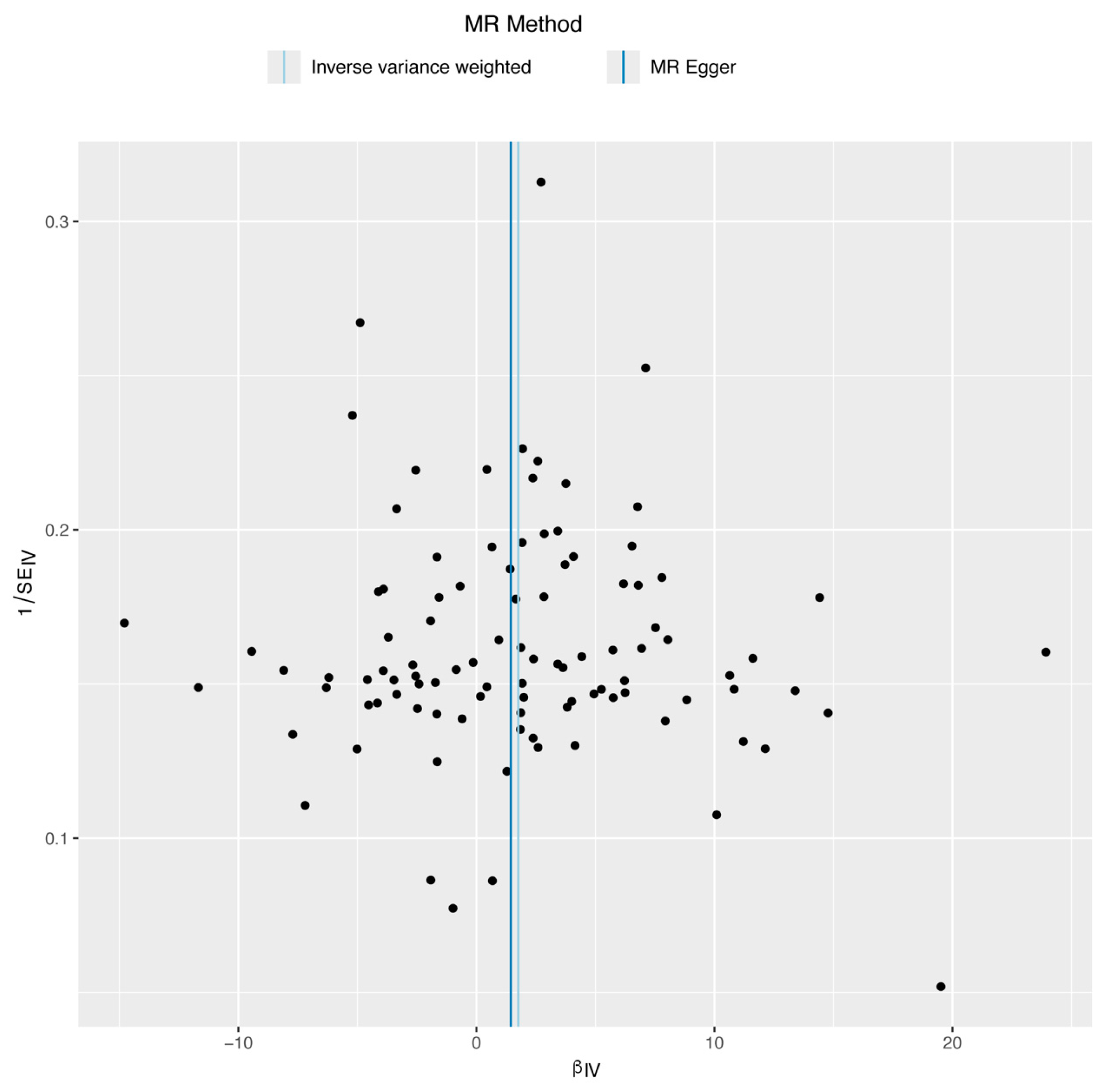
3.3. Sensitivity Analysis
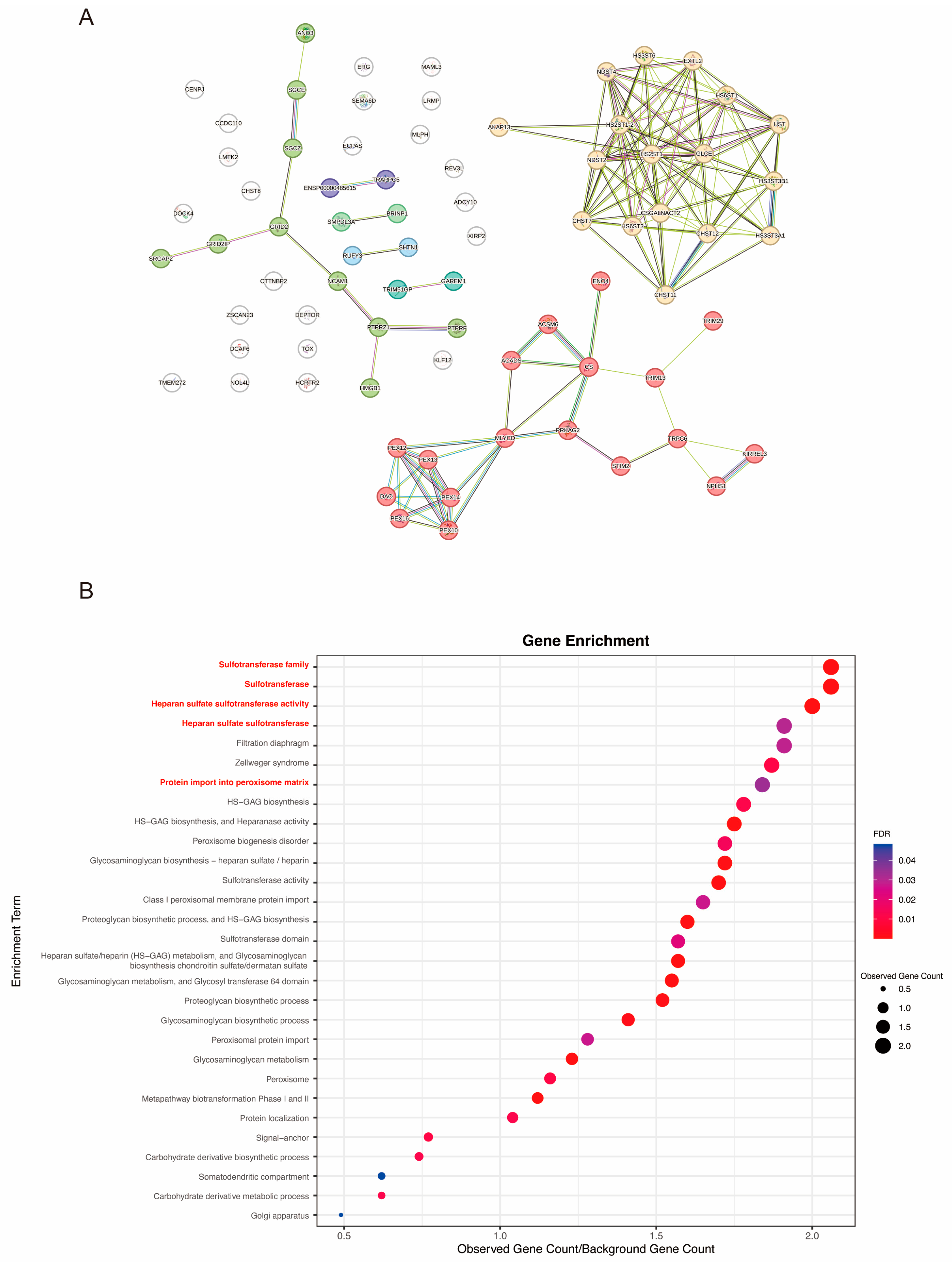
3.4. Bioinformatical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- AIDS and Hepatitis C Professional Group; Society of Infectious Diseases; Chinese Medical Association; Chinese Center for Disease Control and Prevention. Chinese Guidelines for the Diagnosis and Treatment of HIV/AIDS (2021 Edition). Infect. Dis. Immun. 2022, 2, 145–167. [Google Scholar] [CrossRef]
- Ghebreyesus, T.A. Progress in beating the tobacco epidemic. Lancet 2019, 394, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Mdodo, R.; Frazier, E.L.; Dube, S.R.; Mattson, C.L.; Sutton, M.Y.; Brooks, J.T.; Skarbinski, J. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: Cross-sectional surveys. Ann. Intern. Med. 2015, 162, 335–344. [Google Scholar] [CrossRef]
- Rahmanian, S.; Wewers, M.E.; Koletar, S.; Reynolds, N.; Ferketich, A.; Diaz, P. Cigarette smoking in the HIV-infected population. Proc. Am. Thorac. Soc. 2011, 8, 313–319. [Google Scholar] [CrossRef]
- Mdege, N.D.; Shah, S.; Ayo-Yusuf, O.A.; Hakim, J.; Siddiqi, K. Tobacco use among people living with HIV: Analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries. Lancet Glob. Health 2017, 5, e578–e592. [Google Scholar] [CrossRef]
- Tsima, B.M.; Motlhatlhedi, K.; Sharma, K.; Rantshabeng, P.; Ndlovu, A.; Gaolathe, T.; Kyokunda, L.T. The association between smoking and cervical human papillomavirus infection among women from indigenous communities in western Botswana. PLoS ONE 2024, 19, e0302153. [Google Scholar] [CrossRef]
- Helleberg, M.; Afzal, S.; Kronborg, G.; Larsen, C.S.; Pedersen, G.; Pedersen, C.; Gerstoft, J.; Nordestgaard, B.G.; Obel, N. Mortality attributable to smoking among HIV-1-infected individuals: A nationwide, population-based cohort study. Clin. Infect. Dis. 2013, 56, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Altekruse, S.F.; Shiels, M.S.; Modur, S.P.; Land, S.R.; Crothers, K.A.; Kitahata, M.M.; Thorne, J.E.; Mathews, W.C.; Fernández-Santos, D.M.; Mayor, A.M.; et al. Cancer burden attributable to cigarette smoking among HIV-infected people in North America. AIDS 2018, 32, 513–521. [Google Scholar] [CrossRef]
