Abstract
Background: An antidiabetic medication regimen is crucial for maintaining glycemic control. Type 2 diabetes mellitus (T2DM) and cognitive dysfunction have a bidirectional relationship. This study aims to explore the impact that adjusting antidiabetic medication regimens has on medication adherence, glycemic control, and cognitive function in patients with T2DM and mild cognitive impairment (MCI). Methods: This is an observational cross-sectional analysis that includes 364 consecutive inpatients with T2DM. Clinical data were collected, medication adherence was assessed using the Medication Adherence Report Scale (MARS-5), and cognitive status was evaluated using the Chinese version of the Montreal Cognitive Assessment (MoCA) and Mini-mental State Examination (MMSE). These data were obtained both during hospitalization and at a three-month follow-up. Multivariable logistic regression analysis was applied to determine the association between changes in medication regimens and medication adherence, glycemic control, and cognitive function. Results: Baseline medication adherence was high across all three different cognitive status groups, with no significant difference in MARS-5 scores. At the 3-month follow-up, the group with a high adjustment ratio of antidiabetic medication regimens showed an increase in their hemoglobin A1c (HbA1c) level compared to the baseline, while the group with a low adjustment ratio showed a decrease in this level. In addition, the MoCA, MMSE, and MARS-5 scores of the high-adjustment group were significantly lower than those of the low-adjustment group. Conclusions: A high ratio of medication adjustment was significantly associated with worse medication adherence and glycemic control in T2DM patients with MCI. Patients with a low ratio of medication adjustment had good adherence and better glycemic control. Clinicians should take cognitive status into account when adjusting antidiabetic regimens for T2DM patients and may need to provide additional guidance to patients with cognitive impairment to improve adherence and glycemic outcomes.
1. Introduction
The global prevalence of diabetes mellitus is rapidly increasing, with an estimated 783 million people expected to be affected worldwide by 2045 [1]. The primary therapeutic goal for individuals with diabetes is to achieve and maintain glycemic control and prevent diabetes-related complications, morbidity, and mortality [2]. Proper management through antidiabetic medications and recommended lifestyle changes is crucial for maintaining glycemic control and preventing diabetes-related complications [3]. As diabetes progresses, many patients often require ongoing adjustments to their medication regimens to achieve or maintain glycemic control, making medication adherence a critical aspect of diabetes management [4].
Cognitive dysfunctions, including mild cognitive impairment (MCI) and dementia (including conditions such as Alzheimer’s disease), are gaining increasing recognition as common complications of Type 2 diabetes mellitus (T2DM) [5,6,7]. Glycemic control in T2DM patients is closely related to cognitive function changes. Poor glycemic control is associated with cognitive decline [8,9], while cognitive impairment can make self-care and glycemic control more challenging [10]. Thus, stable glucose control is essential for T2DM patients with mild cognitive impairment.
Poor medication adherence can result in inadequate glycemic control, increased use of medical resources, higher medical costs, and significantly higher mortality [11,12,13]. Previous research has identified several factors that influence long-term medication adherence, including patient factors (such as age, education level, and depression), environmental factors (such as social support and socioeconomic status), and treatment regimen factors (such as drug burden, regimen complexity, side effects, required treatment duration, and administration time) [14,15]. Recent studies have primarily focused on the impact of medication regimen complexity on adherence and glycemic control [14,16,17]. Additionally, there is limited research on medication adherence in diabetes patients with cognitive impairment. Some studies have found no association between dementia and adherence to antidiabetic medications [18], while others have highlighted how negative beliefs about medications and regimen-related distress affect adherence and glycemic control in patients with diabetes and mild cognitive impairment (MCI) [19]. Currently, there is a lack of research on the impact of medication regimen adjustments on adherence, glycemic control, and cognitive status in T2DM patients with cognitive impairment. Understanding the relationship between medication regimen adjustments and adherence and glycemic control may help with the development of future interventions to improve treatment outcomes. This study aims to explore the impact of changes in antidiabetic medication regimens on medication adherence, glycemic control, and cognitive status in T2DM patients with MCI.
