The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure?
Abstract
:1. Introduction
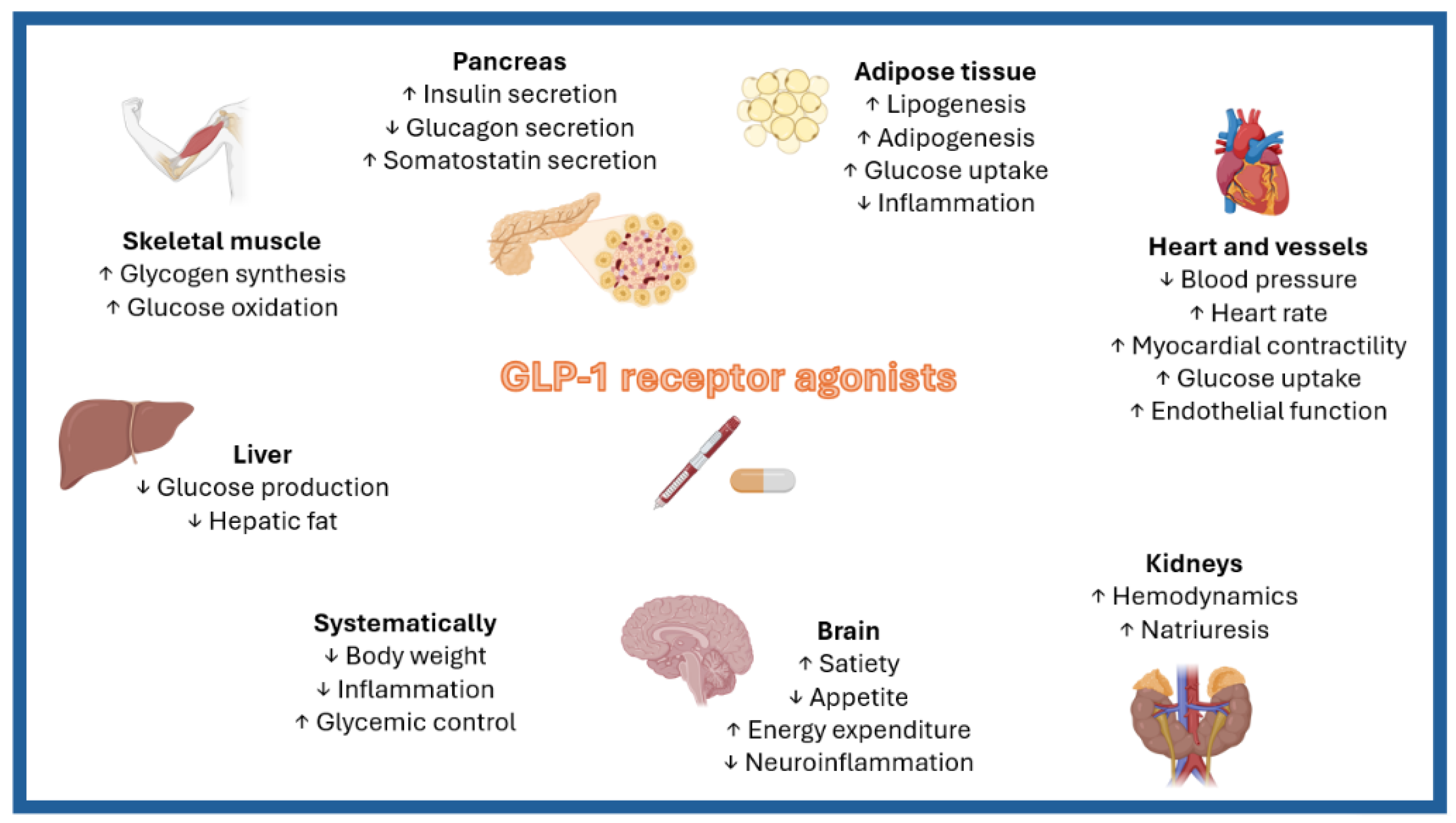
2. Potential Mechanisms Underlying the Effects of GLP-1RAs on Obesity-Related HFpEF
2.1. GLP-1RAs and Epicardial Adipose Tissue
2.2. GLP-1RAs, Renal Function, and Diuretic Resistance
2.3. GLP-1RAs and the Renin–Angiotensin System
2.4. GLP-1RAs and Microvascular Dysfunction
2.5. GLP-1RAs and Atrial Fibrillation
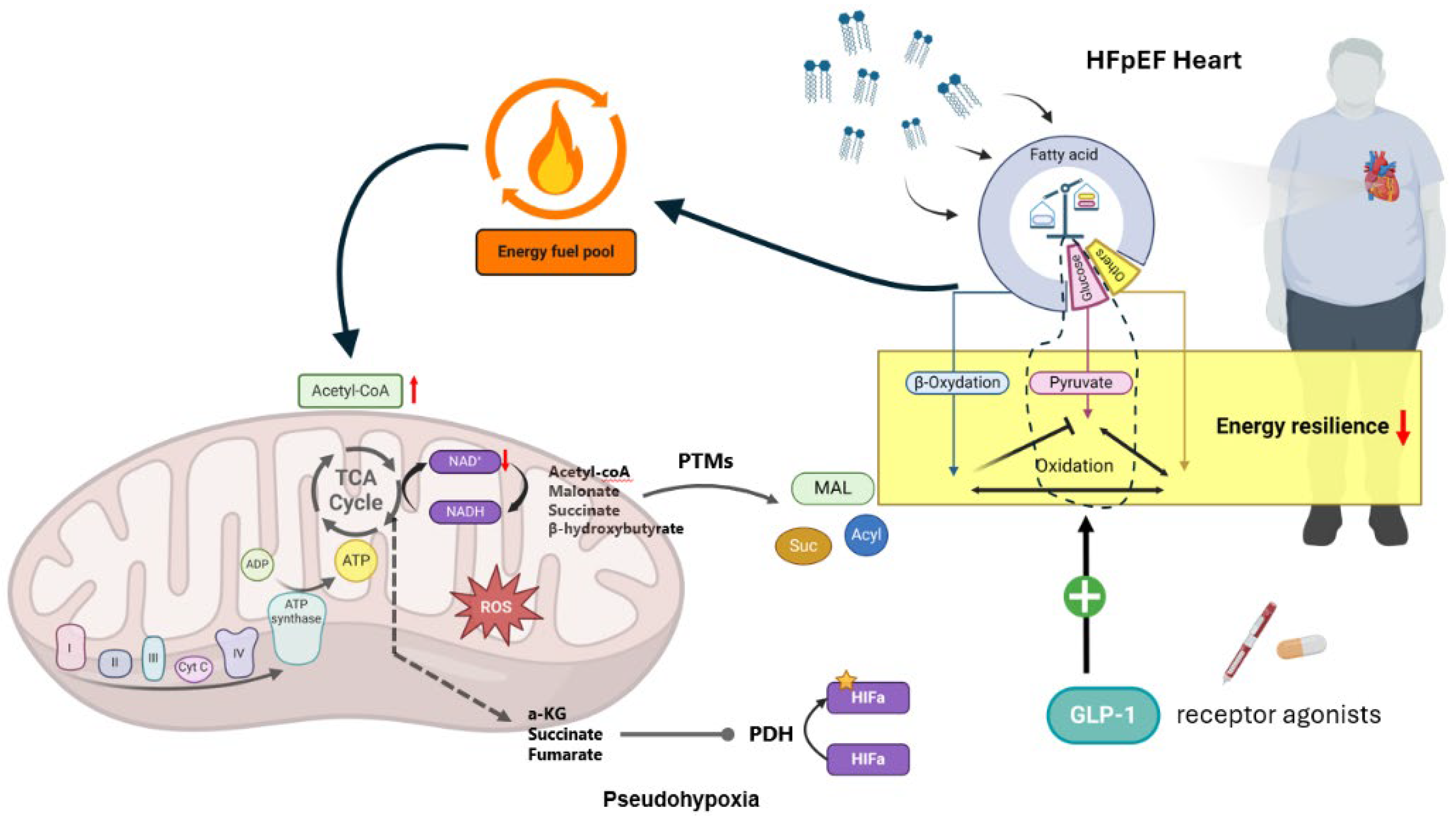
2.6. GLP-1RAs and Myocardial Energetics
3. GLP-1RAs and Clinical Outcomes in Obesity-Related HFpEF
3.1. GLP-1RAs and Clinical Outcomes in Obesity-Related HFpEF in Patients without Diabetes
3.2. GLP-1RAs and Clinical Outcomes in Obesity-Related HFpEF in Patients with Diabetes
3.3. GLP-1RAs and Clinical Outcomes in Obesity-Related HFpEF Irrespective of T2DM Status
3.3.1. Effect of GLP-1RAs According to Baseline Diuretic Use and Diuretic Resistance
3.3.2. Efficacy of GLP-1RAs by Sex in Obesity-Related HFpEF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mensah, G.A.; Fuster, V.; Murray, C.J.L.; Roth, G.A.; Mensah, G.A.; Abate, Y.H.; Abbasian, M.; Abd-Allah, F.; Abdollahi, A.; Abdollahi, M.; et al. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, E153–E639. [Google Scholar] [CrossRef] [PubMed]
- Kapelios, C.J.; Shahim, B.; Lund, L.H.; Savarese, G. Epidemiology, Clinical Characteristics and Cause-Specific Outcomes in Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2023, 9, e14. [Google Scholar] [CrossRef]
- Tsao, C.W.; Lyass, A.; Enserro, D.; Larson, M.G.; Ho, J.E.; Kizer, J.R.; Gottdiener, J.S.; Psaty, B.M.; Vasan, R.S. Temporal Trends in the Incidence of and Mortality Associated with Heart Failure with Preserved and Reduced Ejection Fraction. JACC. Heart Fail. 2018, 6, 678–685. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of Heart Failure with Preserved Ejection Fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Ng, A.C.T.; Delgado, V.; Borlaug, B.A.; Bax, J.J. Diabesity: The Combined Burden of Obesity and Diabetes on Heart Disease and the Role of Imaging. Nat. Rev. Cardiol. 2021, 18, 291–304. [Google Scholar] [CrossRef]
- Global Burden and Strength of Evidence for 88 Risk Factors in 204 Countries and 811 Subnational Locations, 1990-2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [CrossRef]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L.; Projected, U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- Rao, V.N.; Zhao, D.; Allison, M.A.; Guallar, E.; Sharma, K.; Criqui, M.H.; Cushman, M.; Blumenthal, R.S.; Michos, E.D. Adiposity and Incident Heart Failure and Its Subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC. Heart Fail. 2018, 6, 999–1007. [Google Scholar] [CrossRef]
- Pandey, A.; LaMonte, M.; Klein, L.; Ayers, C.; Psaty, B.M.; Eaton, C.B.; Allen, N.B.; de Lemos, J.A.; Carnethon, M.; Greenland, P.; et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1129–1142. [Google Scholar] [CrossRef]
- Brouwers, F.P.; de Boer, R.A.; van der Harst, P.; Voors, A.A.; Gansevoort, R.T.; Bakker, S.J.; Hillege, H.L.; van Veldhuisen, D.J.; van Gilst, W.H. Incidence and Epidemiology of New Onset Heart Failure with Preserved vs. Reduced Ejection Fraction in a Community-Based Cohort: 11-Year Follow-up of PREVEND. Eur. Heart J. 2013, 34, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Lyass, A.; Lee, D.S.; Vasan, R.S.; Kannel, W.B.; Larson, M.G.; Levy, D. Predictors of New-Onset Heart Failure: Differences in Preserved versus Reduced Ejection Fraction. Circ. Heart Fail. 2013, 6, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Matsushita, K.; Lazo, M.; Bello, N.; Blumenthal, R.S.; Gerstenblith, G.; Nambi, V.; Ballantyne, C.M.; Solomon, S.D.; Selvin, E.; et al. Obesity and Subtypes of Incident Cardiovascular Disease. J. Am. Heart Assoc. 2016, 5, e003921. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Sorimachi, H.; Jarolim, P.; Borlaug, B.A. Uncoupling between Intravascular and Distending Pressures Leads to Underestimation of Circulatory Congestion in Obesity. Eur. J. Heart Fail. 2022, 24, 353–361. [Google Scholar] [CrossRef]
- Clemenza, F.; Citarrella, R.; Patti, A.; Rizzo, M. Obesity and HFpEF. J. Clin. Med. 2022, 11, 3858. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Omote, K.; Reddy, Y.N.V.; Sorimachi, H.; Obokata, M.; Borlaug, B.A. Heart Failure with Preserved Ejection Fraction in Patients with Normal Natriuretic Peptide Levels Is Associated with Increased Morbidity and Mortality. Eur. Heart J. 2022, 43, 1941–1951. [Google Scholar] [CrossRef]
- Kitzman, D.W.; Lam, C.S.P. Obese Heart Failure with Preserved Ejection Fraction Phenotype: From Pariah to Central Player. Circulation 2017, 136, 20–23. [Google Scholar] [CrossRef]
- Anker, S.D.; Usman, M.S.; Anker, M.S.; Butler, J.; Böhm, M.; Abraham, W.T.; Adamo, M.; Chopra, V.K.; Cicoira, M.; Cosentino, F.; et al. Patient Phenotype Profiling in Heart Failure with Preserved Ejection Fraction to Guide Therapeutic Decision Making. A Scientific Statement of the Heart Failure Association, the European Heart Rhythm Association of the European Society of Cardiology, And. Eur. J. Heart Fail. 2023, 25, 936–955. [Google Scholar] [CrossRef]
- Karakasis, P.; Pamporis, K.; Stachteas, P.; Patoulias, D.; Bougioukas, K.I.; Fragakis, N. Efficacy and Safety of Sodium-Glucose Cotransporter-2 Inhibitors in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: An Overview of 36 Systematic Reviews. Heart Fail. Rev. 2023, 28, 1033–1051. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Giannakoulas, G.; Rosenkranz, S.; Fragakis, N. Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Pulmonary Arterial Wedge Pressure. Eur. J. Intern. Med. 2024, 124, 147–149. [Google Scholar] [CrossRef]