- Freibott, C.E.; Biondi, B.E.; Rao, S.R.; Blokhina, E.; Dugas, J.N.; Patts, G.; Bendiks, S.; Krupitsky, E.; Chichetto, N.E.; Samet, J.H.; et al. Is Abstinence from Alcohol and Smoking Associated with Less Anxiety and Depressive Symptoms Among People with HIV? AIDS Behav. 2024, 28, 1447–1455. [Google Scholar] [CrossRef]
- Toikumo, S.; Jennings, M.V.; Pham, B.K.; Lee, H.; Mallard, T.T.; Bianchi, S.B.; Meredith, J.J.; Vilar-Ribó, L.; Xu, H.; Hatoum, A.S.; et al. Multi-ancestry meta-analysis of tobacco use disorder identifies 461 potential risk genes and reveals associations with multiple health outcomes. Nat. Hum. Behav. 2024, 8, 1177–1193. [Google Scholar] [CrossRef]
- Sekula, P.; Del Greco, M.F.; Pattaro, C.; Köttgen, A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J. Am. Soc. Nephrol. 2016, 27, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Au Yeung, S.L.; Li, A.M.; He, B.; Kwok, K.O.; Schooling, C.M. Association of smoking, lung function and COPD in COVID-19 risk: A two-step Mendelian randomization study. Addiction 2022, 117, 2027–2036. [Google Scholar] [CrossRef] [PubMed]
- Rosoff, D.B.; Yoo, J.; Lohoff, F.W. Smoking is significantly associated with increased risk of COVID-19 and other respiratory infections. Commun. Biol. 2021, 4, 1230. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Real, L.M.; Sáez, M.E.; Corma-Gómez, A.; Gonzalez-Pérez, A.; Thorball, C.; Ruiz, R.; Jimenez-Leon, M.R.; Gonzalez-Serna, A.; Gasca-Capote, C.; Bravo, M.J.; et al. A metagenome-wide association study of HIV disease progression in HIV controllers. Iscience 2023, 26, 107214. [Google Scholar] [CrossRef]
- He, J.; Ding, Y.; Lin, H.; Liu, X.; Chen, X.; Shen, W.; Zhou, S.; Feng, C.; Wang, M.; Xia, J.; et al. Differential genome-wide associated variants and enriched pathways of ECG parameters among people with versus without HIV. AIDS 2023, 37, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Small, D.S.; Thompson, S.G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 2017, 26, 2333–2355. [Google Scholar] [CrossRef]
- Burgess, S.; Scott, R.A.; Timpson, N.J.; Davey Smith, G.; Thompson, S.G. Using published data in Mendelian randomization: A blueprint for efficient identification of causal risk factors. Eur. J. Epidemiol. 2015, 30, 543–552. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Connell, B.J.; Lortat-Jacob, H. Human immunodeficiency virus and heparan sulfate: From attachment to entry inhibition. Front. Immunol. 2013, 4, 385. [Google Scholar] [CrossRef] [PubMed]
