COVID-19 Inflammatory Syndrome: Lessons from TNFRI and CRP about the Risk of Death in Severe Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design Study and Participants
2.2. Procedures and Cytokine Measurement Methods
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. TNF Family Soluble Factors
3.3. Effects of Biomarkers among COVID-19 Patients
3.4. Soluble TNFRs Predict Prognosis of Patients with Severe COVID-19
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clementi, N.; Ghosh, S.; De Santis, M.; Castelli, M.; Criscuolo, E.; Zanoni, I.; Clementi, M.; Mancini, N. Viral Respiratory Pathogens and Lung Injury. Clin. Microbiol. Rev. 2021, 34, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Akbari, H.; Tabrizi, R.; Lankarani, K.B.; Aria, H.; Vakili, S.; Asadian, F.; Noroozi, S.; Keshavarz, P.; Faramarz, S. The Role of Cytokine Profile and Lymphocyte Subsets in the Severity of Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. Life Sci. 2020, 258, 118167. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yin, Z.; Hu, Y.; Mei, H. Controlling Cytokine Storm Is Vital in COVID-19. Front. Immunol. 2020, 11, 3158. [Google Scholar] [CrossRef] [PubMed]
- Leisman, D.E.; Ronner, L.; Pinotti, R.; Taylor, M.D.; Sinha, P.; Calfee, C.S.; Hirayama, A.V.; Mastroiani, F.; Turtle, C.J.; Harhay, M.O.; et al. Cytokine Elevation in Severe and Critical COVID-19: A Rapid Systematic Review, Meta-Analysis, and Comparison with Other Inflammatory Syndromes. Lancet Respir. Med. 2020, 8, 1233–1244. [Google Scholar] [CrossRef]
- Herbein, G.; O’Brien, W.A. Tumor Necrosis Factor (TNF)-Alpha and TNF Receptors in Viral Pathogenesis. Proc. Soc. Exp. Biol. Med. 2000, 223, 241–257. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Eisenhut, M.; Shin, J. Il Pathways in the Pathophysiology of Coronavirus 19 Lung Disease Accessible to Prevention and Treatment. Front. Physiol. 2020, 11, 872. [Google Scholar] [CrossRef]
- Vanamee, É.S.; Faustman, D.L. Structural Principles of Tumor Necrosis Factor Superfamily Signaling. Sci. Signal. 2018, 11, eaao4910. [Google Scholar] [CrossRef]
- Cabal-Hierro, L.; Lazo, P.S. Signal Transduction by Tumor Necrosis Factor Receptors. Cell Signal. 2012, 24, 1297–1305. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Ellis, P.D. The Essential Guide to Effect Sizes; Cambridge University Press: Cambridge, UK, 2010; ISBN 9780521194235. [Google Scholar]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect Size Estimates: Current Use, Calculations, and Interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, W.F.; Miguel, C.B.; Marques, L.C.; da Costa, T.A.; de Abreu, M.C.M.; Oliveira, C.J.F.; Lazo-Chica, J.E. Predicting Blood Parasite Load and Influence of Expression of INOS on the Effect Size of Clinical Laboratory Parameters in Acute Trypanosoma Cruzi Infection with Different Inoculum Concentrations in C57BL/6 Mice. Front. Immunol. 2022, 13, 850037. [Google Scholar] [CrossRef] [PubMed]
- Arango, H.G. Bioestatística: Teórica E Computacional; Guanabara Koogan SA: Rio de Janeiro, Brazil, 2001. [Google Scholar]
- Casella, G.; Berger, R. Statistical Inference; Chapman and Hall/CRC: Boca Raton, FL, USA, 2024; ISBN 9781003456285. [Google Scholar]