2. Materials and Methods
2.1. Study Design and Participants
A total of 521 patients with T2DM who were hospitalized at Tongji Hospital (Wuhan, China) between September 2023 and December 2023 were enrolled in this study. And 157 patients were excluded based on the exclusion criteria, leaving 364 patients included in this study. Baseline interviews assessed the patients’ cognition status and medication adherence to antidiabetic drugs upon admission, and follow-up interviews were conducted 3 months post-discharge. Exclusion criteria included (1) other types of diabetes; (2) age under 18 years; (3) acute cardiovascular and cerebrovascular events (such as cerebral infarction); (4) metabolic diseases that may have affected cognitive function in the last 3 months, such as severe hypoglycemia, diabetic ketoacidosis, diabetic hyperglycemia hypertonic coma, hypothyroidism, etc.; (5) combined with head trauma that might affect cognitive function; mental and neurological disorders such as depression, anxiety, delirium; severe lung or kidney diseases, history of heart failure, malignant tumors, etc.; (6) missing 3-month follow-up data.
A total of 194 T2DM patients with mild cognitive impairment were followed up for data on medication adherence, glycemic control, and cognitive status three months after their initial hospitalization.
The study was approved by the Ethics Committee of Tongji Hospital and was conducted according to the Declaration of Helsinki. Appropriate consent and assent were acquired from all participants.
2.2. Data Collection Procedure and Methods
Clinical and demographic data and diabetes-related information were obtained from Tongji Hospital’s hospitalization medical record system. The collected data included the patient’s sex, age, weight, height, education level, diabetes duration, any hypoglycemic events they had experienced in the last 3 months, any history of hyperlipidemia or hypertension, any history of smoking and drinking, coronary heart disease, or cerebrovascular disease, and details of antidiabetic medication regimens (including before hospitalization and at discharge, and the drugs types and dosages prescribed).
2.3. Glycemic Control
Hemoglobin A1c (HbA1c) values were retrieved from the patients’ medical records to determine the levels at the baseline interview and at the 3-month follow-up. Higher HbA1c levels indicate worse glycemic control.
2.4. Medication Adherence
Medication adherence was estimated by the Medication Adherence Report Scale (MARS-5). The MARS-5 was developed by Horne et al. [20] and has been widely used in studies on various chronic illnesses, including T2DM, hypertension, and asthma [21,22,23]. The MARS-5 has good reliability and validity and might be the most accurate self-report [24,25]. The score ranges from 5 to 25, and a higher MARS-5 score indicates higher self-reported adherence. In the study, a cut-off point of 90% [26] was used and the patients with total scores ≥ 23 were considered adherent, while those with scores of less than 23 were considered non-adherent [26].
2.5. Cognition Status
Cognition was assessed using the Chinese versions of the Montreal Cognitive Assessment (MoCA) and Mini-mental State Examination (MMSE), which are both valid and reliable in Chinese populations [27,28,29,30]. The scores of the MMSE and MoCA, respectively, range from 0 to 30; higher scores indicate better cognition. The MMSE score was bounded by 23/24 for dementia [31] and the MOCA score was bounded by 25/26 for MCI; 1 point was added to the total MoCA score for participants with 12 years of education or fewer [32,33].
2.6. Measures
Medication adherence and cognition status were assessed through interviews at admission and at a 3-month follow-up assessment.
2.6.1. The MoCA and MMSE Assessments Were Conducted as Follows
(1) The assessments took place in a dedicated, quiet room with necessary materials provided, and no clocks or calendars present.
(2) Each participant took the test face to face.
(3) A 5 min calming conversation preceded the assessment to help the participant relax.
(4) Each test item was attempted only once, with neutral feedback.
(5) The assessment lasted about 10 min with uniform instructions and adherence to “Scoring Criteria”.
2.6.2. MARS-5 Assessment
(1) Medication adherence was assessed based on the responses to five questions (e.g., “I forget to take my antidiabetic drugs”; “I alter the dose of my antidiabetic drugs”; “I stop taking my antidiabetic drugs for a while/sometimes”; “I decide to skip one of my antidiabetic drugs dosages”; “I use my antidiabetic drugs less than is prescribed”, using a 5-level response format (1—always, 2—often, 3—sometimes, 4—rarely, and 5—never).
(2) Scores ranged from 5 to 25, with higher scores indicating better adherence.
All assessments were performed by clinicians who are trained to carry out these tasks.
2.7. Adjustment Ratio of Medication
Adjustments in the antidiabetic medication regimen at discharge were quantified using the medication adjustment ratio, which was calculated as follows:
Adjustment ratio = (Number of Newly Added Antidiabetic Drug Types)/(Total Number of the Antidiabetic Drug Types at discharge) × 100%.
Ratios were categorized as low (0–33.3%), moderate (33.4–66.6%), and high (66.7–100%).