- Obel, N.; Omland, L.H.; Kronborg, G.; Larsen, C.S.; Pedersen, C.; Pedersen, G.; Sørensen, H.T.; Gerstoft, J. Impact of non-HIV and HIV risk factors on survival in HIV-infected patients on HAART: A population-based nationwide cohort study. PLoS ONE 2011, 6, e22698. [Google Scholar] [CrossRef]
- Bronner Murrison, L.; Martinson, N.; Moloney, R.M.; Msandiwa, R.; Mashabela, M.; Samet, J.M.; Golub, J.E. Tobacco Smoking and Tuberculosis among Men Living with HIV in Johannesburg, South Africa: A Case-Control Study. PLoS ONE 2016, 11, e0167133. [Google Scholar] [CrossRef]
- Gordin, F.M.; Roediger, M.P.; Girard, P.M.; Lundgren, J.D.; Miro, J.M.; Palfreeman, A.; Rodriguez-Barradas, M.C.; Wolff, M.J.; Easterbrook, P.J.; Clezy, K.; et al. Pneumonia in HIV-infected persons: Increased risk with cigarette smoking and treatment interruption. Am. J. Respir. Crit. Care Med. 2008, 178, 630–636. [Google Scholar] [CrossRef]
- Valiathan, R.; Miguez, M.J.; Patel, B.; Arheart, K.L.; Asthana, D. Tobacco smoking increases immune activation and impairs T-cell function in HIV infected patients on antiretrovirals: A cross-sectional pilot study. PLoS ONE 2014, 9, e97698. [Google Scholar] [CrossRef]
- Pollack, T.M.; Duong, H.T.; Pham, T.T.; Do, C.D.; Colby, D. Cigarette smoking is associated with high HIV viral load among adults presenting for antiretroviral therapy in Vietnam. PLoS ONE 2017, 12, e0173534. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, Q.; Xie, M. Smoking increases the risk of infectious diseases: A narrative review. Tob. Induc. Dis. 2020, 18, 60. [Google Scholar] [CrossRef]
- McGeoch, L.J.; Ross, S.; Massa, M.S.; Lewington, S.; Clarke, R. Cigarette smoking and risk of severe infectious respiratory diseases in UK adults: 12-year follow-up of UK biobank. J. Public Health 2023, 45, e621–e629. [Google Scholar] [CrossRef]