- Stachteas, P.; Nasoufidou, A.; Patoulias, D.; Karakasis, P.; Karagiannidis, E.; Mourtzos, M.-A.; Samaras, A.; Apostolidou, X.; Fragakis, N. The Role of Sodium-Glucose Co-Transporter-2 Inhibitors on Diuretic Resistance in Heart Failure. Int. J. Mol. Sci. 2024, 25, 3122. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, N.; Nikolaou, P.E.; Karakasis, P.; Stachteas, P.; Fragakis, N.; Andreadou, I. Endothelial Protection by Sodium-Glucose Cotransporter 2 Inhibitors: A Literature Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2024, 25, 7274. [Google Scholar] [CrossRef]
- Patoulias, D.; Fragakis, N.; Rizzo, M. The Therapeutic Role of SGLT-2 Inhibitors in Acute Heart Failure: From Pathophysiologic Mechanisms to Clinical Evidence with Pooled Analysis of Relevant Studies across Safety and Efficacy Endpoints of Interest. Life 2022, 12, 2062. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J. Card. Fail. 2022, 28, e1–e167. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Karagiannis, T.; Malandris, K.; Avgerinos, I.; Stamati, A.; Kakotrichi, P.; Liakos, A.; Vasilakou, D.; Kakaletsis, N.; Tsapas, A.; Bekiari, E. Subcutaneously Administered Tirzepatide vs Semaglutide for Adults with Type 2 Diabetes: A Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. Diabetologia 2024, 67, 1206–1222. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Pamporis, K.; Stachteas, P.; Bougioukas, K.I.; Klisic, A.; Fragakis, N.; Rizzo, M. Safety and Efficacy of the New, Oral, Small-Molecule, GLP-1 Receptor Agonists Orforglipron and Danuglipron for the Treatment of Type 2 Diabetes and Obesity: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Metabolism 2023, 149, 155710. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Klisic, A.; Rizzo, M. Effect of Tirzepatide on Albuminuria Levels and Renal Function in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Multilevel Meta-Analysis. Diabetes. Obes. Metab. 2024, 26, 1090–1104. [Google Scholar] [CrossRef]
- Karakasis, P.; Patoulias, D.; Fragakis, N.; Bernal-López, M.R.; Gómez-Huelgas, R. Glucagon-like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors Combination Therapy versus Monotherapy and Major Adverse Cardiovascular Events: Do the Benefits Add Up? Eur. J. Intern. Med. 2024. [Google Scholar] [CrossRef]
- Patoulias, D.; Karakasis, P.; El-Tanani, M.; Rizzo, M. Can Tirzepatide Untie the Gordian Knot of Eating Disorders among Individuals with Type 2 Diabetes and Obesity? J. Diabetes Complicat. 2024, 38, 108812. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, A.; Li, D.; Wu, Y.; Wang, C.-Z.; Wan, J.-Y.; Yuan, C.-S. Comparative Effectiveness of GLP-1 Receptor Agonists on Glycaemic Control, Body Weight, and Lipid Profile for Type 2 Diabetes: Systematic Review and Network Meta-Analysis. BMJ 2024, 384, e076410. [Google Scholar] [CrossRef] [PubMed]
- de Mesquita, Y.L.L.; Pera Calvi, I.; Reis Marques, I.; Almeida Cruz, S.; Padrao, E.M.H.; de Carvalho, P.E.P.; da Silva, C.H.A.; Cardoso, R.; Moura, F.A.; Rafalskiy, V.V. Efficacy and Safety of the Dual GIP and GLP-1 Receptor Agonist Tirzepatide for Weight Loss: A Meta-Analysis of Randomized Controlled Trials. Int. J. Obes. 2023, 47, 883–892. [Google Scholar] [CrossRef]
- Muzurović, E.M.; Volčanšek, Š.; Tomšić, K.Z.; Janež, A.; Mikhailidis, D.P.; Rizzo, M.; Mantzoros, C.S. Glucagon-Like Peptide-1 Receptor Agonists and Dual Glucose-Dependent Insulinotropic Polypeptide/Glucagon-Like Peptide-1 Receptor Agonists in the Treatment of Obesity/Metabolic Syndrome, Prediabetes/Diabetes and Non-Alcoholic Fatty Liver Disease-Current, E.J. Cardiovasc. Pharmacol. Ther. 2022, 27, 10742484221146372. [Google Scholar] [CrossRef]
- Jackson, A.M.; Rørth, R.; Liu, J.; Kristensen, S.L.; Anand, I.S.; Claggett, B.L.; Cleland, J.G.F.; Chopra, V.K.; Desai, A.S.; Ge, J.; et al. Diabetes and Pre-Diabetes in Patients with Heart Failure and Preserved Ejection Fraction. Eur. J. Heart Fail. 2022, 24, 497–509. [Google Scholar] [CrossRef]
- Sorimachi, H.; Omote, K.; Omar, M.; Popovic, D.; Verbrugge, F.H.; Reddy, Y.N.V.; Lin, G.; Obokata, M.; Miles, J.M.; Jensen, M.D.; et al. Sex and Central Obesity in Heart Failure with Preserved Ejection Fraction. Eur. J. Heart Fail. 2022, 24, 1359–1370. [Google Scholar] [CrossRef]
- Obokata, M.; Reddy, Y.N.V.; Pislaru, S.V.; Melenovsky, V.; Borlaug, B.A. Evidence Supporting the Existence of a Distinct Obese Phenotype of Heart Failure with Preserved Ejection Fraction. Circulation 2017, 136, 6–19. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Jensen, M.D.; Kitzman, D.W.; Lam, C.S.P.; Obokata, M.; Rider, O.J. Obesity and Heart Failure with Preserved Ejection Fraction: New Insights and Pathophysiological Targets. Cardiovasc. Res. 2023, 118, 3434–3450. [Google Scholar] [CrossRef]
- Janez, A.; Muzurovic, E.; Bogdanski, P.; Czupryniak, L.; Fabryova, L.; Fras, Z.; Guja, C.; Haluzik, M.; Kempler, P.; Lalic, N.; et al. Modern Management of Cardiometabolic Continuum: From Overweight/Obesity to Prediabetes/Type 2 Diabetes Mellitus. Recommendations from the Eastern and Southern Europe Diabetes and Obesity Expert Group. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2024, 15, 1865–1892. [Google Scholar] [CrossRef]
- Benn, M.; Marott, S.C.W.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Obesity Increases Heart Failure Incidence and Mortality: Observational and Mendelian Randomization Studies Totalling over 1 Million Individuals. Cardiovasc. Res. 2023, 118, 3576–3585. [Google Scholar] [CrossRef]
- Haass, M.; Kitzman, D.W.; Anand, I.S.; Miller, A.; Zile, M.R.; Massie, B.M.; Carson, P.E. Body Mass Index and Adverse Cardiovascular Outcomes in Heart Failure Patients with Preserved Ejection Fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) Trial. Circ. Heart Fail. 2011, 4, 324–331. [Google Scholar] [CrossRef]