The adjustment of antidiabetic medications for all inpatients was a comprehensive evaluation performed by attending physicians with over 10 years of clinical experience, based on the latest diabetes treatment guidelines [34,35,36], clinical experience, and the patient’s blood glucose and HbA1c levels during hospitalization.
2.8. Statistical Analyses
Data are presented as the median with the interquartile range for non-normally distributed continuous variables or means with standard deviations (SD) for normally distributed continuous variables and as counts (percentages) for categorical variables. The modified Kolmogorov–Smirnov test was used to test the normal distribution of continuous variables. The Mann–Whitney U-test was used for the two-group comparison for non-normally distributed variables. The χ2 test or Fisher’s exact test was used to determine the categorical variables. The Kruskal–Wallis test was used to evaluate the differences between groups with different cognitive statuses or different ratios of antidiabetic medication adjustment. To identify the independent associated variable for non-adherence or worse glycemic control, we used a univariate logistic regression model to identify multiple variables. Then, we applied a multivariate stepwise logistic regression model to exclude confounding factors and identify independent factors that were significantly associated with medication non-adherence or poor glycemic control. After collinearity was excluded, the variables selected for inclusion in the multivariate stepwise model were sex, age, low education, HbA1c%, MoCA, insulin use, and the adjustment ratio of the medication regimen. Statistical analyses were performed using SPSS 26.0 software (IBM Corp, Armonk, NY, USA). p < 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of Study Population
The flow chart of our study is shown in Figure 1. Among the 521 inpatients diagnosed with T2DM, 157 patients were excluded according to the exclusion criteria and 364 patients were included in the study. The included participants were categorized into three groups based on their cognition status: normal cognition (78, 21.4%), mild cognitive impairment (194, 53.3%), or dementia (92, 25.3%). The baseline characteristics of the three groups are summarized in Table 1. Significant differences were observed among the groups concerning the patients’ age, gender, diabetes duration, hyperlipidemia, hypertension, education level, and HbA1c (all p < 0.05). Patients with worse cognitive status were older and had a longer duration of diabetes, lower education levels, and higher rates of hyperlipidemia and hypertension. No significant differences were found for BMI, hypoglycemic events, smoking, or drinking status (all p > 0.05). The MARS-5 scores were high across all groups (93.6% for normal, 96.9% for MCI, and 94.6% for dementia) with no significant difference (p = 0.412), indicating good medication adherence in all groups.
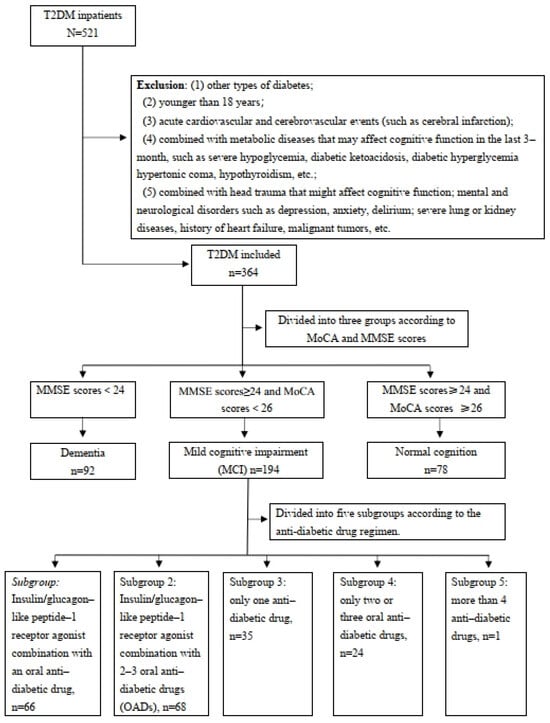
Figure 1.
Flow chart of the study population.

Table 1.
Baseline demographic, clinical, and laboratory characteristics of the type 2 diabetic patients categorized by cognition status (n = 364).