- Ferretti, V.V.; Klersy, C.; Bruno, R.; Cutti, S.; Nappi, R.E. More on Age and Gender in COVID-19. Maturitas 2022, 163, 89. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Soto, M.C.; Ortega-Cáceres, G.; Arroyo-Hernández, H. Sex Differences in COVID-19 Fatality Rate and Risk of Death: An Analysis in 73 Countries, 2020–2021. Infez. Med. 2021, 29, 402. [Google Scholar] [CrossRef]
- Teodoro, A.G.F.; Rodrigues, W.F.; Farnesi-de-Assunção, T.S.; Borges, A.V.B.E.; Obata, M.M.S.; Neto, J.R.D.C.; da Silva, D.A.A.; Andrade-Silva, L.E.; Desidério, C.S.; Costa-Madeira, J.C.; et al. Inflammatory Response and Activation of Coagulation after COVID-19 Infection. Viruses 2023, 15, 938. [Google Scholar] [CrossRef]
- da Silva, D.A.A.; de Andrade-Silva, L.E.; Desidério, C.S.; Assunção, T.S.F.d.; Oliveira, A.C.d.M.; Trevisan, R.O.; Santos, M.M.; Helmo, F.R.; Barbosa, L.M.; Costa-Madeira, J.C.; et al. Relation between Hematological and Biochemical Parameters per Days of Symptoms in Hospitalized Patients with Flu-like Syndrome and COVID-19. Res. Soc. Dev. 2022, 11, e54411427439. [Google Scholar] [CrossRef]
- Jiang, S.Q.; Huang, Q.F.; Xie, W.M.; Lv, C.; Quan, X.Q. The Association between Severe COVID-19 and Low Platelet Count: Evidence from 31 Observational Studies Involving 7613 Participants. Br. J. Haematol. 2020, 190, e29–e33. [Google Scholar] [CrossRef]
- Gohda Id, T.; Murakoshi, M.; Suzuki, Y.; Hiki, M.; Naito, T.; Takahashi, K.; Tabe, Y.; Chen, R.J. Circulating Tumor Necrosis Factor Receptors Are Associated with Mortality and Disease Severity in COVID-19 Patients. PLoS ONE 2022, 17, e0275745. [Google Scholar] [CrossRef]
- Mortaz, E.; Tabarsi, P.; Jamaati, H.; Dalil Roofchayee, N.; Dezfuli, N.K.; Hashemian, S.M.R.; Moniri, A.; Marjani, M.; Malekmohammad, M.; Mansouri, D.; et al. Increased Serum Levels of Soluble TNF-α Receptor Is Associated with ICU Mortality in COVID-19 Patients. Front. Immunol. 2021, 12, 592727. [Google Scholar] [CrossRef]
- Palacios, Y.; Ruiz, A.; Ramón-Luing, L.A.; Ocaña-Guzman, R.; Barreto-Rodriguez, O.; Sánchez-Monciváis, A.; Tecuatzi-Cadena, B.; Regalado-García, A.G.; Pineda-Gudiño, R.D.; García-Martínez, A.; et al. Severe COVID-19 Patients Show an Increase in Soluble TNFR1 and ADAM17, with a Relationship to Mortality. Int. J. Mol. Sci. 2021, 22, 8423. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Buendía-Roldán, I.; Ruiz, A.; Palacios, Y.; Pérez-Rubio, G.; de Jesus Hernández-Zenteno, R.; Reyes-Melendres, F.; Zazueta-Márquez, A.; Alarcón-Dionet, A.; Guzmán-Vargas, J.; et al. TNFRSF1B and TNF Variants Are Associated with Differences in Levels of Soluble Tumor Necrosis Factor Receptors in Patients With Severe COVID-19. J. Infect. Dis. 2022, 226, 778. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Ramiro, B.; García-Weber, D.; Millán, J. TNF-Induced Endothelial Barrier Disruption: Beyond Actin and Rho. Thromb. Haemost. 2014, 112, 1088–1102. [Google Scholar] [CrossRef] [PubMed]
- Van Hauwermeiren, F.; Vandenbroucke, R.E.; Libert, C. Treatment of TNF Mediated Diseases by Selective Inhibition of Soluble TNF or TNFR1. Cytokine Growth Factor Rev. 2011, 22, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.P.; Pober, J.S.; Bradley, J.R. Tumour Necrosis Factor in Infectious Disease. J. Pathol. 2013, 230, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hua, S. Transcription Factor-Mediated Signaling Pathways’ Contribution to the Pathology of Acute Lung Injury and Acute Respiratory Distress Syndrome. Am. J. Transl. Res. 2020, 12, 5608–5618. [Google Scholar]