- Kark, J.D.; Lebiush, M.; Rannon, L. Cigarette smoking as a risk factor for epidemic a(h1n1) influenza in young men. N. Engl. J. Med. 1982, 307, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.R.; Yao, W.; Dulin, H.; Castellanos, J.; Xu, D.; Hai, R. Modeling the effects of cigarette smoke extract on influenza B virus infections in mice. Front. Immunol. 2023, 14, 1083251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ninaber, D.K.; Faiz, A.; van der Linden, A.C.; van Schadewijk, A.; Lutter, R.; Hiemstra, P.S.; van der Does, A.M.; Ravi, A. Acute cigarette smoke exposure leads to higher viral infection in human bronchial epithelial cultures by altering interferon, glycolysis and GDF15-related pathways. Respir. Res. 2023, 24, 207. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Kivimäki, M.; Gale, C.R.; Batty, G.D. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: A community-based cohort study of 387,109 adults in UK. Brain Behav. Immun. 2020, 87, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, M.; De Marco, L.; Spadoni, J.L.; Tison, M.; Medina-Santos, R.; Labib, T.; Noirel, J.; Tamouza, R.; Limou, S.; Delaneau, O.; et al. The HLA-B*57:01 allele corresponds to a very large MHC haploblock likely explaining its massive effect for HIV-1 elite control. Front. Immunol. 2023, 14, 1305856. [Google Scholar] [CrossRef]
- Limou, S.; Le Clerc, S.; Coulonges, C.; Carpentier, W.; Dina, C.; Delaneau, O.; Labib, T.; Taing, L.; Sladek, R.; Deveau, C.; et al. Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 02). J. Infect. Dis. 2009, 199, 419–426. [Google Scholar] [CrossRef]
- Fellay, J.; Shianna, K.V.; Ge, D.; Colombo, S.; Ledergerber, B.; Weale, M.; Zhang, K.; Gumbs, C.; Castagna, A.; Cossarizza, A.; et al. A whole-genome association study of major determinants for host control of HIV-1. Science 2007, 317, 944–947. [Google Scholar] [CrossRef]
- Suhre, K.; Arnold, M.; Bhagwat, A.M.; Cotton, R.J.; Engelke, R.; Raffler, J.; Sarwath, H.; Thareja, G.; Wahl, A.; DeLisle, R.K.; et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat. Commun. 2017, 8, 14357. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Petersen, J.; Rossjohn, J.; Fugger, L. HLA variation and disease. Nat. Rev. Immunol. 2018, 18, 325–339. [Google Scholar] [CrossRef]
- Saaoud, F.; Shao, Y.; Cornwell, W.; Wang, H.; Rogers, T.J.; Yang, X. Cigarette Smoke Modulates Inflammation and Immunity via Reactive Oxygen Species-Regulated Trained Immunity and Trained Tolerance Mechanisms. Antioxid. Redox Signal. 2023, 38, 1041–1069. [Google Scholar] [CrossRef]