3.2. Characteristics of MCI Group after MedicationAdjustment
Since some MCI patients might revert to normal cognitive function, we examined changes in HbA1c, MoCA, MMSE, and MARS-5 scores at a 3-month follow-up post-medication change in T2DM patients with MCI. To assess the association between these factors and medication adjustment, MCI patients were divided into three groups based on the medication adjustment ratio at discharge (Table 2). No significant differences in age, sex, diabetes duration, hyperlipidemia, hypertension, history of smoking and drinking, and diabetes complications were observed among the groups (p > 0.05). Significant differences in baseline HbA1c levels were noted among the groups (p < 0.001), with higher adjustment ratios corresponding to higher baseline HbA1c levels. No significant differences were found in MoCA scores (p = 0.794), MMSE scores (p = 0.421), or education levels (p = 0.554). MARS-5 scores were similarly high across groups (96.0%, 98.0%, and 96.8%) with no significant difference (p = 0.844). At the 3-month follow-up, significant changes were observed in HbA1c, MoCA, MMSE, and MARS-5 scores across the groups. The high-ratio group exhibited a higher increase (0.4 (−0.6,0.6)) in HbA1c than the moderate-ratio group (0.1 (−0.5,0.5)), while the low-ratio group exhibited a decrease (−0.2 (−0.7,0.2)) in HbA1c. The MoCA, MMSE, and MARS-5 scores were significantly lower in the high-ratio group compared to the other two groups (all p < 0.05). To control for medication regimen differences, MCI patients were further subdivided based on glucose-lowering medication regimens. Analyses of subgroup1 and subgroup2 revealed similar changing trends in HbA1c, MoCA, and MARS-5 scores (Table 3). Analyses of subgroups 3 and 4 revealed no significant differences in cognitive or adherence scores (Supplementary Tables S1 and S2).

Table 2.
Characteristics of the MCI group categorized by adjustment ratio of antidiabetic drugs (n = 194).

Table 3.
Subgroup analysis of the MCI patients categorized by antidiabetic medication regimen.
3.3. Factors Influencing Medication Adherence
Logistic regression analysis was performed to identify factors associated with non-adherence in T2DM patients with MCI following medication changes. The baseline variables of MoCA score, Hb1Ac, level of education, insulin use, and adjustment ratio were included in the logistic regression, as these variables differed significantly among patients with different medication adherence (Figure 2). The MoCA score, Hb1Ac level, low education, insulin use, and medication adjustment ratio were related to the risk of non-adherence in T2DM patients with MCI in the univariate analysis (p < 0.05 for each) (Table 4), while multivariate analysis confirmed that the MoCA score (OR: 0.73, 95% CI: 0.62–0.87, p < 0.001) and medication adjustment ratio were independently associated with medication adherence after adjusting for age, sex, education, insulin use, and Hb1Ac level. There was a nearly 3-fold and 11-fold increased risk of non-adherence in patients with adjustment ratios of 33.4–66.6% (OR: 3.22, 95% CI: 1.01–10.24, p = 0.048) and adjustment ratios of 66.7–100% (OR: 10.90, 95% CI: 3.63–32.76, p < 0.001), respectively, compared with patients with adjustment ratios of 0–33.3%.
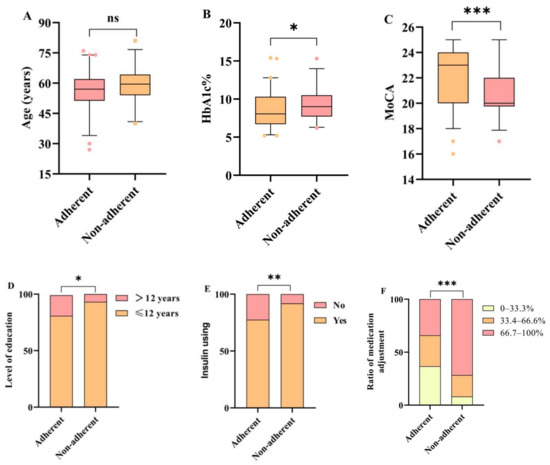
Figure 2.
Comparisons of baseline variables in two groups classified by level of medication adherence in T2DM patients with MCI. (A–F) Age, HbA1c%, MoCA, level of education, insulin using and ratio of medication adjustment in the two groups were assessed. Adherent—MARS-5 scores ≥ 23; Non-adherent—MARS-5 scores < 23. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant.

Table 4.
Logistic regression analysis of factors associated with medication non-adherence.
3.4. Factors Influencing Glycemic Control
Logistic regression analysis was conducted to identify factors affecting glycemic control in T2DM patients with MCI following medication changes. Patients were categorized into two groups based on ΔHbA1c values, using 0 as the cutoff value (Table 5). Univariate analysis indicated that higher HbA1c (OR: 0.87, 95% CI 0.76–0.99, p = 0.039) at baseline and a higher medication adjustment ratio were associated with worse glycemic control. Multivariate analysis demonstrated that HbA1c at baseline and the medication adjustment ratio remained significant predicators after adjusting for age, sex, and MoCA. Patients with adjustment ratios of 33.4–66.6% (OR: 2.70, 95% CI: 1.13–6.41, p = 0.025) and adjustment ratios of 66.7–100% (OR: 4.57, 95% CI: 2.01–10.40, p < 0.001) had nearly 3-fold and 4.5-fold increased risk of worse glycemic control, respectively, compared with patients with adjustment ratios of 0–33.3% after multiple adjustments.

