Omega-3 Fatty Acids Modify Drp1 Expression and Activate the PINK1-Dependent Mitophagy Pathway in the Kidney and Heart of Adenine-Induced Uremic Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Western Blotting and Immunoprecipitation
2.3. Measurement of mtDNA Content
2.4. Statistics
3. Results
3.1. Laboratory Data
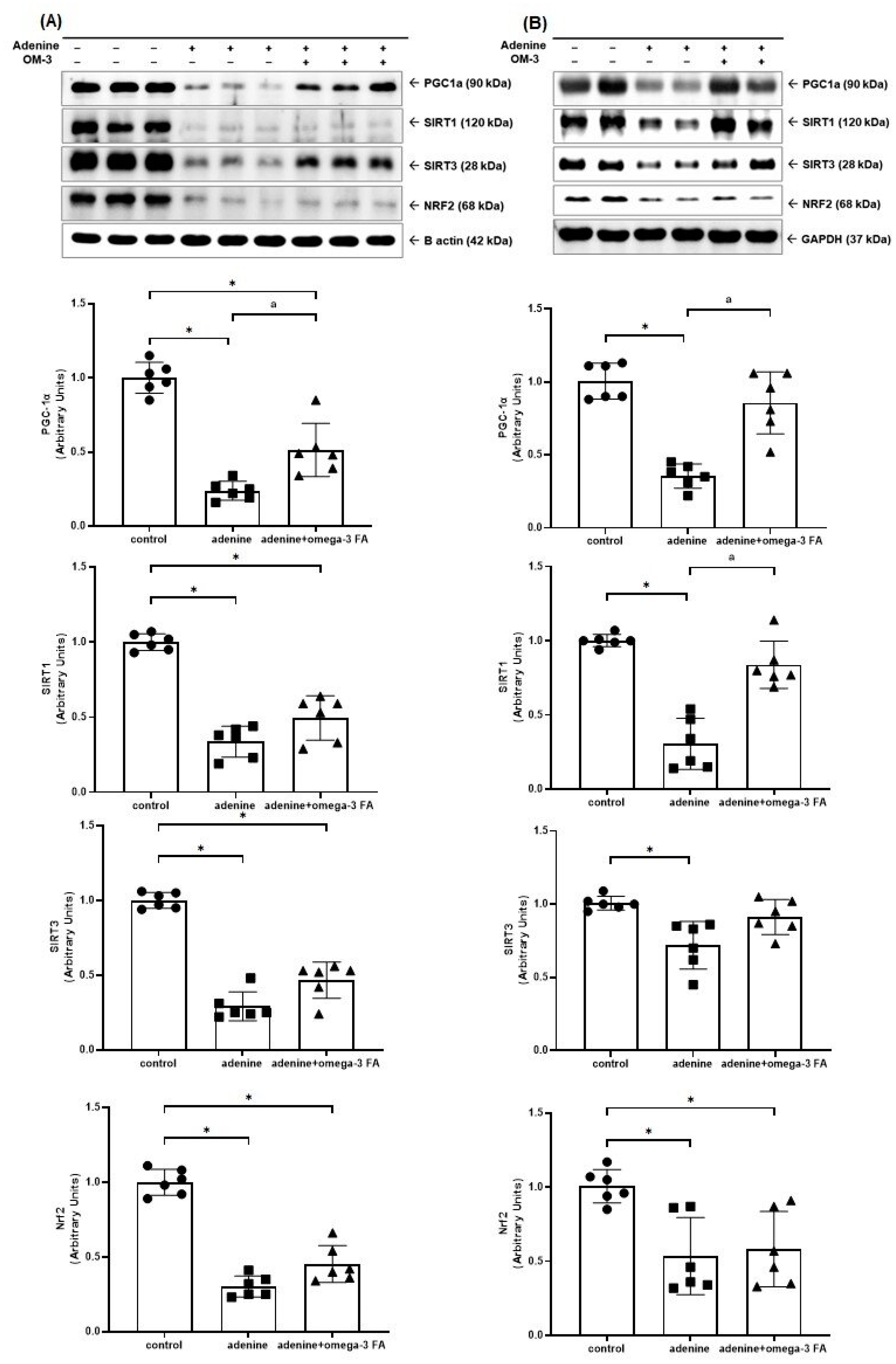
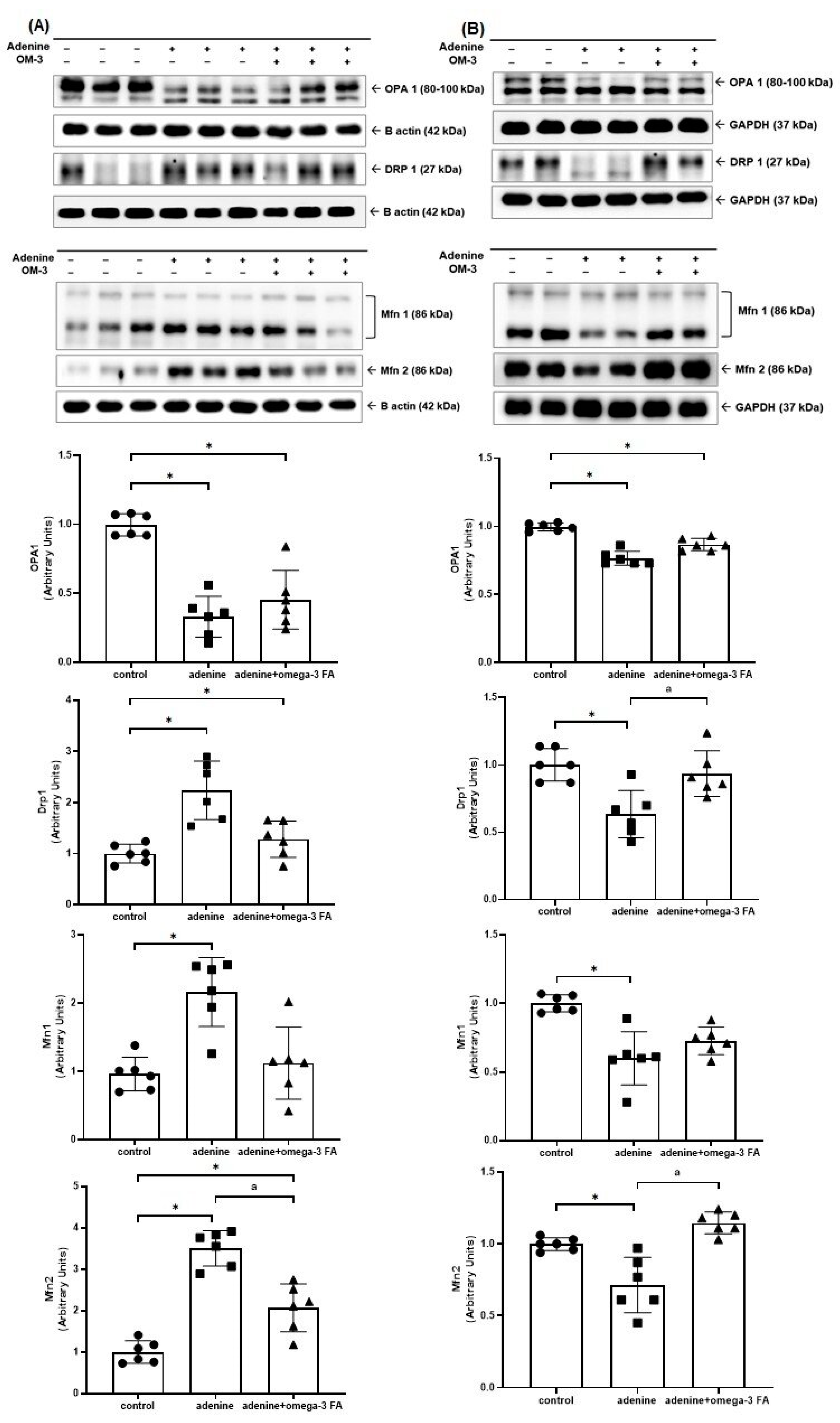
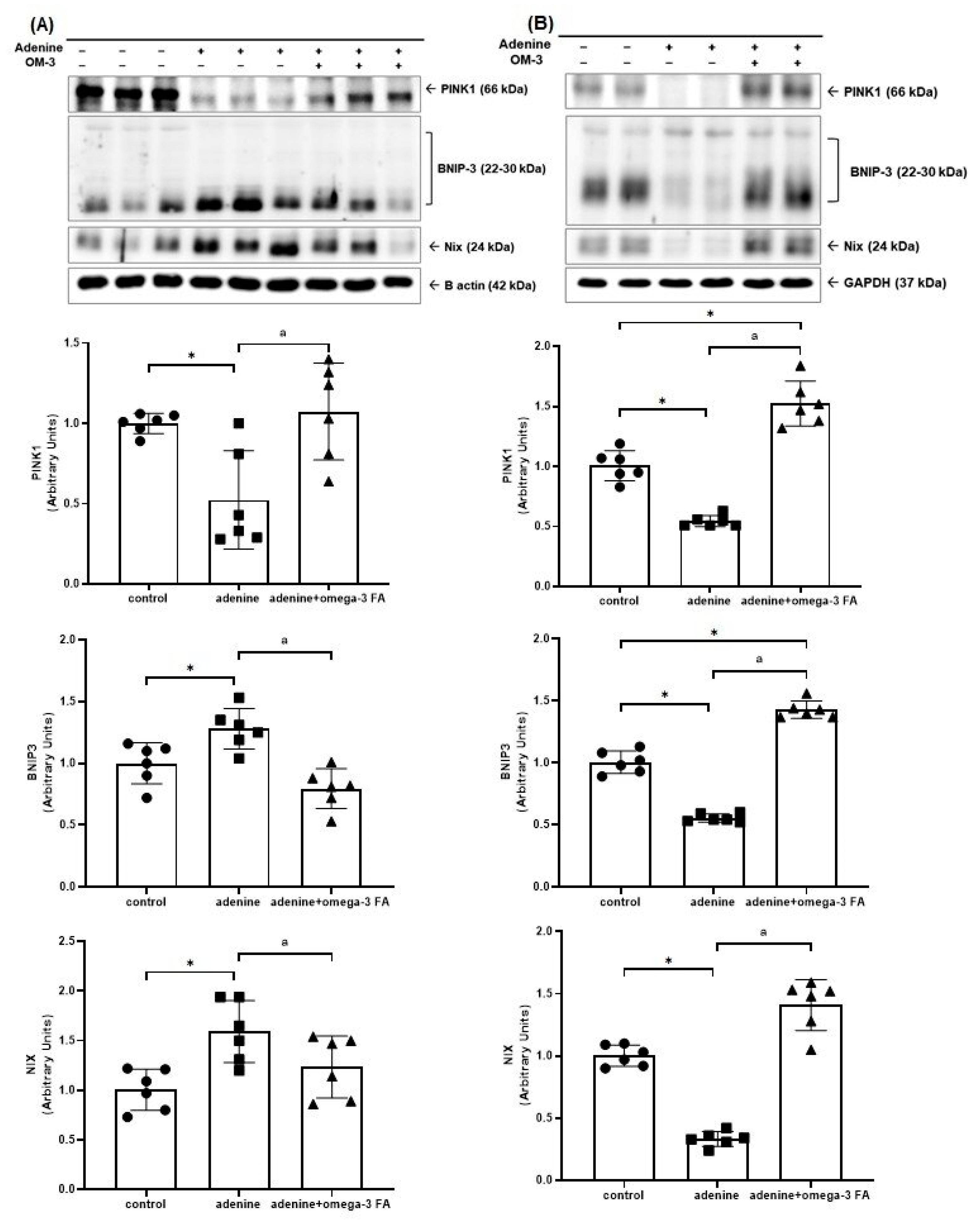
3.2. Changes in Factors Related to Mitochondrial Biogenesis, Dynamics, and Mitophagy
3.2.1. Mitochondrial Biogenesis-Related Molecules
3.2.2. Mitochondrial Fusion- and Fission-Related Molecules
3.2.3. Mitochondrial Mitophagy-Related Molecules
3.2.4. Content of Mitochondrial DNA (mtDNA)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2022. [Google Scholar]
- Carracedo, J.; Alique, M.; Vida, C.; Bodega, G.; Ceprian, N.; Morales, E.; Praga, M.; de Sequera, P.; Ramirez, R. Mechanisms of Cardiovascular Disorders in Patients With Chronic Kidney Disease: A Process Related to Accelerated Senescence. Front. Cell Dev. Biol. 2020, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Bohm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Testani, J.M. The kidney in heart failure: An update. Eur. Heart J. 2015, 36, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Hadjiphilippou, S.; Kon, S.P. Cardiorenal syndrome: Review of our current understanding. J. R. Soc. Med. 2016, 109, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Korea Disease Control and Prevention Agency. Korea Health Statistics 2022: Korea National Health and Nutrition Examination Survey (KNHANES IX-1); Korea Disease Control and Prevention Agency: Sejong, Republic of Korea, 2023. [Google Scholar]
- Lee, C.J.; Lee, H.; Yoon, M.; Chun, K.H.; Kong, M.G.; Jung, M.H.; Kim, I.C.; Cho, J.Y.; Kang, J.; Park, J.J.; et al. Heart Failure Statistics 2024 Update: A Report From the Korean Society of Heart Failure. Int. J. Heart Fail. 2024, 6, 56–69. [Google Scholar] [CrossRef]
