Changes in the Release of Endothelial Extracellular Vesicles CD144+, CCR6+, and CXCR3+ in Individuals with Acute Myocardial Infarction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Echocardiography
2.3. Samples and Analytical Procedures
2.4. Isolation and Characterization of Human Plasma EVs
2.5. Size Exclusion Chromatography (SEC)
2.6. Extracellular Vesicles Characterization
2.7. Flow Cytometry of EVs
2.8. Statistical Analysis
3. Results
3.1. Studied Group
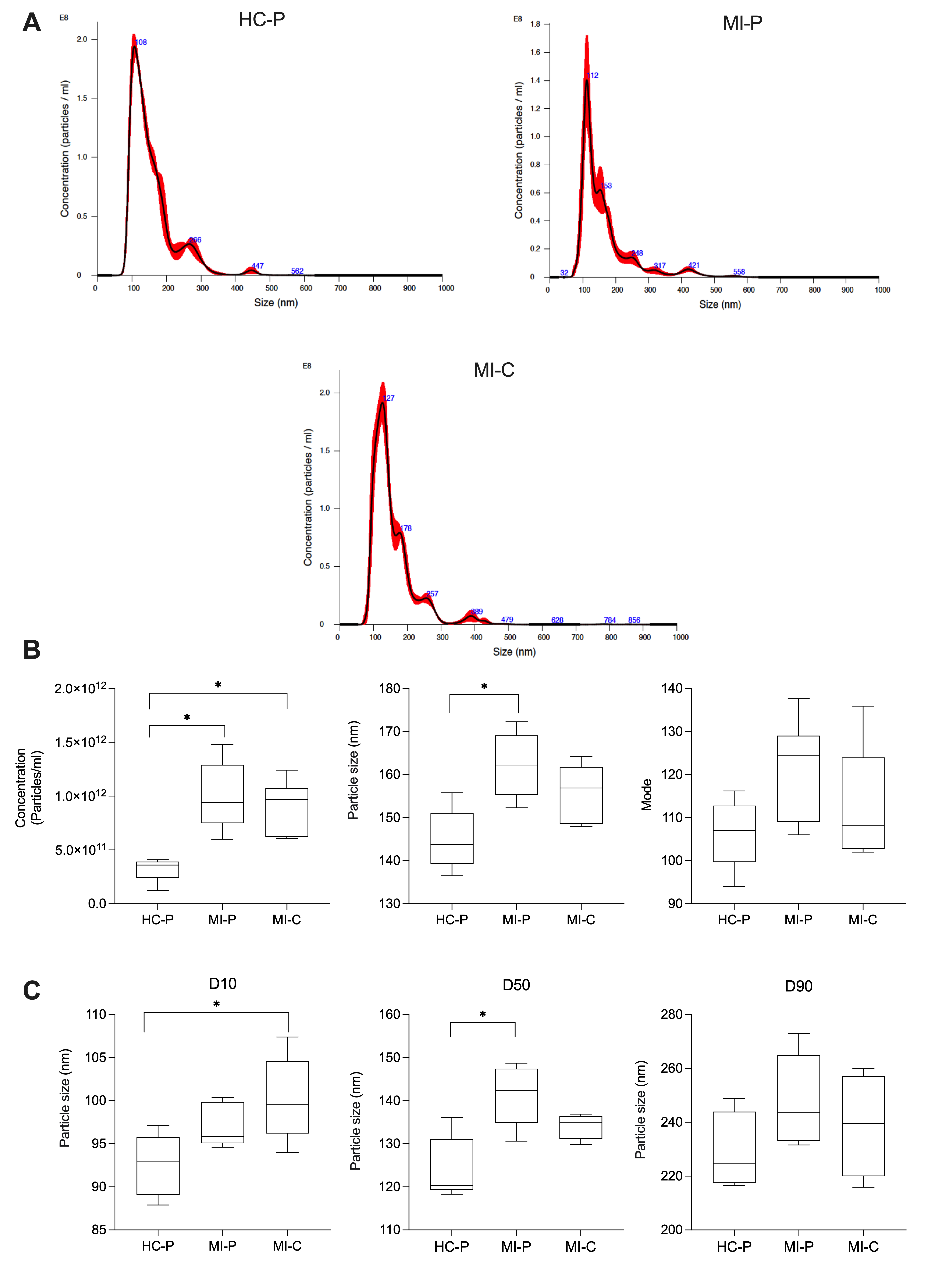
3.2. Characteristics of Peripheric and Coronary EVs from AMI (MI-P and MI-C) and Controls (HC-P)
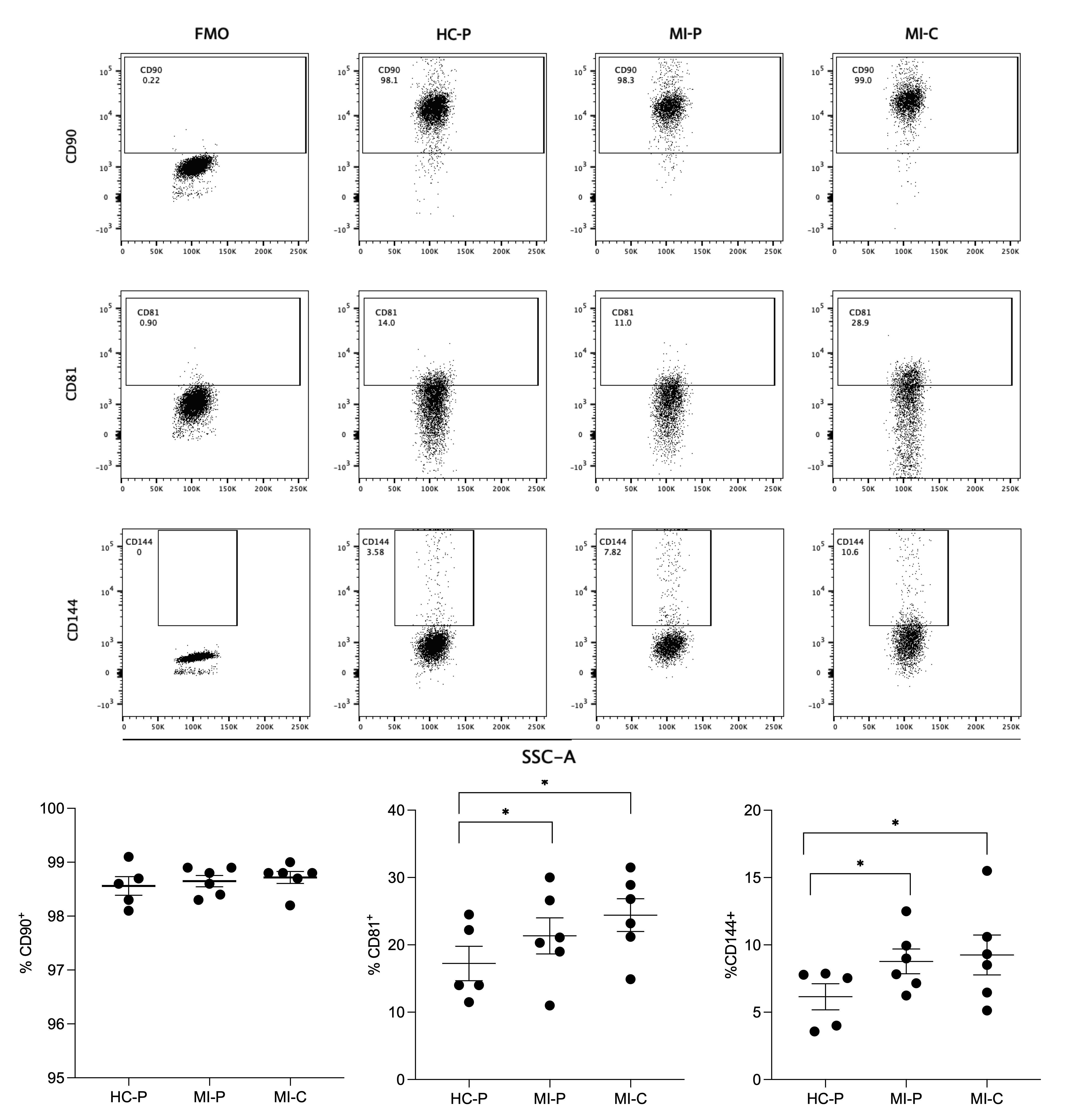
3.3. Immunophenotype Characterization of Extracellular Vesicles by Flow Cytometry
3.4. Characterization of the Chemokine Receptor Profile of Extracellular Vesicles by Flow Cytometry
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleh, M.; Ambrose, J.A. Understanding myocardial infarction. F1000Research 2018, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, H.; Minami, E.; Zhu, W.Z.; Laflamme, M.A. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ. Res. 2008, 103, 1058–1071. [Google Scholar] [CrossRef] [PubMed]
- Moreno Ruiz, N. Modificacioόn de los criterios de Sgarbossa para el diagnόstico de infarto agudo de miocardio en presencia de bloqueo de rama izquierda. Revista de la Facultad de Medicina 2015, 63, 151–154. [Google Scholar] [CrossRef]