Table 5.
Logistic regression analysis of factors associated with worse glycemic control.
4. Discussion
The current study focused on the impact of medication adjustments on glycemic control, cognitive status, and medication adherence in patients with Type 2 diabetes mellitus and mild cognitive impairment. The results indicated that a high ratio of adjustments to antidiabetic medications was associated with worse medication adherence, poorer glycemic control, and cognitive decline in patients with T2DM and mild cognitive impairment. Patients with a low ratio of medication adjustment had good adherence and better glycemic control.
More than half of the enrolled T2DM patients had MCI, and nearly a quarter had dementia, which was similar to our previous findings [37]. Consistent with previous studies, diabetic patients with more severe cognitive impairment were older, had a higher probability of hyperlipidemia and hypertension, and had a longer disease duration [38,39]. Consistent with previous studies, we also noticed that despite cognitive impairment, medication adherence remained high [19,40], possibly due to medication habits or support from family members.
Our finding suggested that higher medication adjustment ratios were associated with poorer medication adherence, worse glycemic control, and worse cognitive status in T2DM patients with MCI. The significant association between medication adjustment ratio and glycemic control observed in our study is a relatively novel finding. T2DM patients with high medication regimen complexity had poor medication adherence and this was associated with poor glycemic control [14]. The increased risk of worse glycemic control and worse cognitive status with higher medication adjustment ratios suggests that a high ratio of adjustments to the medication regimen may disrupt the stability of diabetes management, particularly in patients with MCI, highlighting the importance of cautious medication management and personalized treatment plans that consider the patient’s cognitive function.
Subgroup analyses were in line with previous findings [41] that patients receiving insulin were at higher risk of non-adherence to medication following a high ratio of medication regimen adjustments. This might be due to the fact that insulin regimens are generally more complex, requiring greater management skills, as well as greater family/social support. The need for insulin injections and the technical skill required for insulin administration may pose challenges for patients, particularly those with cognitive impairment. Simplifying insulin regimens and providing ongoing support might be necessary to improve adherence in patients with cognitive dysfunction.
Additionally, our results also suggested that the adjustment ratio of medication was a significant risk factor for non-adherence, a finding that is consistent with a recently published study about medication regimen complexity [14]. In diabetes treatment, in addition to factors related to the treatment regimen, many other factors affect medication adherence. This study showed that age, education level, baseline HbA1c levels, and insulin use were also identified as risk factors for adherence, while the MoCA score was a protective factor for adherence.
Changes in medication regimens also pose a risk for poor glycemic control. The association between an increased ratio of diabetes regimen adjustment and poor glycemic control suggests that a high ratio of modifications to glycemic regimens may exacerbate the disease burden in patients with diabetes without improving their glycemic outcomes. Similar previous studies support the finding that high diabetes-specific medication regimen complexity for antidiabetic agents is associated with poorer glycemic control, which is possibly related to decreased adherence [14,18].
Changes in medication regimens may lead to increased confusion or difficulty adhering to prescribed treatments for patients with T2DM and MCI. Additionally, poor medication adherence itself leads to worse glycemic control. Previous studies have reported that long-term diabetes and poor blood glucose control (including hyperglycemia, hypoglycemia, and glycemic fluctuations) can lead to neuronal damage and inflammation-related glial activation by disrupting the blood–brain barrier and altering the brain’s metabolism, resulting in progressive neuropathy and ultimately leading to cognitive impairment [42,43].
Our results highlight that considering cognitive impairment in T2DM patients is crucial for effective management. Cognitive impairment, such as MCI, can affect a patient’s ability to follow complex medication regimens. Therefore, clinicians should take into account the cognitive status of patients when making adjustments to antidiabetic medications. Meanwhile, the MARS-5 we used has been proven to have good validity and reliability in patients with diabetes [24,25]. However, our study also had several limitations. First, MARS-5 scores were used to assess medication adherence, due to the lack of more objective assessment methods to confirm medication adherence. Second, the relatively short follow-up period may have limited our ability to detect substantial changes in medication adherence, glycemic control, and MoCA scores between enrollment and follow-up. Third, some potential confounding variables that might influence medication adherence or glycemic control, such as socioeconomic status, other comorbidities, and their related medications, were not available for analysis. Finally, this is a single-center study with a single source of patients, and the patient’s clinical characteristics and hospital environment may not be representative of the broader population of T2DM patients with mild cognitive impairment. Admittedly, a 3-month follow-up with a single center may not reflect long-term glycemic control and cognitive function, but studies on how medication adjustments affect glycemic control, cognitive status, and medication adherence are lacking. Future research will require prospective multicenter studies with larger sample sizes to better investigate the long-term effects of adjusting antidiabetic medication regimens on diabetes management and cognitive function in patients with T2DM and mild cognitive impairment.