- Popescu, I.; Snyder, M.E.; Iasella, C.J.; Hannan, S.J.; Koshy, R.; Burke, R.; Das, A.; Brown, M.J.; Lyons, E.J.; Lieber, S.C.; et al. CD41 T-Cell Dysfunction in Severe COVID-19 Disease Is Tumor Necrosis Factor-a/Tumor Necrosis Factor Receptor 1–Dependent. Am. J. Respir. Crit. Care Med. 2022, 205, 1403–1418. [Google Scholar] [CrossRef]
- Sharma, D.; Malik, A.; Guy, C.; Vogel, P.; Kanneganti, T.D. TNF/TNFR Axis Promotes Pyrin Inflammasome Activation and Distinctly Modulates Pyrin Inflammasomopathy. J. Clin. Investig. 2019, 129, 150–162. [Google Scholar] [CrossRef]
- Su, Z.; Wu, Y. A Systematic Test of Receptor Binding Kinetics for Ligands in Tumor Necrosis Factor Superfamily by Computational Simulations. Int. J. Mol. Sci. 2020, 21, 1778. [Google Scholar] [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF Cytokine Triad Is Associated with Post-Acute Sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef]
- Pober, J.S. Activation and Injury of Endothelial Cells by Cytokines. Pathol. Biol. 1998, 46, 159–163. [Google Scholar]
- Okuyama, M.; Yamaguchi, S.; Yamaoka, M.; Nitobe, J.; Fujii, S.; Yoshimura, T.; Tomoike, H. Nitric Oxide Enhances Expression and Shedding of Tumor Necrosis Factor Receptor I (P55) in Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Kleymenov, D.A.; Bykonia, E.N.; Popova, L.I.; Mazunina, E.P.; Gushchin, V.A.; Kolobukhina, L.V.; Burgasova, O.A.; Kruzhkova, I.S.; Kuznetsova, N.A.; Shidlovskaya, E.V.; et al. A Deep Look Into COVID-19 Severity Through Dynamic Changes in Blood Cytokine Levels. Front. Immunol. 2021, 12, 771609. [Google Scholar] [CrossRef] [PubMed]
- Onuk, S.; Sipahioğlu, H.; Karahan, S.; Yeşiltepe, A.; Kuzugüden, S.; Karabulut, A.; Beştepe Dursun, Z.; Akın, A. Cytokine Levels and Severity of Illness Scoring Systems to Predict Mortality in COVID-19 Infection. Healthcare 2023, 11, 387. [Google Scholar] [CrossRef] [PubMed]
 survivor and
survivor and  death). Patients with active disease and evolution to death (DP/COVID-19pos group, n = 168) and patients with active disease and who survived (SP/COVID-19pos group, n = 154) are also presented. The points represent standard error of the mean (SEM). The statistical differences between groups during the weeks are expressed in the graphic. Multiple t-tests were used, and the results were considered statistically significant at p < 0.05.
death). Patients with active disease and evolution to death (DP/COVID-19pos group, n = 168) and patients with active disease and who survived (SP/COVID-19pos group, n = 154) are also presented. The points represent standard error of the mean (SEM). The statistical differences between groups during the weeks are expressed in the graphic. Multiple t-tests were used, and the results were considered statistically significant at p < 0.05.
 survivor and
survivor and  death). Patients with active disease and evolution to death (DP/COVID-19pos group, n = 168) and patients with active disease and who survived (SP/COVID-19pos group, n = 154) are also presented. The points represent standard error of the mean (SEM). The statistical differences between groups during the weeks are expressed in the graphic. Multiple t-tests were used, and the results were considered statistically significant at p < 0.05.