- Martinez-Greene, J.A.; Hernandez-Ortega, K.; Quiroz-Baez, R.; Resendis-Antonio, O.; Pichardo-Casas, I.; Sinclair, D.A.; Budnik, B.; Hidalgo-Miranda, A.; Uribe-Querol, E.; Ramos-Godinez, M.D.P.; et al. Quantitative proteomic analysis of extracellular vesicle subgroups isolated by an optimized method combining polymer-based precipitation and size exclusion chromatography. J. Extracell. Vesicles 2021, 10, e12087. [Google Scholar] [CrossRef]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [CrossRef]
- Giricz, Z.; Varga, Z.V.; Baranyai, T.; Sipos, P.; Paloczi, K.; Kittel, A.; Buzas, E.I.; Ferdinandy, P. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J. Mol. Cell Cardiol. 2014, 68, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.R.; Klyachko, E.; Thorne, T.; Schultz, K.M.; Millay, M.; Ito, A.; Kamide, C.E.; Liu, T.; Gupta, R.; Sahoo, S.; et al. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ. Res. 2012, 111, 312–321. [Google Scholar] [CrossRef]
- de Couto, G.; Gallet, R.; Cambier, L.; Jaghatspanyan, E.; Makkar, N.; Dawkins, J.F.; Berman, B.P.; Marban, E. Exosomal MicroRNA Transfer Into Macrophages Mediates Cellular Postconditioning. Circulation 2017, 136, 200–214. [Google Scholar] [CrossRef]
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 2014, 114, 333–344. [Google Scholar] [CrossRef]