5. Conclusions
In summary, our study is the first to reveal a significant association between a high ratio of adjustment of the antidiabetic medication regimens and poor medication adherence, worse glycemic control, and decreased cognitive status in T2DM patients with mild cognitive impairment. This study offers valuable insights into the cognitive challenges faced by T2DM patients with mild cognitive impairment when it comes to managing their diabetes. For patients with a high ratio of adjustment in their medication regimen, more education and support are needed to improve medication adherence and glycemic control and to facilitate the recovery of cognitive function or delay cognitive decline.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biomedicines12092110/s1 Supplemental Table S1. Subgroup analysis of a single anti-diabetic drug in MCI patients (n = 35). Supplemental Table S2. Subgroup analysis of two or three oral anti-diabetic drugs in MCI patients (n = 24).
Author Contributions
X.S. (Xiaoqing Song), J.W., Y.Y., D.M. and J.T.: investigation, methodology, data curation, formal analysis, conceptualization, drafting, and revision of the manuscript. W.X., X.S. (Xiaoli Shi), K.D., M.L., X.C., Y.W., X.B., L.G. and X.Y.: patient enrollment, data curation, formal analysis, conceptualization, and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from 2023 Tongji Hospital Scientific Research Fund Project (grant number: 2023C06).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and has received ethical approval from the Medical Ethics Committee of Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology (August 2023, protocol code: TJ-IRB20230809).
Informed Consent Statement
Each participant signed written informed consent approved by the Medical Ethics Committee of Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, following the Declaration of Helsinki’s principles.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the Tongji Hospital Scientific Research Fund Project. We also thank all the doctors, nurses, technicians, and participating patients for their dedication to this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
HbA1c: hemoglobin A1c; MCI: mild cognitive impairment; MARS-5: Medication Adherence Report Scale; MMSE: Mini-mental State Examination; MoCA: Montreal Cognitive Assessment; T2DM: Type 2 diabetes mellitus.
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Magliano, D.J.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Arifulla, M.; John, L.J.; Sreedharan, J.; Muttappallymyalil, J.; Basha, S.A. Patients’ Adherence to Anti-Diabetic Medications in a Hospital at Ajman, UAE. Malays. J. Med. Sci. MJMS 2014, 21, 44–49. [Google Scholar] [PubMed]
- Doyle-Delgado, K.; Chamberlain, J.J.; Shubrook, J.H.; Skolnik, N.; Trujillo, J. Pharmacologic Approaches to Glycemic Treatment of Type 2 Diabetes: Synopsis of the 2020 American Diabetes Association’s Standards of Medical Care in Diabetes Clinical Guideline. Ann. Intern. Med. 2020, 173, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Sun, Y.; Heng, B.H.; Chew, D.E.K.; Chong, P. Medication adherence and glycemic control among newly diagnosed diabetes patients. BMJ Open Diabetes Res. Care 2017, 5, e429. [Google Scholar] [CrossRef]
- Biessels, G.J.; Nobili, F.; Teunissen, C.E.; Simó, R.; Scheltens, P. Understanding multifactorial brain changes in type 2 diabetes: A biomarker perspective. Lancet Neurol. 2020, 19, 699–710. [Google Scholar] [CrossRef]
- Battle, D.E. Diagnostic and Statistical Manual of Mental Disorders (DSM). Codas 2013, 25, 191–192. [Google Scholar]
- Hugo, J.; Ganguli, M. Dementia and cognitive impairment: Epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes-2021 Abridged for Primary Care Providers. Clin. Diabetes 2021, 39, 14–43. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 13. Older Adults: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45 (Suppl. S1), S195–S207. [Google Scholar]