- Kerdidani, D.; Magkouta, S.; Chouvardas, P.; Karavana, V.; Glynos, K.; Roumelioti, F.; Zakynthinos, S.; Wauters, E.; Janssens, W.; Lambrechts, D.; et al. Cigarette Smoke-Induced Emphysema Exhausts Early Cytotoxic CD8+ T Cell Responses against Nascent Lung Cancer Cells. J. Immunol. 2018, 201, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, X.; Schmalen, A.; Haines, S.; Wolff, M.; Ma, H.; Zhang, H.; Stoleriu, M.G.; Nowak, J.; Nakayama, M.; et al. Antiviral CD8+ T-cell immune responses are impaired by cigarette smoke and in COPD. Eur. Respir. J. 2023, 62, 2201374. [Google Scholar] [CrossRef]
- Shao, Y.; Cornwell, W.; Xu, K.; Kirchhoff, A.; Saasoud, F.; Lu, Y.; Jiang, X.; Criner, G.J.; Wang, H.; Rogers, T.J.; et al. Chronic Exposure to the Combination of Cigarette Smoke and Morphine Decreases CD4+ Regulatory T Cell Numbers by Reprogramming the Treg Cell Transcriptome. Front. Immunol. 2022, 13, 887681. [Google Scholar] [CrossRef] [PubMed]
- Pinto, T.N.C.; da Silva, C.; Pinto, R.M.C.; da Silva Duarte, A.J.; Benard, G.; Fernandes, J.R. Tobacco exposure, but not aging, shifts the frequency of peripheral blood B cell subpopulations. Geroscience 2024, 46, 2729–2738. [Google Scholar] [CrossRef]
- Feldman, C.; Anderson, R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J. Infect. 2013, 67, 169–184. [Google Scholar] [CrossRef]
- Słomian, D.; Szyda, J.; Dobosz, P.; Stojak, J.; Michalska-Foryszewska, A.; Sypniewski, M.; Liu, J.; Kotlarz, K.; Suchocki, T.; Mroczek, M.; et al. Better safe than sorry-Whole-genome sequencing indicates that missense variants are significant in susceptibility to COVID-19. PLoS ONE 2023, 18, e0279356. [Google Scholar] [CrossRef]
- Ye, Z.; Vasco, D.A.; Carter, T.C.; Brilliant, M.H.; Schrodi, S.J.; Shukla, S.K. Genome wide association study of SNP-, gene-, and pathway-based approaches to identify genes influencing susceptibility to Staphylococcus aureus infections. Front. Genet. 2014, 5, 125. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Hromatka, B.S.; Kiefer, A.K.; Eriksson, N.; Noble, S.M.; Tung, J.Y.; Hinds, D.A. Genome-wide association and HLA region fine-mapping studies identify susceptibility loci for multiple common infections. Nat. Commun. 2017, 8, 599. [Google Scholar] [CrossRef]