- Duann, P.; Lin, P.H. Mitochondria Damage and Kidney Disease. Adv. Exp. Med. Biol. 2017, 982, 529–551. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Ivanova, E.A.; Sobenin, I.A.; Yet, S.F.; Orekhov, A.N. The Role of Mitochondria in Cardiovascular Diseases. Biology 2020, 9, 137. [Google Scholar] [CrossRef]
- Fontecha-Barriuso, M.; Martin-Sanchez, D.; Martinez-Moreno, J.M.; Monsalve, M.; Ramos, A.M.; Sanchez-Nino, M.D.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A.B. The Role of PGC-1alpha and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347. [Google Scholar] [CrossRef]
- Chen, L.; Qin, Y.; Liu, B.; Gao, M.; Li, A.; Li, X.; Gong, G. PGC-1alpha-Mediated Mitochondrial Quality Control: Molecular Mechanisms and Implications for Heart Failure. Front. Cell Dev. Biol. 2022, 10, 871357. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Zhou, F.; Wang, W.; Chen, N. PGC-1alpha ameliorates kidney fibrosis in mice with diabetic kidney disease through an antioxidative mechanism. Mol. Med. Rep. 2018, 17, 4490–4498. [Google Scholar] [CrossRef]
- Brooks, C.; Wei, Q.; Cho, S.G.; Dong, Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J. Clin. Investig. 2009, 119, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, M.; Xiong, L.; Fan, J.; Zhou, Y.; Li, H.; Peng, X.; Zhong, Z.; Wang, Y.; Huang, F.; et al. Drp1-mediated mitochondrial fission promotes renal fibroblast activation and fibrogenesis. Cell Death Dis. 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Shirakabe, A.; Maejima, Y.; Zhai, P.; Sciarretta, S.; Toli, J.; Nomura, M.; Mihara, K.; Egashira, K.; Ohishi, M.; et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 2015, 116, 264–278. [Google Scholar] [CrossRef]
- Bhatia, D.; Chung, K.P.; Nakahira, K.; Patino, E.; Rice, M.C.; Torres, L.K.; Muthukumar, T.; Choi, A.M.; Akchurin, O.M.; Choi, M.E. Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis. JCI Insight 2019, 4, e132826. [Google Scholar] [CrossRef]
- Li, S.; Lin, Q.; Shao, X.; Zhu, X.; Wu, J.; Wu, B.; Zhang, M.; Zhou, W.; Zhou, Y.; Jin, H.; et al. Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruction. Free. Radic. Biol. Med. 2020, 152, 632–649. [Google Scholar] [CrossRef]