- Lazar, E.; Benedek, T.; Korodi, S.; Rat, N.; Lo, J.; Benedek, I. Stem cell-derived exosomes—An emerging tool for myocardial regeneration. World J. Stem Cells 2018, 10, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Shen, Y.; Ma, G.; Liu, Y.; Cai, J.; Kim, I.M.; Weintraub, N.L.; Liu, N.; Tang, Y. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes Promotes Repair in Ischemic Myocardium. J. Cardiovasc. Transl. Res. 2018, 11, 420–428. [Google Scholar] [CrossRef]
- Yao, J.; Huang, K.; Zhu, D.; Chen, T.; Jiang, Y.; Zhang, J.; Mi, L.; Xuan, H.; Hu, S.; Li, J.; et al. A Minimally Invasive Exosome Spray Repairs Heart after Myocardial Infarction. ACS Nano 2021, 15, 11099–11111. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, L.; Ding, Y.; Jiang, X.; Xia, Z.; You, Z. Human umbilical cord mesenchymal stem cells-derived exosomes transfers microRNA-19a to protect cardiomyocytes from acute myocardial infarction by targeting SOX6. Cell Cycle 2020, 19, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef]
- Sinning, J.M.; Losch, J.; Walenta, K.; Bohm, M.; Nickenig, G.; Werner, N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur. Heart J. 2011, 32, 2034–2041. [Google Scholar] [CrossRef]
- Nozaki, T.; Sugiyama, S.; Koga, H.; Sugamura, K.; Ohba, K.; Matsuzawa, Y.; Sumida, H.; Matsui, K.; Jinnouchi, H.; Ogawa, H. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J. Am. Coll. Cardiol. 2009, 54, 601–608. [Google Scholar] [CrossRef]
- Jimenez, J.J.; Jy, W.; Mauro, L.M.; Soderland, C.; Horstman, L.L.; Ahn, Y.S. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb. Res. 2003, 109, 175–180. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 15 March 2020).