- Buscetta, M.; Di Vincenzo, S.; Miele, M.; Badami, E.; Pace, E.; Cipollina, C. Cigarette smoke inhibits the NLRP3 inflammasome and leads to caspase-1 activation via the TLR4-TRIF-caspase-8 axis in human macrophages. Faseb J. 2020, 34, 1819–1832. [Google Scholar] [CrossRef]
- Buscetta, M.; Cristaldi, M.; Cimino, M.; La Mensa, A.; Dino, P.; Bucchieri, F.; Rappa, F.; Amato, S.; Aronica, T.S.; Pace, E.; et al. Cigarette smoke promotes inflammasome-independent activation of caspase-1 and -4 leading to gasdermin D cleavage in human macrophages. Faseb J. 2022, 36, e22525. [Google Scholar] [CrossRef]
- Pauwels, N.S.; Bracke, K.R.; Dupont, L.L.; Van Pottelberge, G.R.; Provoost, S.; Vanden Berghe, T.; Vandenabeele, P.; Lambrecht, B.N.; Joos, G.F.; Brusselle, G.G. Role of IL-1α and the Nlrp3/caspase-1/IL-1β axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur. Respir. J. 2011, 38, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Suhre, K. Genetic associations with ratios between protein levels detect new pQTLs and reveal protein-protein interactions. Cell Genom. 2024, 4, 100506. [Google Scholar] [CrossRef] [PubMed]
- Orrù, V.; Steri, M.; Sidore, C.; Marongiu, M.; Serra, V.; Olla, S.; Sole, G.; Lai, S.; Dei, M.; Mulas, A.; et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 2020, 52, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, D.; Bao, E.L.; Akbari, P.; Lareau, C.A.; Mousas, A.; Jiang, T.; Chen, M.H.; Raffield, L.M.; Tardaguila, M.; Huffman, J.E.; et al. The Polygenic and Monogenic Basis of Blood Traits and Diseases. Cell 2020, 182, 1214–1231.e1211. [Google Scholar] [CrossRef]
- Chen, M.H.; Raffield, L.M.; Mousas, A.; Sakaue, S.; Huffman, J.E.; Moscati, A.; Trivedi, B.; Jiang, T.; Akbari, P.; Vuckovic, D.; et al. Trans-ethnic and Ancestry-Specific Blood-Cell Genetics in 746,667 Individuals from 5 Global Populations. Cell 2020, 182, 1198–1213.e1114. [Google Scholar] [CrossRef]
- Bomsel, M.; Alfsen, A. Entry of viruses through the epithelial barrier: Pathogenic trickery. Nat. Rev. Mol. Cell Biol. 2003, 4, 57–68. [Google Scholar] [CrossRef]