- Henao Agudelo, J.S.; Baia, L.C.; Ormanji, M.S.; Santos, A.R.P.; Machado, J.R.; Saraiva Camara, N.O.; Navis, G.J.; de Borst, M.H.; Heilberg, I.P. Fish Oil Supplementation Reduces Inflammation but Does Not Restore Renal Function and Klotho Expression in an Adenine-Induced CKD Model. Nutrients 2018, 10, 1283. [Google Scholar] [CrossRef]
- Yamamoto, T.; Isaka, Y. Dietary Omega-3 Polyunsaturated Fatty Acids and Amelioration of CKD: Possible Cellular Mechanisms. Kidney360 2023, 4, 1661–1662. [Google Scholar] [CrossRef]
- Ong, K.L.; Marklund, M.; Huang, L.; Rye, K.A.; Hui, N.; Pan, X.F.; Rebholz, C.M.; Kim, H.; Steffen, L.M.; van Westing, A.C.; et al. Association of omega 3 polyunsaturated fatty acids with incident chronic kidney disease: Pooled analysis of 19 cohorts. BMJ 2023, 380, e072909. [Google Scholar] [CrossRef]
- Mason, R.P.; Libby, P.; Bhatt, D.L. Emerging Mechanisms of Cardiovascular Protection for the Omega-3 Fatty Acid Eicosapentaenoic Acid. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Lee, S.M.; Lee, M.H.; Son, Y.K.; Kim, S.E.; An, W.S. Omega-3 Fatty Acids Upregulate SIRT1/3, Activate PGC-1alpha via Deacetylation, and Induce Nrf1 Production in 5/6 Nephrectomy Rat Model. Mar. Drugs 2021, 19, 182. [Google Scholar] [CrossRef]
- Shirakabe, A.; Zhai, P.; Ikeda, Y.; Saito, T.; Maejima, Y.; Hsu, C.P.; Nomura, M.; Egashira, K.; Levine, B.; Sadoshima, J. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation 2016, 133, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.R.; Burke, N.; Dongworth, R.K.; Hausenloy, D.J. Mitochondrial fusion and fission proteins: Novel therapeutic targets for combating cardiovascular disease. Br. J. Pharmacol. 2014, 171, 1890–1906. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, N.; Eura, Y.; Mihara, K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J. Cell Sci. 2004, 117, 6535–6546. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.M.; Wang, Z.; Liesa, M.; Molina, A.; Havasi, A.; Schwartz, J.H.; Shirihai, O.; Borkan, S.C.; Bonegio, R.G. Role of mitofusin 2 in the renal stress response. PLoS ONE 2012, 7, e31074. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Y.; Mi, L.; Wang, X.; Li, L.; Gao, W. Mitofusin 2 inhibits angiotensin II-induced myocardial hypertrophy. J. Cardiovasc. Pharmacol. Ther. 2011, 16, 205–211. [Google Scholar] [CrossRef]
- Gao, S.; Hu, J. Mitochondrial Fusion: The Machineries In and Out. Trends Cell Biol. 2021, 31, 62–74. [Google Scholar] [CrossRef]
- Chen, L.; Gong, Q.; Stice, J.P.; Knowlton, A.A. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc. Res. 2009, 84, 91–99. [Google Scholar] [CrossRef]
- Huang, C.; Yi, H.; Shi, Y.; Cao, Q.; Shi, Y.; Cheng, D.; Braet, F.; Chen, X.M.; Pollock, C.A. KCa3.1 Mediates Dysregulation of Mitochondrial Quality Control in Diabetic Kidney Disease. Front. Cell Dev. Biol. 2021, 9, 573814. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Choi, M.E. The Emerging Role of Mitophagy in Kidney Diseases. J. Life Sci. 2019, 1, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, T.; Li, Z.; Liu, N.; Yan, Y.; Liu, B. Role of Mitophagy in Cardiovascular Disease. Aging Dis. 2020, 11, 419–437. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.W.; Zhao, G. The mitophagy pathway and its implications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.M.; Yang, Y.; He, L.; Tang, C.; Zhan, M.; Dong, Z. Mitochondrial function and disturbances in the septic kidney. Semin. Nephrol. 2015, 35, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, Y.; Inagi, R. Mitochondria: A therapeutic target in acute kidney injury. Nephrol. Dial. Transplant. 2016, 31, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W., 2nd. Mitochondrial pruning by Nix and BNip3: An essential function for cardiac-expressed death factors. J. Cardiovasc. Transl. Res. 2010, 3, 374–383. [Google Scholar] [CrossRef]
- Billia, F.; Hauck, L.; Konecny, F.; Rao, V.; Shen, J.; Mak, T.W. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc. Natl. Acad. Sci. USA 2011, 108, 9572–9577. [Google Scholar] [CrossRef]