- Otero-Ortega, L.; Alonso-Lopez, E.; Perez-Mato, M.; Laso-Garcia, F.; Gomez-de Frutos, M.C.; Diekhorst, L.; Garcia-Bermejo, M.L.; Conde-Moreno, E.; Fuentes, B.; Alonso de Lecinana, M.; et al. Similarities and Differences in Extracellular Vesicle Profiles between Ischaemic Stroke and Myocardial Infarction. Biomedicines 2020, 9, 8. [Google Scholar] [CrossRef]
- Barile, L.; Lionetti, V.; Cervio, E.; Matteucci, M.; Gherghiceanu, M.; Popescu, L.M.; Torre, T.; Siclari, F.; Moccetti, T.; Vassalli, G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014, 103, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Oporto, K.; Radojkovic, C.; Mellisho, E.A.; Zuniga, F.; Ormazabal, V.; Guzman-Gutierrez, E.; Nova-Lamperti, E.; Rodriguez-Alvarez, L.; Aranda, M.; Escudero, C.; et al. Adenosine promoted angiogenesis mediated by the release of small extracellular vesicles from human endothelial progenitor cells. Microvasc. Res. 2023, 148, 104498. [Google Scholar] [CrossRef] [PubMed]
- Contreras, H.; Alarcón-Zapata, A.; Nova-Lamperti, E.; Ormazabal, V.; Varas-Godoy, M.; Salomon, C.; Zuniga, F. Comparative study of size exclusion chromatography for isolation of small extracellular vesicle from cell-conditioned media, plasma, urine, and saliva. Front. Nanotechnol. 2023, 5, 1146772. [Google Scholar] [CrossRef]
- Bryl-Gorecka, P.; James, K.; Torngren, K.; Haraldsson, I.; Gan, L.M.; Svedlund, S.; Olde, B.; Laurell, T.; Omerovic, E.; Erlinge, D. Microvesicles in plasma reflect coronary flow reserve in patients with cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H2147–H2160. [Google Scholar] [CrossRef]
- Boulanger, C.M.; Loyer, X.; Rautou, P.E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef]
- Mallat, Z.; Benamer, H.; Hugel, B.; Benessiano, J.; Steg, P.G.; Freyssinet, J.M.; Tedgui, A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000, 101, 841–843. [Google Scholar] [CrossRef]
- Jung, C.; Sorensson, P.; Saleh, N.; Arheden, H.; Ryden, L.; Pernow, J. Circulating endothelial and platelet derived microparticles reflect the size of myocardium at risk in patients with ST-elevation myocardial infarction. Atherosclerosis 2012, 221, 226–231. [Google Scholar] [CrossRef]
- Vion, A.C.; Ramkhelawon, B.; Loyer, X.; Chironi, G.; Devue, C.; Loirand, G.; Tedgui, A.; Lehoux, S.; Boulanger, C.M. Shear stress regulates endothelial microparticle release. Circ. Res. 2013, 112, 1323–1333. [Google Scholar] [CrossRef]
- Al Faraj, A.; Gazeau, F.; Wilhelm, C.; Devue, C.; Guerin, C.L.; Pechoux, C.; Paradis, V.; Clement, O.; Boulanger, C.M.; Rautou, P.E. Endothelial cell-derived microparticles loaded with iron oxide nanoparticles: Feasibility of MR imaging monitoring in mice. Radiology 2012, 263, 169–178. [Google Scholar] [CrossRef]
- Werner, N.; Wassmann, S.; Ahlers, P.; Kosiol, S.; Nickenig, G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 112–116. [Google Scholar] [CrossRef]
- van Ierssel, S.H.; Hoymans, V.Y.; Van Craenenbroeck, E.M.; Van Tendeloo, V.F.; Vrints, C.J.; Jorens, P.G.; Conraads, V.M. Endothelial microparticles (EMP) for the assessment of endothelial function: An in vitro and in vivo study on possible interference of plasma lipids. PLoS ONE 2012, 7, e31496. [Google Scholar] [CrossRef] [PubMed]