- Ather, S.; Chan, W.; Bozkurt, B.; Aguilar, D.; Ramasubbu, K.; Zachariah, A.A.; Wehrens, X.H.T.; Deswal, A. Impact of Noncardiac Comorbidities on Morbidity and Mortality in a Predominantly Male Population with Heart Failure and Preserved versus Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2012, 59, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Ngwa, J.; Kebede, S.; Lu, D.; Schulte, P.J.; Bhatt, D.L.; Yancy, C.; Fonarow, G.C.; Albert, M.A. Impact of Body Mass Index on Heart Failure by Race/Ethnicity from the Get with The Guidelines-Heart Failure (GWTG-HF) Registry. JACC. Heart Fail. 2018, 6, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Iorio, A.; Senni, M.; Barbati, G.; Greene, S.J.; Poli, S.; Zambon, E.; Di Nora, C.; Cioffi, G.; Tarantini, L.; Gavazzi, A.; et al. Prevalence and Prognostic Impact of Non-Cardiac Co-Morbidities in Heart Failure Outpatients with Preserved and Reduced Ejection Fraction: A Community-Based Study. Eur. J. Heart Fail. 2018, 20, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Padwal, R.; McAlister, F.A.; McMurray, J.J.V.; Cowie, M.R.; Rich, M.; Pocock, S.; Swedberg, K.; Maggioni, A.; Gamble, G.; Ariti, C.; et al. The Obesity Paradox in Heart Failure Patients with Preserved versus Reduced Ejection Fraction: A Meta-Analysis of Individual Patient Data. Int. J. Obes. 2014, 38, 1110–1114. [Google Scholar] [CrossRef]
- Zhang, J.; Begley, A.; Jackson, R.; Harrison, M.; Pellicori, P.; Clark, A.L.; Cleland, J.G.F. Body Mass Index and All-Cause Mortality in Heart Failure Patients with Normal and Reduced Ventricular Ejection Fraction: A Dose-Response Meta-Analysis. Clin. Res. Cardiol. 2019, 108, 119–132. [Google Scholar] [CrossRef]
- Gentile, F.; Sciarrone, P.; Zamora, E.; De Antonio, M.; Santiago, E.; Domingo, M.; Aimo, A.; Giannoni, A.; Passino, C.; Codina, P.; et al. Body Mass Index and Outcomes in Ischaemic versus Non-Ischaemic Heart Failure across the Spectrum of Ejection Fraction. Eur. J. Prev. Cardiol. 2021, 28, 948–955. [Google Scholar] [CrossRef]
- Prausmüller, S.; Weidenhammer, A.; Heitzinger, G.; Spinka, G.; Goliasch, G.; Arfsten, H.; Abdel Mawgoud, R.; Gabler, C.; Strunk, G.; Hengstenberg, C.; et al. Obesity in Heart Failure with Preserved Ejection Fraction with and without Diabetes: Risk Factor or Innocent Bystander? Eur. J. Prev. Cardiol. 2023, 30, 1247–1254. [Google Scholar] [CrossRef]
- Katsiki, N.; Rizzo, M.; Mikhailidis, D.P. Epicardial, Peripancreatic and Other “Orthotopic” Excessive Fat Deposition in South Asians and Europeans: Are Differences Clinically Relevant? J. Diabetes Complicat. 2023, 37, 108419. [Google Scholar] [CrossRef]
- Koepp, K.E.; Obokata, M.; Reddy, Y.N.V.; Olson, T.P.; Borlaug, B.A. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure with Preserved Ejection Fraction. JACC. Heart Fail. 2020, 8, 657–666. [Google Scholar] [CrossRef]
- van Woerden, G.; van Veldhuisen, D.J.; Manintveld, O.C.; van Empel, V.P.M.; Willems, T.P.; de Boer, R.A.; Rienstra, M.; Westenbrink, B.D.; Gorter, T.M. Epicardial Adipose Tissue and Outcome in Heart Failure with Mid-Range and Preserved Ejection Fraction. Circ. Heart Fail. 2022, 15, e009238. [Google Scholar] [CrossRef]
- Tromp, J.; Packer, M.; Lam, C.S. The Diverging Role of Epicardial Adipose Tissue in Heart Failure with Reduced and Preserved Ejection Fraction: Not All Fat Is Created Equal. Eur. J. Heart Fail. 2021, 23, 1872–1874. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Mohseni, M.; Bianco, S.D.; Banga, P.K. Liraglutide Causes Large and Rapid Epicardial Fat Reduction. Obesity 2017, 25, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Morano, S.; Romagnoli, E.; Filardi, T.; Nieddu, L.; Mandosi, E.; Fallarino, M.; Turinese, I.; Dagostino, M.P.; Lenzi, A.; Carnevale, V. Short-Term Effects of Glucagon-like Peptide 1 (GLP-1) Receptor Agonists on Fat Distribution in Patients with Type 2 Diabetes Mellitus: An Ultrasonography Study. Acta Diabetol. 2015, 52, 727–732. [Google Scholar] [CrossRef]
- Iacobellis, G.; Villasante Fricke, A.C. Effects of Semaglutide Versus Dulaglutide on Epicardial Fat Thickness in Subjects with Type 2 Diabetes and Obesity. J. Endocr. Soc. 2020, 4, bvz042. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Li, G.; Zhang, P. Effect of Liraglutide on Epicardial Adipose Tissue Thickness with Echocardiography in Patients with Obese Type 2 Diabetes Mellitus. Int. J. Diabetes Dev. Ctries. 2020, 40, 500–506. [Google Scholar] [CrossRef]
- Wajdlich, M.; Nowicki, M. The Impact of GLP-1 Receptor Agonist Liraglutide on Blood Pressure Profile, Hydration, Natriuresis in Diabetic Patients with Severely Impaired Kidney Function. Sci. Rep. 2024, 14, 5002. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Holstein-Rathlou, N.-H.; Sosnovtseva, O.; Sørensen, C.M. Renoprotective Effects of GLP-1 Receptor Agonists and SGLT-2 Inhibitors-Is Hemodynamics the Key Point? Am. J. Physiol. Cell Physiol. 2023, 325, C243–C256. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Wilbon, S.S.; Kolonin, M.G. GLP1 Receptor Agonists-Effects beyond Obesity and Diabetes. Cells 2023, 13, 65. [Google Scholar] [CrossRef]
- Yuan, D.; Sharma, H.; Krishnan, A.; Vangaveti, V.N.; Malabu, U.H. Effect of Glucagon-like Peptide 1 Receptor Agonists on Albuminuria in Adult Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes. Obes. Metab. 2022, 24, 1869–1881. [Google Scholar] [CrossRef]
- Skov, J.; Dejgaard, A.; Frøkiær, J.; Holst, J.J.; Jonassen, T.; Rittig, S.; Christiansen, J.S. Glucagon-like Peptide-1 (GLP-1): Effect on Kidney Hemodynamics and Renin-Angiotensin-Aldosterone System in Healthy Men. J. Clin. Endocrinol. Metab. 2013, 98, E664–E671. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Zheng, Z.; Xue, J.; Cheng, M.; Guan, M.; Xue, Y. Effects of Exendin-4 on the Intrarenal Renin-Angiotensin System and Interstitial Fibrosis in Unilateral Ureteral Obstruction Mice: Exendin-4 and Unilateral Ureteral Obstruction. J. Renin. Angiotensin. Aldosterone. Syst. 2016, 17, 1470320316677918. [Google Scholar] [CrossRef] [PubMed]