- National Institute on Aging. Assessing Cognitive Impairment in Older Patients. In 2021: Get Practical Information and Tips for Assessing Patients with Memory Loss or Other Signs of Cognitive Impairment with Brief, Easy-to-Use Tools; National Institute on Aging: Bethesda, MD, USA, 2021. [Google Scholar]
- Evans, M.; Engberg, S.; Faurby, M.; Fernandes, J.D.D.R.; Hudson, P.; Polonsky, W. Adherence to and persistence with antidiabetic medications and associations with clinical and economic outcomes in people with type 2 diabetes mellitus: A systematic literature review. Diabetes Obes. Metab. 2022, 24, 377–390. [Google Scholar] [CrossRef]
- Shalaeva, E.V.; Bano, A.; Kasimov, U.; Janabaev, B.; Laimer, M.; Saner, H. Impact of Persistent Medication Adherence and Compliance with Lifestyle Recommendations on Major Cardiovascular Events and One-Year Mortality in Patients with Type 2 Diabetes and Advanced Stages of Atherosclerosis: Results From a Prospective Cohort Study. Glob. Heart 2023, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Egede, L.E.; Gebregziabher, M.; Echols, C.; Lynch, C.P. Longitudinal effects of medication nonadherence on glycemic control. Ann. Pharmacother. 2014, 48, 562–570. [Google Scholar] [CrossRef]
- Ayele, A.A.; Tegegn, H.G.; Ayele, T.A.; Ayalew, M.B. Medication regimen complexity and its impact on medication adherence and glycemic control among patients with type 2 diabetes mellitus in an Ethiopian general hospital. BMJ Open Diabetes Res. Care 2019, 7, e685. [Google Scholar] [CrossRef] [PubMed]
- Baghikar, S.; Benitez, A.; Fernandez Piñeros, P.; Gao, Y.; Baig, A.A. Factors Impacting Adherence to Diabetes Medication Among Urban, Low Income Mexican-Americans with Diabetes. J. Immigr. Minor. Health 2019, 21, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Ab Rahman, N.; Lim, M.T.; Thevendran, S.; Ahmad Hamdi, N.; Sivasampu, S. Medication Regimen Complexity and Medication Burden Among Patients With Type 2 Diabetes Mellitus: A Retrospective Analysis. Front. Pharmacol. 2022, 13, 808190. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.M.; Opsasnick, L.; Yoon, E.; Bailey, S.C.; O’Brien, M.; Wolf, M.S. Association between medication regimen complexity and glycemic control among patients with type 2 diabetes. J. Am. Pharm. Assoc. 2023, 63, 769–777. [Google Scholar] [CrossRef]
- Mirghani, H.; Aljohani, S.; Albalawi, A. Dementia and Adherence to Anti-Diabetic Medications: A Meta-Analysis. Cureus 2021, 13, e14611. [Google Scholar] [CrossRef]
- Rovner, B.W.; Casten, R.J. Health Beliefs and Medication Adherence in Black Patients with Diabetes and Mild Cognitive Impairment. Am. J. Geriatr. Psychiatry 2018, 26, 812–816. [Google Scholar] [CrossRef]
- Horne, R.; Weinman, J. Self-regulation and Self-management in Asthma: Exploring The Role of Illness Perceptions and Treatment Beliefs in Explaining Non-adherence to Preventer Medication. Psychol. Health 2002, 17, 17–32. [Google Scholar] [CrossRef]
- Elander, A.; Gustafsson, M. Inhaler Technique and Self-reported Adherence to Medications Among Hospitalised People with Asthma and COPD. Drugs Real World Outcomes 2020, 7, 317–323. [Google Scholar] [CrossRef]
- Kang, G.C.Y.; Koh, E.Y.L.; Tan, N.C. Prevalence and factors associated with adherence to anti-hypertensives among adults with hypertension in a developed Asian community: A cross-sectional study. Proc. Singap. Healthc. 2020, 29, 167–175. [Google Scholar] [CrossRef]
- Baah-Nyarkoh, E.; Alhassan, Y.; Dwomoh, A.K.; Kretchy, I.A. Medicated-related burden and adherence in patients with co-morbid type 2 diabetes mellitus and hypertension. Heliyon 2023, 9, e15448. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.H.Y.; Horne, R.; Hankins, M.; Chisari, C. The Medication Adherence Report Scale: A measurement tool for eliciting patients’ reports of nonadherence. Br. J. Clin. Pharmacol. 2020, 86, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, P.; Kasinopoulos, O.; Karashiali, C.; Georgiou, G.; Panayides, A.; Papageorgiou, A.; Wozniak, G.; Kassianos, A.P.; Karekla, M. A Scoping Review of Methods Used to Assess Medication Adherence in Patients with Chronic Conditions. Ann. Behav. Med. 2022, 56, 1201–1217. [Google Scholar] [CrossRef]