- Amabile, N.; Heiss, C.; Real, W.M.; Minasi, P.; McGlothlin, D.; Rame, E.J.; Grossman, W.; De Marco, T.; Yeghiazarians, Y. Circulating endothelial microparticle levels predict hemodynamic severity of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2008, 177, 1268–1275. [Google Scholar] [CrossRef]
- Zacharia, E.; Antonopoulos, A.S.; Oikonomou, E.; Papageorgiou, N.; Pallantza, Z.; Miliou, A.; Mystakidi, V.C.; Simantiris, S.; Kriebardis, A.; Orologas, N.; et al. Plasma signature of apoptotic microvesicles is associated with endothelial dysfunction and plaque rupture in acute coronary syndromes. J. Mol. Cell Cardiol. 2020, 138, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bi, L.; He, X.; Wang, Z.; Qian, Y.; Xiao, L.; Shi, B. Follicular Helper T Cell Derived Exosomes Promote B Cell Proliferation and Differentiation in Antibody-Mediated Rejection after Renal Transplantation. BioMed Res. Int. 2019, 2019, 6387924. [Google Scholar] [CrossRef]
- Altara, R.; Gu, Y.M.; Struijker-Boudier, H.A.; Thijs, L.; Staessen, J.A.; Blankesteijn, W.M. Left ventricular dysfunction and CXCR3 ligands in hypertension: From animal experiments to a population-based pilot study. PLoS ONE 2015, 10, e0141394. [Google Scholar] [CrossRef] [PubMed]
- Altara, R.; Gu, Y.M.; Struijker-Boudier, H.A.; Staessen, J.A.; Blankesteijn, W.M. Circulating CXCL-9,−10 and−11 levels improve the discrimination of risk prediction models for left ventricular dysfunction. FASEB J. 2015, 29, 46.2. [Google Scholar] [CrossRef]
- Altara, R.; Manca, M.; Hessel, M.H.; Gu, Y.; van Vark, L.C.; Akkerhuis, K.M.; Staessen, J.A.; Struijker-Boudier, H.A.J.; Booz, G.W.; Blankesteijn, W.M. CXCL10 is a circulating inflammatory marker in patients with advanced heart failure: A pilot study. J. Cardiovasc. Trans. Res. 2016, 9, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Sanhueza, S.; Vidal, M.A.; Hernandez, M.A.; Henriquez-Beltran, M.E.; Cabrera, C.; Quiroga, R.; Antilef, B.E.; Aguilar, K.P.; Castillo, D.A.; Llerena, F.; et al. Clinical and pulmonary function analysis in long-COVID revealed that long-term pulmonary dysfunction is associated with vascular inflammation pathways and metabolic syndrome. Front. Med. 2023, 10, 1271863. [Google Scholar] [CrossRef]
- Ciullo, A.; Biemmi, V.; Milano, G.; Bolis, S.; Cervio, E.; Fertig, E.T.; Gherghiceanu, M.; Moccetti, T.; Camici, G.G.; Vassalli, G.; et al. Exosomal Expression of CXCR4 Targets Cardioprotective Vesicles to Myocardial Infarction and Improves Outcome after Systemic Administration. Int. J. Mol. Sci. 2019, 20, 468. [Google Scholar] [CrossRef]
- Wan, W.; Murphy, P.M. Regulation of atherogenesis by chemokine receptor CCR6. Trends Cardiovasc. Med. 2011, 21, 140–144. [Google Scholar] [CrossRef]