- Kan, M.; Fu, H.; Xu, Y.; Yue, Z.; Du, B.; Chen, Q.; Wang, X.; Yu, S.; Zhang, Z. Effects of Once-Weekly Glucagon-like Peptide-1 Receptor Agonists on Type 2 Diabetes Mellitus Complicated with Coronary Artery Disease: Potential Role of the Renin-Angiotensin System. Diabetes. Obes. Metab. 2023, 25, 3223–3234. [Google Scholar] [CrossRef]
- van Heerebeek, L.; Hamdani, N.; Handoko, M.L.; Falcao-Pires, I.; Musters, R.J.; Kupreishvili, K.; Ijsselmuiden, A.J.J.; Schalkwijk, C.G.; Bronzwaer, J.G.F.; Diamant, M.; et al. Diastolic Stiffness of the Failing Diabetic Heart: Importance of Fibrosis, Advanced Glycation End Products, and Myocyte Resting Tension. Circulation 2008, 117, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac Inflammation Contributes to Changes in the Extracellular Matrix in Patients with Heart Failure and Normal Ejection Fraction. Circ. Heart Fail. 2011, 4, 44–52. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef]
- Wang, R.; Schiattarella, G.G. Tackling Metabolic Defects in HFpEF. Eur. Heart J. 2024, 45, 1494–1496. [Google Scholar] [CrossRef]
- Sanders-van Wijk, S.; Tromp, J.; Beussink-Nelson, L.; Hage, C.; Svedlund, S.; Saraste, A.; Swat, S.A.; Sanchez, C.; Njoroge, J.; Tan, R.-S.; et al. Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure with Preserved Ejection Fraction: Results from the PROMIS-HFpEF Study. Circulation 2020, 142, 2029–2044. [Google Scholar] [CrossRef]
- Jensen, J.K.; Zobel, E.H.; von Scholten, B.J.; Rotbain Curovic, V.; Hansen, T.W.; Rossing, P.; Kjaer, A.; Ripa, R.S. Effect of 26 Weeks of Liraglutide Treatment on Coronary Artery Inflammation in Type 2 Diabetes Quantified by [(64)Cu]Cu-DOTATATE PET/CT: Results from the LIRAFLAME Trial. Front. Endocrinol. 2021, 12, 790405. [Google Scholar] [CrossRef]
- Sukumaran, V.; Tsuchimochi, H.; Sonobe, T.; Waddingham, M.T.; Shirai, M.; Pearson, J.T. Liraglutide Treatment Improves the Coronary Microcirculation in Insulin Resistant Zucker Obese Rats on a High Salt Diet. Cardiovasc. Diabetol. 2020, 19, 24. [Google Scholar] [CrossRef]
- Wang, D.; Luo, P.; Wang, Y.; Li, W.; Wang, C.; Sun, D.; Zhang, R.; Su, T.; Ma, X.; Zeng, C.; et al. Glucagon-like Peptide-1 Protects against Cardiac Microvascular Injury in Diabetes via a CAMP/PKA/Rho-Dependent Mechanism. Diabetes 2013, 62, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Lam, C.S.P.; Van Veldhuisen, D.J.; Van Gelder, I.C.; Voors, A.A.; Rienstra, M. Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J. Am. Coll. Cardiol. 2016, 68, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Sartipy, U.; Dahlström, U.; Fu, M.; Lund, L.H. Atrial Fibrillation in Heart Failure with Preserved, Mid-Range, and Reduced Ejection Fraction. JACC. Heart Fail. 2017, 5, 565–574. [Google Scholar] [CrossRef]
- Zafrir, B.; Lund, L.H.; Laroche, C.; Ruschitzka, F.; Crespo-Leiro, M.G.; Coats, A.J.S.; Anker, S.D.; Filippatos, G.; Seferovic, P.M.; Maggioni, A.P.; et al. Prognostic Implications of Atrial Fibrillation in Heart Failure with Reduced, Mid-Range, and Preserved Ejection Fraction: A Report from 14 964 Patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur. Heart J. 2018, 39, 4277–4284. [Google Scholar] [CrossRef]
- Olsson, L.G.; Swedberg, K.; Ducharme, A.; Granger, C.B.; Michelson, E.L.; McMurray, J.J.V.; Puu, M.; Yusuf, S.; Pfeffer, M.A. Atrial Fibrillation and Risk of Clinical Events in Chronic Heart Failure with and without Left Ventricular Systolic Dysfunction: Results from the Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity (CHARM) Program. J. Am. Coll. Cardiol. 2006, 47, 1997–2004. [Google Scholar] [CrossRef]
- Butt, J.H.; Kondo, T.; Jhund, P.S.; Comin-Colet, J.; de Boer, R.A.; Desai, A.S.; Hernandez, A.F.; Inzucchi, S.E.; Janssens, S.P.; Kosiborod, M.N.; et al. Atrial Fibrillation and Dapagliflozin Efficacy in Patients with Preserved or Mildly Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2022, 80, 1705–1717. [Google Scholar] [CrossRef]
- Sha, R.; Baines, O.; Hayes, A.; Tompkins, K.; Kalla, M.; Holmes, A.P.; O’Shea, C.; Pavlovic, D. Impact of Obesity on Atrial Fibrillation Pathogenesis and Treatment Options. J. Am. Heart Assoc. 2024, 13, e032277. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Zhong, G. Glycemic Variability and the Risk of Atrial Fibrillation: A Meta-Analysis. Front. Endocrinol. 2023, 14, 1126581. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.-S.; Joung, B. Optimal Rhythm Control Strategy in Patients with Atrial Fibrillation. Korean Circ. J. 2022, 52, 496–512. [Google Scholar] [CrossRef]
- Ussher, J.R.; Drucker, D.J. Cardiovascular Actions of Incretin-Based Therapies. Circ. Res. 2014, 114, 1788–1803. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, W.; Zhang, D.; Ren, G.; Wang, P.; Gao, L.; Chen, H.; Ding, C. Comparison of the Effect of Glucose-Lowering Agents on the Risk of Atrial Fibrillation: A Network Meta-Analysis. Heart Rhythm 2021, 18, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Patoulias, D.; Tzeis, S.; Fragakis, N. Glucagon-Like Peptide-1 Receptor Agonists and Atrial Fibrillation Recurrence After Ablation: A Fire without the Smoke? Clin. Electrophysiol. 2024, 10, 1940–1941. [Google Scholar] [CrossRef] [PubMed]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Kassimis, G.; Karamitsos, T.; El-Tanani, M.; Rizzo, M. Effects of Glucagon-Like Peptide 1 Receptor Agonists on Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-Analysis. Adv. Ther. 2024. [Google Scholar] [CrossRef]
- RANDLE, P.J.; GARLAND, P.B.; HALES, C.N.; NEWSHOLME, E.A. The Glucose Fatty-Acid Cycle. Its Role in Insulin Sensitivity and the Metabolic Disturbances of Diabetes Mellitus. Lancet 1963, 1, 785–789. [Google Scholar] [CrossRef]
- Stanley, W.C.; Meadows, S.R.; Kivilo, K.M.; Roth, B.A.; Lopaschuk, G.D. Beta-Hydroxybutyrate Inhibits Myocardial Fatty Acid Oxidation in Vivo Independent of Changes in Malonyl-CoA Content. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1626–H1631. [Google Scholar] [CrossRef]