- Bhandari, P.; Song, M.; Chen, Y.; Burelle, Y.; Dorn, G.W., 2nd. Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ. Res. 2014, 114, 257–265. [Google Scholar] [CrossRef]
- Wang, B.; Nie, J.; Wu, L.; Hu, Y.; Wen, Z.; Dong, L.; Zou, M.H.; Chen, C.; Wang, D.W. AMPKalpha2 Protects Against the Development of Heart Failure by Enhancing Mitophagy via PINK1 Phosphorylation. Circ. Res. 2018, 122, 712–729. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Zhang, J.; Ney, P.A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009, 16, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.J.; Wang, Z.Y.; Xu, L.; Chen, X.H.; Li, X.X.; Liao, W.T.; Ma, H.K.; Jiang, M.D.; Xu, T.T.; Xu, J.; et al. HIF-1alpha-BNIP3-mediated mitophagy in tubular cells protects against renal ischemia/reperfusion injury. Redox Biol. 2020, 36, 101671. [Google Scholar] [CrossRef]
- Schweers, R.L.; Zhang, J.; Randall, M.S.; Loyd, M.R.; Li, W.; Dorsey, F.C.; Kundu, M.; Opferman, J.T.; Cleveland, J.L.; Miller, J.L.; et al. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19500–19505. [Google Scholar] [CrossRef] [PubMed]
- Quinsay, M.N.; Thomas, R.L.; Lee, Y.; Gustafsson, A.B. Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophagy 2010, 6, 855–862. [Google Scholar] [CrossRef]
- Tran, M.; Tam, D.; Bardia, A.; Bhasin, M.; Rowe, G.C.; Kher, A.; Zsengeller, Z.K.; Akhavan-Sharif, M.R.; Khankin, E.V.; Saintgeniez, M.; et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Investig. 2011, 121, 4003–4014. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Andres, O.; Suarez-Alvarez, B.; Sanchez-Ramos, C.; Monsalve, M.; Sanchez-Nino, M.D.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A.; Sanz, A.B. The inflammatory cytokine TWEAK decreases PGC-1alpha expression and mitochondrial function in acute kidney injury. Kidney Int. 2016, 89, 399–410. [Google Scholar] [CrossRef]