- Vluggen, S.; Hoving, C.; Schaper, N.C.; De Vries, H. Psychological predictors of adherence to oral hypoglycaemic agents: An application of the ProMAS questionnaire. Psychol. Health 2020, 35, 387–404. [Google Scholar] [CrossRef]
- Hawkins, M.A.; Gathright, E.C.; Gunstad, J.; Dolansky, M.A.; Redle, J.D.; Josephson, R.; Moore, S.M.; Hughes, J.W. The MoCA and MMSE as screeners for cognitive impairment in a heart failure population: A study with comprehensive neuropsychological testing. Heart Lung J. Crit. Care 2014, 43, 462–468. [Google Scholar] [CrossRef]
- Lim, M.Y.L.; Loo, J.H.Y. Screening an elderly hearing impaired population for mild cognitive impairment using Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). Int. J. Geriatr. Psychiatry 2018, 33, 972–979. [Google Scholar] [CrossRef]
- Katzman, R.; Zhang, M.; Wang, Z.; Liu, W.T.; Yu, E.; Wong, S.C.; Salmon, D.P.; Grant, I. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J. Clin. Epidemiol. 1988, 41, 971–978. [Google Scholar] [CrossRef]
- Lu, J.; Li, D.; Li, F.; Zhou, A.; Wang, F.; Zuo, X.; Jia, X.-F.; Song, H.; Jia, J. Montreal cognitive assessment in detecting cognitive impairment in Chinese elderly individuals: A population-based study. J. Geriatr. Psychiatry Neurol. 2011, 24, 184–190. [Google Scholar] [CrossRef]
- Moyer, V.A. Screening for cognitive impairment in older adults: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 2014, 160, 791–797. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.K.F.; Chan, J.Y.C.; Hirai, H.W.; Wong, S.Y.S.; Kwok, T.C.Y. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2015, 175, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Care in Diabetes-2023 Abridged for Primary Care Providers. Clin. Diabetes. 2022, 41, 4–31, Erratum in Clin. Diabetes 2023, 41, 328. https://doi.org/10.2337/cd23-er02a. [Google Scholar] [CrossRef]
- Chinese Diabetes Society. Guideline for the Prevention and Treatment of Type 2 Diabetes Mellitus in China (2020 edition). Chin. Diabetes Mellit. 2021, 13, 315–409. [Google Scholar] [CrossRef]
- Chinese Society of Endocrinology, The Blood Pressure Control Target in Diabetes (BPROAD) Research Group. Chinese Expert Consensus on the Prevention and Management of Cognitive Impairment in Patients with Type 2 Diabetes Mellitus. Chin. J. Endocrinol. Metab. 2022, 38, 453–464. [Google Scholar]
- Xia, S.; Xia, W.; Huang, J.; Zou, H.; Tao, J.; Yang, Y. The factors contributing to cognitive dysfunction in type 2 diabetic patients. Ann. Transl. Med. 2020, 8, 104. [Google Scholar] [CrossRef]
- Gómez-Gómez, M.E.; Zapico, S.C. Frailty, Cognitive Decline, Neurodegenerative Diseases and Nutrition Interventions. Int. J. Mol. Sci. 2019, 20, 2842. [Google Scholar] [CrossRef]
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Zhu, M.; Jiao, H.; Qiu, Q.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef]
- Jacob, L.; Adam-Schnepf, L.; Kostev, K. Persistence With Oral Antihyperglycemic Drugs in Type 2 Diabetes Mellitus Patients With Dementia in Germany. J. Diabetes Sci. Technol. 2018, 12, 140–144. [Google Scholar] [CrossRef]
- Mendes, R.; Martins, S.; Fernandes, L. Adherence to Medication, Physical Activity and Diet in Older Adults With Diabetes: Its Association With Cognition, Anxiety and Depression. J. Clin. Med. Res. 2019, 11, 583–592. [Google Scholar] [CrossRef]
- Hsieh, C.F.; Liu, C.K.; Lee, C.T.; Yu, L.E.; Wang, J.Y. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci. Rep. 2019, 9, 840. [Google Scholar] [CrossRef]
- Kawamura, T.; Umemura, T.; Hotta, N. Cognitive impairment in diabetic patients: Can diabetic control prevent cognitive decline? J. Diabetes Investig. 2012, 3, 413–423. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).