- Gormsen, L.C.; Svart, M.; Thomsen, H.H.; Søndergaard, E.; Vendelbo, M.H.; Christensen, N.; Tolbod, L.P.; Harms, H.J.; Nielsen, R.; Wiggers, H.; et al. Ketone Body Infusion with 3-Hydroxybutyrate Reduces Myocardial Glucose Uptake and Increases Blood Flow in Humans: A Positron Emission Tomography Study. J. Am. Heart Assoc. 2017, 6, e005066. [Google Scholar] [CrossRef]
- Almutairi, M.; Gopal, K.; Greenwell, A.A.; Young, A.; Gill, R.; Aburasayn, H.; Al Batran, R.; Chahade, J.J.; Gandhi, M.; Eaton, F.; et al. The GLP-1 Receptor Agonist Liraglutide Increases Myocardial Glucose Oxidation Rates via Indirect Mechanisms and Mitigates Experimental Diabetic Cardiomyopathy. Can. J. Cardiol. 2021, 37, 140–150. [Google Scholar] [CrossRef]
- Chan, J.S.F.; Greenwell, A.A.; Saed, C.T.; Stenlund, M.J.; Mangra-Bala, I.A.; Tabatabaei Dakhili, S.A.; Yang, K.; Ferrari, S.R.; Eaton, F.; Gopal, K.; et al. Liraglutide Alleviates Experimental Diabetic Cardiomyopathy in a PDH-Dependent Manner. J. Endocrinol. 2024, 262, e240032. [Google Scholar] [CrossRef]
- Al Batran, R.; Almutairi, M.; Ussher, J.R. Glucagon-like Peptide-1 Receptor Mediated Control of Cardiac Energy Metabolism. Peptides 2018, 100, 94–100. [Google Scholar] [CrossRef]
- Sattar, N.; Lee, M.M.Y.; Kristensen, S.L.; Branch, K.R.H.; Del Prato, S.; Khurmi, N.S.; Lam, C.S.P.; Lopes, R.D.; McMurray, J.J.V.; Pratley, R.E.; et al. Cardiovascular, Mortality, and Kidney Outcomes with GLP-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Trials. Lancet Diabetes Endocrinol. 2021, 9, 653–662. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Muzurović, E.; Yumuk, V.D.; Rizzo, M. GLP-1 and Dual GIP/GLP-1 Receptor Agonists in Overweight/Obese Patients for Atherosclerotic Cardiovascular Disease Prevention: Where Are We Now? J. Diabetes Complicat. 2023, 37, 108647. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Abildstrøm, S.Z.; Borlaug, B.A.; Butler, J.; Christensen, L.; Davies, M.; Hovingh, K.G.; Kitzman, D.W.; Lindegaard, M.L.; Møller, D.V.; et al. Design and Baseline Characteristics of STEP-HFpEF Program Evaluating Semaglutide in Patients with Obesity HFpEF Phenotype. JACC. Heart Fail. 2023, 11, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.N.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Treppendahl, M.B.; Verma, S.; et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N. Engl. J. Med. 2024, 390, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Abildstrøm, S.Z.; Borlaug, B.A.; Davies, M.J.; Kitzman, D.W.; Petrie, M.C.; Shah, S.J.; Verma, S.; Abhayaratna, W.P.; Chopra, V.; et al. Semaglutide in Patients with Obesity and Heart Failure Across Mildly Reduced or Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2023, 82, 2087–2096. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Verma, S.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Jon Jensen, T.; Rasmussen, S.; Erlang Marstrand, P.; Petrie, M.C.; Shah, S.J.; et al. Effects of Semaglutide on Symptoms, Function, and Quality of Life in Patients with Heart Failure with Preserved Ejection Fraction and Obesity: A Prespecified Analysis of the STEP-HFpEF Trial. Circulation 2024, 149, 204–216. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Kitzman, D.W.; Davies, M.J.; Rasmussen, S.; Barros, E.; Butler, J.; Einfeldt, M.N.; Hovingh, G.K.; Møller, D.V.; Petrie, M.C.; et al. Semaglutide in HFpEF across Obesity Class and by Body Weight Reduction: A Prespecified Analysis of the STEP-HFpEF Trial. Nat. Med. 2023, 29, 2358–2365. [Google Scholar] [CrossRef]
- Butler, J.; Shah, S.J.; Petrie, M.C.; Borlaug, B.A.; Abildstrøm, S.Z.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Verma, S.; et al. Semaglutide versus Placebo in People with Obesity-Related Heart Failure with Preserved Ejection Fraction: A Pooled Analysis of the STEP-HFpEF and STEP-HFpEF DM Randomised Trials. Lancet 2024, 403, 1635–1648. [Google Scholar] [CrossRef]
- Schou, M.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Kitzman, D.W.; Shah, S.J.; Verma, S.; Patel, S.; Chinnakondepalli, K.M.; et al. Semaglutide and NYHA Functional Class in Obesity-Related Heart Failure with Preserved Ejection Fraction: The STEP-HFpEF Program. J. Am. Coll. Cardiol. 2024, 84, 247–257. [Google Scholar] [CrossRef]
- Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Kitzman, D.W.; Shah, S.J.; Verma, S.; Jensen, T.J.; Einfeldt, M.N.; Liisberg, K.; et al. Semaglutide and NT-ProBNP in Obesity-Related HFpEF: Insights from the STEP-HFpEF Program. J. Am. Coll. Cardiol. 2024, 84, 27–40. [Google Scholar] [CrossRef]
- Shah, S.J.; Sharma, K.; Borlaug, B.A.; Butler, J.; Davies, M.; Kitzman, D.W.; Petrie, M.C.; Verma, S.; Patel, S.; Chinnakondepalli, K.M.; et al. Semaglutide and Diuretic Use in Obesity-Related Heart Failure with Preserved Ejection Fraction: A Pooled Analysis of the STEP-HFpEF and STEP-HFpEF-DM Trials. Eur. Heart J. 2024. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Belmonte, L.M.; Sanz-Cánovas, J.; García de Lucas, M.D.; Ricci, M.; Avilés-Bueno, B.; Cobos-Palacios, L.; Pérez-Velasco, M.A.; López-Sampalo, A.; Bernal-López, M.R.; Jansen-Chaparro, S.; et al. Efficacy and Safety of Semaglutide for the Management of Obese Patients with Type 2 Diabetes and Chronic Heart Failure in Real-World Clinical Practice. Front. Endocrinol. 2022, 13, 851035. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.Gov. Study Details—A Study of Tirzepatide (LY3298176) in Participants with Heart Failure with Preserved Ejection Fraction (HFpEF) and Obesity: The SUMMIT Trial. Available online: https://clinicaltrials.gov/study/NCT04847557?cond=NCT04847557&rank=1 (accessed on 12 August 2024).
- Pius, R.; Odukudu, G.-D.O.; Olorundare, I.; Makanjuola, D.I.; Komolafe, R.; Njoku, C.; Ubogun, O.E.; Muhammad, R.; Osiogo, E.O.; Anulaobi, C. A Narrative Review on the Efficacy and Safety of Loop Diuretics in Patients with Heart Failure with Reduced Ejection Fraction and Preserved Ejection Fraction. Cureus 2023, 15, e45794. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Butler, J. Expanding the Definition of Worsening Heart Failure and Recognizing the Importance of Outpatient Escalation of Oral Diuretics. Circulation 2023, 148, 1746–1749. [Google Scholar] [CrossRef]