death). Patients with active disease and evolution to death (DP/COVID-19pos group, n = 168) and patients with active disease and who survived (SP/COVID-19pos group, n = 154) are also presented. The points represent standard error of the mean (SEM). The statistical differences between groups during the weeks are expressed in the graphic. Multiple t-tests were used, and the results were considered statistically significant at p < 0.05.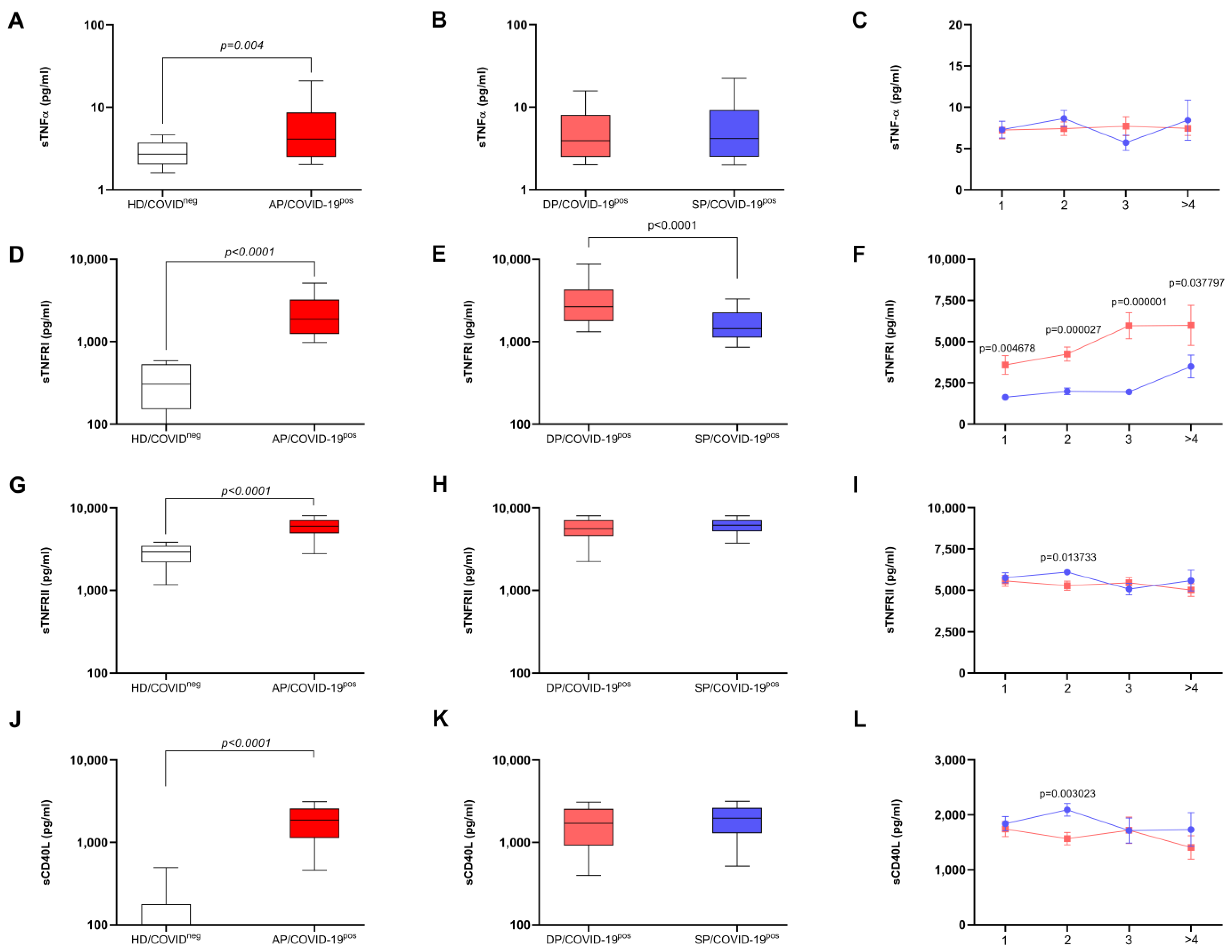

| BLOOD CELLS | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Outcome | N | Ẋ//Md | σ | RBC—% | Power—% | p-Value |
| Erythrocytes—106/mm3 | Survivor | 139 | 4.34//4.41 | 0.68 | 35.85 | 90.00 | <0.001 |
| Deceased | 142 | 3.88//3.81 | 0.85 | ||||
| Hemoglobin—g% | Survivor | 139 | 12.95//13.10 | 1.91 | 34.25 | 87.56 | <0.001 |
| Deceased | 142 | 11.63//11.80 | 2.41 | ||||
| Hematocrit—% | Survivor | 139 | 38.98//39.50 | 5.67 | 29.24 | 77.15 | <0.001 |
| Deceased | 142 | 54.45//35.55 | 223.78 | ||||
| Platelets—103/mm3 | Survivor | 139 | 226.00//205.00 | 89.3 | 17.15 | 40.36 | 0.821 |
| Deceased | 142 | 212.00//205.00 | 83.6 | ||||
| Leukocytes—mm3 | Survivor | 139 | 10,498.13//8800.00 | 5852.82 | 25.19 | 66.01 | <0.001 |
| Deceased | 142 | 13,000.22//11,735.00 | 6877.21 | ||||
| Neutrophils—mm3 | Survivor | 139 | 8179.47//6966.00 | 4985.90 | 28.66 | 75.69 | <0.001 |
| Deceased | 141 | 10,748.47//9877.00 | 6038.81 | ||||
| Band cell—mm3 | Survivor | 139 | 489.47//261.00 | 890.07 | 14.81 | 33.18 | 0.031 |
| Deceased | 141 | 730.42//392.00 | 1036.80 | ||||
| Lymphocytes—mm3 | Survivor | 139 | 1221.66//1014.00 | 679.17 | 32.16 | 83.70 | <0.001 |
| Deceased | 142 | 881.98//815.50 | 468.95 | ||||