- Bobardt, M.D.; Salmon, P.; Wang, L.; Esko, J.D.; Gabuzda, D.; Fiala, M.; Trono, D.; Van der Schueren, B.; David, G.; Gallay, P.A. Contribution of proteoglycans to human immunodeficiency virus type 1 brain invasion. J. Virol. 2004, 78, 6567–6584. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, F.; Maeyama, J.I.; Kubota, A.; Nishimune, A.; Horiguchi, S.; Takii, T.; Urasaki, Y.; Shimada, I.; Iho, S. Effect of cigarette smoke on mucosal vaccine response with activation of plasmacytoid dendritic cells: The outcomes of in vivo and in vitro experiments. Vaccine 2023, 41, 1447–1456. [Google Scholar] [CrossRef]
- Nelson, T.M.; Borgogna, J.C.; Michalek, R.D.; Roberts, D.W.; Rath, J.M.; Glover, E.D.; Ravel, J.; Shardell, M.D.; Yeoman, C.J.; Brotman, R.M. Cigarette smoking is associated with an altered vaginal tract metabolomic profile. Sci. Rep. 2018, 8, 852. [Google Scholar] [CrossRef]
- Ponomarova, I.G.; Lisyana, T.O.; Matyashova, O.I.; Krishchuk, S.U. The state of the microbiota of the genital tract in women who smoke. Med. Res. J. 2023, 8, 147–151. [Google Scholar] [CrossRef]


| Methods | Regression Coefficient (SE) | OR (95% CIs) | N of IVs | p | Q | p for Q |
|---|---|---|---|---|---|---|
| Inverse variance weighted | 1.756 (0.600) | 5.790 (1.785, 18.787) | 100 | 0.003 | 93.182 | 0.620 |
| MR Egger | 1.444 (1.462) | 4.237 (0.241, 74.448) | 100 | 0.326 | 93.127 | 0.646 |
| Weighted median | 1.928 (0.905) | 6.876 (1.166, 40.556) | 100 | 0.033 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.-R.; Hu, W.; Yan, S.; Qu, M.-M.; Jiao, Y.-M.; Wang, F.-S. The Relationship between Smoking and Susceptibility to HIV Infection: A Two-Sample Mendelian Randomization Analysis. Biomedicines 2024, 12, 2060. https://doi.org/10.3390/biomedicines12092060
Yu M-R, Hu W, Yan S, Qu M-M, Jiao Y-M, Wang F-S. The Relationship between Smoking and Susceptibility to HIV Infection: A Two-Sample Mendelian Randomization Analysis. Biomedicines. 2024; 12(9):2060. https://doi.org/10.3390/biomedicines12092060
Chicago/Turabian StyleYu, Min-Rui, Wei Hu, Song Yan, Meng-Meng Qu, Yan-Mei Jiao, and Fu-Sheng Wang. 2024. "The Relationship between Smoking and Susceptibility to HIV Infection: A Two-Sample Mendelian Randomization Analysis" Biomedicines 12, no. 9: 2060. https://doi.org/10.3390/biomedicines12092060
APA StyleYu, M.-R., Hu, W., Yan, S., Qu, M.-M., Jiao, Y.-M., & Wang, F.-S. (2024). The Relationship between Smoking and Susceptibility to HIV Infection: A Two-Sample Mendelian Randomization Analysis. Biomedicines, 12(9), 2060. https://doi.org/10.3390/biomedicines12092060