- Ferreira, J.P.; Liu, J.; Claggett, B.L.; Vardeny, O.; Pitt, B.; Pfeffer, M.A.; Solomon, S.D.; Zannad, F. Outpatient Diuretic Intensification as Endpoint in Heart Failure with Preserved Ejection Fraction Trials: An Analysis from TOPCAT. Eur. J. Heart Fail. 2022, 24, 378–384. [Google Scholar] [CrossRef]
- Chatur, S.; Vaduganathan, M.; Claggett, B.L.; Cunningham, J.W.; Docherty, K.F.; Desai, A.S.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; et al. Outpatient Worsening Among Patients with Mildly Reduced and Preserved Ejection Fraction Heart Failure in the DELIVER Trial. Circulation 2023, 148, 1735–1745. [Google Scholar] [CrossRef]
- Litwin, S.E.; Komtebedde, J.; Seidler, T.; Borlaug, B.A.; Winkler, S.; Solomon, S.D.; Eicher, J.-C.; Mazimba, S.; Khawash, R.; Sverdlov, A.L.; et al. Obesity in Heart Failure with Preserved Ejection Fraction: Insights from the REDUCE LAP-HF II Trial. Eur. J. Heart Fail. 2024, 26, 177–189. [Google Scholar] [CrossRef]
- Morgen, C.S.; Haase, C.L.; Oral, T.K.; Schnecke, V.; Varbo, A.; Borlaug, B.A. Obesity, Cardiorenal Comorbidities, and Risk of Hospitalization in Patients with Heart Failure with Preserved Ejection Fraction. Mayo Clin. Proc. 2023, 98, 1458–1468. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Testani, J.M.; Felker, G.M.; Tang, W.H.W.; Abou-Ezzeddine, O.F.; Sun, J.-L.; Chakrabothy, H.; McNulty, S.; Shah, S.J.; et al. Adverse Renal Response to Decongestion in the Obese Phenotype of Heart Failure with Preserved Ejection Fraction. J. Card. Fail. 2020, 26, 101–107. [Google Scholar] [CrossRef]
- Chatur, S.; Claggett, B.L.; Vardeny, O.; Jering, K.; Desai, A.S.; Pfeffer, M.A.; Lefkowitz, M.; McMurray, J.J.V.; Solomon, S.D.; Vaduganathan, M. Sacubitril/Valsartan and Loop Diuretic Requirement in Heart Failure with Preserved Ejection Fraction in the PARAGON-HF Trial. Eur. J. Heart Fail. 2023, 25, 87–94. [Google Scholar] [CrossRef]
- Chatur, S.; Vaduganathan, M.; Claggett, B.; Vardeny, O.; Desai, A.S.; Jhund, P.S.; de Boer, R.A.; Lam, C.S.P.; Kosiborod, M.N.; Shah, S.J.; et al. Dapagliflozin and Diuretic Utilization in Heart Failure with Mildly Reduced or Preserved Ejection Fraction: The DELIVER Trial. Eur. Heart J. 2023, 44, 2930–2943. [Google Scholar] [CrossRef] [PubMed]
- Chyou, J.Y.; Qin, H.; Butler, J.; Voors, A.A.; Lam, C.S.P. Sex-Related Similarities and Differences in Responses to Heart Failure Therapies. Nat. Rev. Cardiol. 2024, 21, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure with Preserved Ejection Fraction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef]
- Butler, J.; Filippatos, G.; Siddiqi, T.J.; Ferreira, J.P.; Brueckmann, M.; Bocchi, E.; Böhm, M.; Chopra, V.K.; Giannetti, N.; Iwata, T.; et al. Effects of Empagliflozin in Women and Men with Heart Failure and Preserved Ejection Fraction. Circulation 2022, 146, 1046–1055. [Google Scholar] [CrossRef]
- Sorimachi, H.; Obokata, M.; Takahashi, N.; Reddy, Y.N.V.; Jain, C.C.; Verbrugge, F.H.; Koepp, K.E.; Khosla, S.; Jensen, M.D.; Borlaug, B.A. Pathophysiologic Importance of Visceral Adipose Tissue in Women with Heart Failure and Preserved Ejection Fraction. Eur. Heart J. 2021, 42, 1595–1605. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Jackson, A.M.; Lam, C.S.P.; Redfield, M.M.; Anand, I.S.; Ge, J.; Lefkowitz, M.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; et al. Effects of Sacubitril-Valsartan Versus Valsartan in Women Compared with Men with Heart Failure and Preserved Ejection Fraction: Insights from PARAGON-HF. Circulation 2020, 141, 338–351. [Google Scholar] [CrossRef]
- Jensterle, M.; Rizzo, M.; Janež, A. Weight Response to GLP-1 Receptor Agonists: Why Women Do It Better? J. Diabetes Complications 2022, 36, 108310. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef]
- Davies, M.; Færch, L.; Jeppesen, O.K.; Pakseresht, A.; Pedersen, S.D.; Perreault, L.; Rosenstock, J.; Shimomura, I.; Viljoen, A.; Wadden, T.A.; et al. Semaglutide 2·4 Mg Once a Week in Adults with Overweight or Obesity, and Type 2 Diabetes (STEP 2): A Randomised, Double-Blind, Double-Dummy, Placebo-Controlled, Phase 3 Trial. Lancet 2021, 397, 971–984. [Google Scholar] [CrossRef]

| Study/Author | Study Design | Sample Size | LVEF | Follow-up Duration | Main Findings |
|---|---|---|---|---|---|
| I-PRESERVE, 2011 [40] | RCT | 4109 | ≥50% | 49.5 months | After adjusting for 21 risk factors, including age, sex, and N-terminal pro-brain natriuretic peptide levels, the risk of the primary outcome was significantly higher in patients with a BMI below 23.5 (HR 1.27; 95% CI 1.04 to 1.56) and in those with a BMI of 35 kg/m2 or more (HR 1.27; 95% CI 1.06 to 1.52) compared to the reference group. A similar pattern was observed for both all-cause mortality and heart failure hospitalization. |
| Ather et al., 2011 [41] | Observational cohort study | 2843 patients with HFpEF &&&and 6599 with HFrEF | ≥50% and <50% | 24 months | The lack of obesity was identified as an independent predictor of all-cause mortality in both HFpEF and HFrEF patients. |
| Powell-Wiley et al., 2018 [42] | Observational cohort study | 39,647 | ≥50% and <50% | 1 and 12 months | In individuals with HFpEF, higher BMI was associated with reduced 30-day mortality, showing a protective effect up to a BMI of 30 kg/m2. Above this level, the risk increased slightly (BMI of 30 kg/m2 versus 18.5 kg/m2, HR 0.63; 95% CI 0.54 to 0.73). |
| Iorio et al., 2018 [43] | Observational cohort study | 2314 &&&(1373 with HFpEF) | ≥50% and <50% | 31 months | The absence of obesity was linked to higher overall mortality in both HFpEF and HFrEF groups. |