| Monocytes—mm3 | Survivor | 139 | 561.31//515.00 | 320.52 | 11.32 | 23.57 | 0.101 |
| Deceased | 142 | 545.79//442.00 | 433.50 | ||||
| Eosinophil—mm3 | Survivor | 138 | 25.21//0.00 | 55.43 | 0.54 | 5.47 | 0.915 |
| Deceased | 141 | 35.23//0.00 | 85.32 | ||||
| CRP—mg/dL | Survivor | 87 | 83.10//58.70 | 69.00 | 42.43 | 83.04 | <0.001 |
| Deceased | 73 | 159.00//130.00 | 113.00 | ||||
| INFLAMMATORY MOLECULES | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Outcome | N | Ẋ//Md | σ | RBC—% | Power—% | p-Value |
| IL-12 p70—pg/mL | Survivor | 153 | 4.58//4.05 | 2.51 | 2.58 | 7.78 | 0.690 |
| Deceased | 168 | 4.62//4.10 | 2.42 | ||||
| IL-1 β—pg/mL | Survivor | 153 | 7.14//6.73 | 2.46 | 3.38 | 8.85 | 0.601 |
| Deceased | 168 | 7.40//6.96 | 3.14 | ||||
| IL-8—pg/mL | Survivor | 153 | 178.58//21.95 | 988.86 | 13.9 | 33.28 | 0.032 |
| Deceased | 168 | 239.11//36.11 | 1342.21 | ||||
| IL-17—pg/mL | Survivor | 153 | 54.67//26.50 | 142.13 | 7.72 | 16.57 | 0.232 |
| Deceased | 168 | 65.91//18.87 | 134.45 | ||||
| IFN-γ—pg/mL | Survivor | 153 | 11.52//6.87 | 12.14 | 17.36 | 44.82 | 0.007 |
| Deceased | 168 | 8.85//5.73 | 9.64 | ||||
| IL-10—pg/mL | Survivor | 153 | 14.88//9.18 | 24.97 | 20.25 | 54.85 | 0.002 |
| Deceased | 168 | 30.62//15.15 | 65.37 | ||||
| IL-6—pg/mL | Survivor | 153 | 99.12//25.48 | 471.98 | 30.72 | 84.98 | <0.001 |
| Deceased | 168 | 377.98//73.59 | 1420.58 | ||||
| IL-4—pg/mL | Survivor | 153 | 14.62//10.76 | 10.96 | 19.83 | 53.40 | 0.002 |
| Deceased | 168 | 11.32//8.86 | 7.58 | ||||
| IL-2—pg/mL | Survivor | 153 | 5.95//6.28 | 3.15 | 12.76 | 29.75 | 0.048 |
| Deceased | 168 | 5.37//5.11 | 3.24 | ||||
| Adiponectin—µg/mL | Survivor | 147 | 5.38 × 106//3.85 × 106 | 3.74 × 106 | 6.28 | 13.44 | 0.339 |