- Tran, M.T.; Zsengeller, Z.K.; Berg, A.H.; Khankin, E.V.; Bhasin, M.K.; Kim, W.; Clish, C.B.; Stillman, I.E.; Karumanchi, S.A.; Rhee, E.P.; et al. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 2016, 531, 528–532. [Google Scholar] [CrossRef]
- Lee, M.S.; Shin, Y.; Moon, S.; Kim, S.; Kim, Y. Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Mitochondrial DNA Replication and PGC-1alpha Gene Expression in C(2)C(12) Muscle Cells. Prev. Nutr. Food Sci. 2016, 21, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Chen, Y.-C.; Tzeng, H.-P.; Chiang, M.-T. Fish oil enriched ω-3 fatty acids ameliorates protein synthesis/degradation imbalance, inflammation, and wasting in muscles of diet-induced obese rats. J. Funct. Foods 2021, 87, 104755. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, X.; Hu, X.; Fassett, J.; Zhu, G.; Tao, Y.; Li, J.; Huang, Y.; Zhang, P.; Zhao, B.; et al. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid. Redox Signal. 2010, 13, 1011–1022. [Google Scholar] [CrossRef]
- Bhat, S.; Chin, A.; Shirakabe, A.; Ikeda, Y.; Ikeda, S.; Zhai, P.; Hsu, C.P.; Sayed, D.; Abdellatif, M.; Byun, J.; et al. Recruitment of RNA Polymerase II to Metabolic Gene Promoters Is Inhibited in the Failing Heart Possibly Through PGC-1alpha (Peroxisome Proliferator-Activated Receptor-gamma Coactivator-1alpha) Dysregulation. Circ. Heart Fail. 2019, 12, e005529. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, B.; Li, Y.; Xu, X.; Lv, J.; Jia, Q.; Chai, R.; Xue, W.; Li, Y.; Wang, Y.; et al. Mitochondrial Dysfunction: An Emerging Link in the Pathophysiology of Cardiorenal Syndrome. Front. Cardiovasc. Med. 2022, 9, 837270. [Google Scholar] [CrossRef]
- Toko, H.; Morita, H.; Katakura, M.; Hashimoto, M.; Ko, T.; Bujo, S.; Adachi, Y.; Ueda, K.; Murakami, H.; Ishizuka, M.; et al. Omega-3 fatty acid prevents the development of heart failure by changing fatty acid composition in the heart. Sci. Rep. 2020, 10, 15553. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yang, M.; Lang, Y.; Lu, S.; Kong, Z.; Gao, Y.; Shen, N.; Zhang, D.; Lv, Z. Mitochondrial metabolic reprogramming in diabetic kidney disease. Cell Death Dis. 2024, 15, 442. [Google Scholar] [CrossRef] [PubMed]