| Padwal et al., 2014 [44] | Meta-analysis of patient-level data from 14 heart failure dedicated studies | 23,967 | ≥50% and <50% | 36 months | In patients with chronic heart failure, the obesity paradox was observed in both HFrEF and HFpEF. Mortality in both heart failure subtypes followed a U-shaped curve, with the lowest mortality rates occurring at a BMI of 30.0–34.9 kg/m2. |
| Zhang et al., 2019 [45] | Dose-response meta-analysis of 10 studies | 96,424 (59,263 with HFpEF) | ≥50% and <50% | N/R | In patients with heart failure, the relationship between BMI and mortality follows a U-shaped curve, with the lowest risk observed at a BMI of 32–33 kg/m2 for both HFpEF and HFrEF. |
| Gentile et al., 2021 [46] | Observational cohort study | 5155 (19% HFpEF) | ≥50% and <50% | 40 months | Mild obesity was independently linked to improved survival across the full range of LVEF. However, the survival advantage associated with obesity was preserved only in cases of non-ischemic heart failure. |
| Prausmüller et al., 2023 [47] | Large-scale cohort study | 6744 individuals with HFpEF (25% with T2DM) | >50% | 47 months | In the overall cohort, with a BMI of 22.5–24.9 kg/m2 serving as the reference, the unadjusted HR for all-cause mortality was elevated in patients with a BMI below 22.5 kg/m2 (HR 1.27; 95% CI 1.09–1.48) and decreased in those with a BMI of 25 kg/m2 or higher. After adjusting for multiple variables, BMI continued to show a significant inverse relationship with survival in patients without T2DM. |
| Author | Inclusion Criteria | Sample Size | Follow-up Duration (Weeks) | GLP-1 Receptor Agonist | Imaging Modality | Change (%) in Epicardial Adipose Tissue Thickness |
|---|---|---|---|---|---|---|
| Iacobellis et al., 2017 [52] | T2DM and obesity | 95 | 24 | Liraglutide | Echocardiography | −42 |
| Morano et al., 2015 [53] | T2DM | 27 | 18 | Liraglutide or exenatide | Echocardiography | −13 |
| Iacobellis et al., 2020 [54] | T2DM and obesity | 30 | 12 | Semaglutide | Echocardiography | −20 |
| 30 | 12 | Dulaglutide | Echocardiography | −20 | ||
| Lit et al., 2020 [55] | T2DM and obesity | 21 | 12 | Liraglutide | CMR | −29 |
| Author/Study | Study Design | Participants | Intervention | Primary Endpoint | Outcomes |
|---|---|---|---|---|---|
| Kosiborod et al., 2023 [37] | Placebo-controlled, double-blind RCT | 529 patients with HFpEF and BMI ≥ 30 without T2DM | Once weekly subcutaneously administered semaglutide (2.4 mg) or placebo for 52 weeks | Dual primary end points were the change from baseline in the KCCQ-CSS, and the change in body weight | In patients with HFpEF and obesity, treatment with semaglutide (2.4 mg) resulted in more pronounced reductions in symptoms and physical limitations, greater enhancements in exercise capacity, and more pronounced weight loss compared to placebo |
| Kosiborod et al., 2024 [94] | Placebo-controlled, double-blind RCT | 616 patients with HFpEF, BMI ≥ 30 and T2DM | Once weekly subcutaneously administered semaglutide (2.4 mg) or placebo for 52 weeks | Dual primary end points were the change from baseline in the KCCQ-CSS, and the change in body weight | In patients with obesity-related HFpEF and T2DM, semaglutide led to more significant reductions in heart failure-related manifestations and physical limitations, as well as greater weight loss, compared to placebo over a 1-year period. |
| Butler et al., 2023 [95] | Prespecified analysis of the STEP-HFpEF RCT | 529 patients with HFpEF and BMI ≥ 30 without T2DM | Once weekly subcutaneously administered semaglutide (2.4 mg) or placebo for 52 weeks | Dual primary end points were the change from baseline in the KCCQ-CSS, and the change in body weight | In patients with HFpEF and obesity, semaglutide 2.4 mg led to improvements in symptoms, physical limitations, and exercise capacity, while also reducing inflammation and body weight consistently across different LVEF categories (i) 45% to 49% (n = 85), (ii) 50% to 59% (n = 215), and (iii) ≥60% (n = 229)) |
| Kosiborod et al., 2024 [96] | Prespecified analysis of the STEP-HFpEF RCT | 529 patients with HFpEF and BMI ≥ 30 without T2DM | Once weekly subcutaneously administered semaglutide (2.4 mg) or placebo for 52 weeks | Dual primary end points were the change from baseline in the KCCQ-CSS, and the change in body weight based on KCCQ-CSS tertiles at baseline | In patients with HFpEF and obesity, semaglutide resulted in significant improvements in HF-related symptoms, physical limitations, exercise function, inflammation, body weight, and NT-proBNP levels, regardless of baseline health status |
| Borlaug et al., 2023 [97] | Prespecified analysis of the STEP-HFpEF RCT | 529 patients with HFpEF and BMI ≥ 30 without T2DM | Once weekly subcutaneously administered semaglutide (2.4 mg) or placebo for 52 weeks | Change in the KCCQ-CSS and body weight across obesity classes I-III (BMI 30.0–34.9 kg m2, 35.0–39.9 kg m2 and ≥ 40 kg m2) and according to body weight loss with semaglutide after 52 weeks | In participants with the obesity phenotype of HFpEF, semaglutide enhanced symptoms, physical limitations, and exercise capacity, while also reducing inflammation and body weight across various obesity levels. Among those treated with semaglutide, the degree of benefit was directly proportional to the extent of weight loss achieved. |
| Butler et al., 2024 [98] | Pooled analysis of the STEP-HFpEF and STEP-HFpEF-DM trials | 1145 patients with HFpEF and BMI ≥ 30 with or without T2DM | Once weekly subcutaneously administered semaglutide (2.4 mg) or placebo for 52 weeks | Change from baseline in the KCCQ-CSS, and the change in body weight | In this predetermined combined analysis of the STEP-HFpEF and STEP-HFpEF DM trials, semaglutide demonstrated superior efficacy over placebo in alleviating heart failure-related symptoms, reducing physical limitations, and decreasing body weight in subjects with obesity-related HFpEF. These benefits were largely consistent across various patient demographic and clinical characteristics. Moreover, semaglutide was well tolerated by the participants. |