- Arunachalam, P.; Ludewig, P.; Melich, P.; Arumugam, T.V.; Gerloff, C.; Prinz, I.; Magnus, T.; Gelderblom, M. CCR6 (CC Chemokine Receptor 6) Is Essential for the Migration of Detrimental Natural Interleukin-17-Producing gammadelta T Cells in Stroke. Stroke 2017, 48, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; Frantz, S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ. Res. 2015, 116, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, D.; Liehn, E.A.; Singh, A.; Curaj, A.; Wijnands, E.; Lira, S.A.; Tacke, F.; Jankowski, J.; Biessen, E.A.L.; van der Vorst, E.P.C. CCR6 Deficiency Increases Infarct Size after Murine Acute Myocardial Infarction. Biomedicines 2021, 9, 1532. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Bajpai, G.; Ma, P.; Koenig, A.; Bredemeyer, A.; Lokshina, I.; Lai, L.; Forster, I.; Leuschner, F.; Kreisel, D.; et al. CCL17 Aggravates Myocardial Injury by Suppressing Recruitment of Regulatory T Cells. Circulation 2022, 145, 765–782. [Google Scholar] [CrossRef] [PubMed]


| CONTROL | AMI | |
|---|---|---|
| n | 8 | 10 |
| Gender (F/M) | 5/3 (62%/38%) | 6/4 (60%/40%) |
| Age | 60 ± 2.4 | 64.8 ± 5.1 |
| Weight (kg) | 74.9 ± 5.7 | 70.1 ± 4.2 |
| Height (m) | 1.6 ± 0.1 | 1.6 ± 1.4 |
| BMI | 29.6 ±1.9 | 26.6 ±1.2 * |
| Waist Circumference (cm) | 95.5 ± 4.6 | 102.6 ± 3.3 |
| Glycemia (mg/dL) | 76.4 ± 5.6 | 176.0 ± 45.0 * |
| Total Cholesterol (mg/dL) | 171.7± 14.5 | 181.5± 12.7 |
| Triglycerides | 130.4 ± 7.6 | 149.6 ± 27.9 |
| LDL Cholesterol (mg/dL) | 119.3 ± 20.8 | 107.8 ± 12.4 |
| HDL Cholesterol (mg/dL) | 38.1 ± 5.1 | 43.0 ± 3.9 |
| sLox-1 | 156.7 ± 55 | 294 ± 70.3 * |
| Troponin (ng/L) | 12 ± 3.5 | 238 ± 72.4 * |
| CK-Total (U/L) | 150 ± 20 | 2.205 ± 677 * |
| CK-Mb (ng/mL) | 3.0 ± 3 | 219.8 ± 64.7 * |
| hCRP (mg/dL) | 1.0 ± 0.2 | 1.6 ± 0.5 |
| Previous Myocardial Infarction | ||
| Yes | - | 0 (0%) |
| No | - | 10 (100%) |
| Diagnosis | ||
| AMI with ST-segment elevation in inferior wall | - | 5 (50%) |
| AMI with ST-segment elevation in anterior wall | - | 4 (40%) |
| AMI with ST-segment elevation in anteroseptal area | - | 1 (10%) |
| TIMI Score | ||
| I | - | 0 |
| II | - | 0 |
| III | - | 10 (100%) |
| KILLIP score | ||
| I | - | 5 (50%) |
| II | - | 5 (50%) |
| III | - | 0 (0%) |
| IV | - | 0 (0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, A.; Alarcón-Zapata, P.; Guzmán-Gútierrez, E.; Radojkovic, C.; Contreras, H.; Nova-Lampeti, E.; A. Zúñiga, F.; Rodriguez-Alvárez, L.; Escudero, C.; Lagos, P.; et al. Changes in the Release of Endothelial Extracellular Vesicles CD144+, CCR6+, and CXCR3+ in Individuals with Acute Myocardial Infarction. Biomedicines 2024, 12, 2119. https://doi.org/10.3390/biomedicines12092119
Moreno A, Alarcón-Zapata P, Guzmán-Gútierrez E, Radojkovic C, Contreras H, Nova-Lampeti E, A. Zúñiga F, Rodriguez-Alvárez L, Escudero C, Lagos P, et al. Changes in the Release of Endothelial Extracellular Vesicles CD144+, CCR6+, and CXCR3+ in Individuals with Acute Myocardial Infarction. Biomedicines. 2024; 12(9):2119. https://doi.org/10.3390/biomedicines12092119
Chicago/Turabian StyleMoreno, Alexa, Pedro Alarcón-Zapata, Enrique Guzmán-Gútierrez, Claudia Radojkovic, Héctor Contreras, Estefanía Nova-Lampeti, Felipe A. Zúñiga, Llerenty Rodriguez-Alvárez, Carlos Escudero, Paola Lagos, and et al. 2024. "Changes in the Release of Endothelial Extracellular Vesicles CD144+, CCR6+, and CXCR3+ in Individuals with Acute Myocardial Infarction" Biomedicines 12, no. 9: 2119. https://doi.org/10.3390/biomedicines12092119