| Normal Control (n = 6) | Adenine Control (n = 6) | Adenine with Omega-3 FA (n = 6) | p Value | |
|---|---|---|---|---|
| Blood Urea Nitrogen (mg/dL) | 10.2 ± 4.8 | 217.2 ± 161.5 * | 127.7 ± 124.4 | 0.011 |
| Creatinine (mg/dL) | 0.5 ± 0.1 | 6.2 ± 2.0 * | 5.1 ± 2.0 * | <0.001 |
| Calcium (mg/dL) | 11.4 ± 0.3 | 9.1 ± 1.2 * | 8.8 ± 1.7 * | 0.002 |
| Phosphorus (mg/dL) | 9.4 ± 1.3 | 34.6 ± 13.1 * | 28.5 ± 9.8 * | <0.001 |
| Kidney weight (mg)-to-body weight (g) ratio | 0.71 ± 0.05 | 3.42 ± 0.46 * | 3.54 ± 0.61 * | <0.001 |
| Heart weight (mg)-to-body weight (g) ratio | 0.33 ± 0.03 | 0.48 ± 0.05 * | 0.43 ± 0.04 * | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.H.; Lee, S.M.; Park, B.N.; Lee, M.H.; Yang, D.E.; Son, Y.K.; Kim, S.E.; An, W.S. Omega-3 Fatty Acids Modify Drp1 Expression and Activate the PINK1-Dependent Mitophagy Pathway in the Kidney and Heart of Adenine-Induced Uremic Rats. Biomedicines 2024, 12, 2107. https://doi.org/10.3390/biomedicines12092107
Choi DH, Lee SM, Park BN, Lee MH, Yang DE, Son YK, Kim SE, An WS. Omega-3 Fatty Acids Modify Drp1 Expression and Activate the PINK1-Dependent Mitophagy Pathway in the Kidney and Heart of Adenine-Induced Uremic Rats. Biomedicines. 2024; 12(9):2107. https://doi.org/10.3390/biomedicines12092107
Chicago/Turabian StyleChoi, Dong Ho, Su Mi Lee, Bin Na Park, Mi Hwa Lee, Dong Eun Yang, Young Ki Son, Seong Eun Kim, and Won Suk An. 2024. "Omega-3 Fatty Acids Modify Drp1 Expression and Activate the PINK1-Dependent Mitophagy Pathway in the Kidney and Heart of Adenine-Induced Uremic Rats" Biomedicines 12, no. 9: 2107. https://doi.org/10.3390/biomedicines12092107
APA StyleChoi, D. H., Lee, S. M., Park, B. N., Lee, M. H., Yang, D. E., Son, Y. K., Kim, S. E., & An, W. S. (2024). Omega-3 Fatty Acids Modify Drp1 Expression and Activate the PINK1-Dependent Mitophagy Pathway in the Kidney and Heart of Adenine-Induced Uremic Rats. Biomedicines, 12(9), 2107. https://doi.org/10.3390/biomedicines12092107