| Schou et al., 2024 [99] | Prespecified analysis of pooled data from the the STEP-HFpEF and STEP-HFpEF-DM trials | 1145 patients with HFpEF and BMI ≥ 30 with or without T2DM | Once weekly subcutaneously administered semaglutide (2.4 mg) or placebo for 52 weeks | Change in NYHA functional class from baseline to 52 weeks | In patients with obesity-related HFpEF, those treated with semaglutide experienced less deterioration and greater improvement in NYHA functional class compared to those receiving placebo at 52 weeks. Semaglutide consistently enhanced heart failure-related symptoms, physical limitations, and exercise capacity, while also reducing body weight and biomarkers of inflammation and congestion across all NYHA functional class categories. Notably, the improvements in health status were particularly pronounced in patients with NYHA functional classes III/IV. |
| Verma et al., 2024 [59] | Prespecified secondary analysis of pooled data from STEP-HFpEF and STEP-HFpEF DM | 1145 patients with HFpEF and BMI ≥ 30 with or without T2DM | Once weekly subcutaneously administered semaglutide (2.4 mg) or placebo for 52 weeks | Change from baseline in the KCCQ-CSS, and the change in body weight were compared between sexes | In patients with obesity-related HFpEF, semaglutide 2.4 mg led to a more substantial reduction in body weight in women, while delivering comparable improvements in heart failure-related symptoms, physical limitations, and exercise function across both sexes |
| Petrie et al., 2024 [100] | Prespecified secondary analysis of pooled data from STEP-HFpEF and STEP-HFpEF DM | 1145 patients with HFpEF and BMI ≥ 30 with or without T2DM | Once weekly subcutaneously administered semaglutide (2.4 mg) or placebo for 52 weeks | Change from baseline in the KCCQ-CSS, and the change in body weight were compared by baseline NT-proBNP | In individuals with obesity-related HFpEF, semaglutide led to reductions in NT-proBNP levels. Those with higher baseline NT-proBNP achieved comparable weight loss but experienced more substantial improvements in heart failure symptoms and physical limitations compared to those with lower NT-proBNP. |
| Shah et al., 2024 [101] | Pooled analysis of the STEP-HFpEF and STEP-HFpEF-DM trials | 1145 patients with HFpEF and BMI ≥ 30 with or without T2DM | Once weekly subcutaneously administered semaglutide (2.4 mg) or placebo for 52 weeks | Change from baseline in the KCCQ-CSS, and the change in body weight across baseline diuretic use groups (no diuretics, non-loop diuretics only, and loop diuretics [< 40, 40, and > 40 mg/day furosemide equivalents]); and changes in loop diuretic use and dose over 52 weeks | In patients with obesity-related HFpEF, semaglutide significantly alleviated heart failure-related symptoms and physical limitations across different diuretic use subgroups, with particularly notable benefits observed in those using loop diuretics at baseline. The reductions in weight and enhancements in exercise function with semaglutide compared to placebo were uniform across all diuretic use categories. Additionally, semaglutide treatment was associated with a decrease in both the use and dosage of loop diuretics from baseline to 52 weeks. |
| Pérez-Belmonte et al., 2022 [102] | Real-world retrospective observational study | 136 patients with T2DM, obesity, and heart failure | Once weekly subcutaneously administered semaglutide (maintenance dose 0.5 mg or 1.0 mg) for 12 months | Change from baseline in the KCCQ-CSS, New York Heart Association (NYHA) classification, and in NT-pro-BNP levels | In patients treated with semaglutide, there was a significantly greater improvement in the KCCQ total symptom score (59.0 ± 24.1 vs 79.9 ± 28.4 points, p < 0.01), a decrease in the percentage of patients with NYHA l class III (from 40.4% to 16.2%, p < 0.01), and a decrease in NT-pro-BNP levels compared to semaglutide non-users. Additionally, there was a reduction in emergency department visits, hospitalizations for heart failure, and all-cause hospitalizations in semaglutide group. |
| SUMMIT [103] | Randomized, double-blind, placebo-controlled Phase 3 trial | 700 patients with NYHA Class II–IV and increased NT-proBNP, structural heart disease, or heart failure decompensation within 1 year | Tirzepatide administered subcutaneously vs. placebo | (i) All-cause mortality, heart failure events, 6MWD test, and KCCQ from baseline to week 120; &&&(ii) 6MWD test variation from baseline to week 52 | (Not published yet) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Sagris, M.; Koufakis, T.; Vlachakis, P.K.; Rangraze, I.R.; El Tanani, M.; Tsioufis, K.; et al. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure? Biomedicines 2024, 12, 2112. https://doi.org/10.3390/biomedicines12092112
Karakasis P, Fragakis N, Patoulias D, Theofilis P, Sagris M, Koufakis T, Vlachakis PK, Rangraze IR, El Tanani M, Tsioufis K, et al. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure? Biomedicines. 2024; 12(9):2112. https://doi.org/10.3390/biomedicines12092112
Chicago/Turabian StyleKarakasis, Paschalis, Nikolaos Fragakis, Dimitrios Patoulias, Panagiotis Theofilis, Marios Sagris, Theocharis Koufakis, Panayotis K. Vlachakis, Imran Rashid Rangraze, Mohamed El Tanani, Konstantinos Tsioufis, and et al. 2024. "The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure?" Biomedicines 12, no. 9: 2112. https://doi.org/10.3390/biomedicines12092112
APA StyleKarakasis, P., Fragakis, N., Patoulias, D., Theofilis, P., Sagris, M., Koufakis, T., Vlachakis, P. K., Rangraze, I. R., El Tanani, M., Tsioufis, K., & Rizzo, M. (2024). The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure? Biomedicines, 12(9), 2112. https://doi.org/10.3390/biomedicines12092112