| Deceased | 164 | 6.16 × 106//4.95 × 106 | 4.89 × 106 | ||||
| Leptin—ng/mL | Survivor | 147 | 30,052.30//21,348.80 | 29,388.90 | 16.69 | 41.60 | 0.011 |
| Deceased | 164 | 26,778.00//12,097.00 | 34,571.00 | ||||
| MIF—pg/mL | Survivor | 153 | 5299.00//3655.55 | 5342.10 | 22.12 | 61.22 | <0.001 |
| Deceased | 168 | 7549.00//4732.00 | 7484.00 | ||||
| sCD40L—pg/mL | Survivor | 151 | 1928.80//1952.70 | 979.20 | 19.53 | 52.03 | 0.003 |
| Deceased | 167 | 1623.00//1539.00 | 1082.00 | ||||
| RECEPTOR FOR TNF AND THE RATIO | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Outcome | N | Ẋ//Md | σ | RBC or Cohen’s d—% | Power—% | p-Value |
| sTNF-α—pg/mL | Survivor | 154 | 7.75//4.48 | 7.45 | 2.69 | 7.93 | 0.677 |
| Deceased | 168 | 7.44//5.66 | 6.53 | ||||
| sTNFRI—pg/mL | Survivor | 154 | 2034.00//1508.00 | 1629.00 | 54.58 | 99.91 | <0.001 |
| Deceased | 168 | 5224.00//3260.00 | 5840.00 | ||||
| sTNFRII—pg/mL | Survivor | 154 | 5760.00//5997.00 | 1855.00 | 11.12 | 25.04 | 0.085 |
| Deceased | 168 | 5356.00//5453.00 | 2100.00 | ||||
| sTNFRI/sTNF-α | Survivor | 154 | 496.00//388.00 | 528.00 | 35.80 | 94.03 | <0.001 |
| Deceased | 168 | 1141.00//590.00 | 1392.00 | ||||
| sTNFRII/sTNF-α | Survivor | 154 | 1555.00//1246.00 | 1245.00 | 9.31 | 20.84 | 0.149 |
| Deceased | 168 | 1341.00//1014.00 | 1222.00 | ||||
| sTNFRI/sTNFRII | Survivor | 154 | 0.419//0.245 | 0.49 | 51.90 | 99.86 | <0.001 |
| Deceased | 168 | 1.33//0.590 | 1.70 | ||||
| Predictor (Parameter) 1 | Odds Ratio | 95% Confidence Interval | p-Value | |
| Lower | Upper | |||
| Hemoglobin (g%) | 0.7948 | 0.599 | 1.0600 | 0.112 |
| Neutrophils (%) | 1.0452 | 0.988 | 1.1100 | 0.122 |
| CRP (mg/L) | 1.0073 | 1.002 | 1.0100 | 0.011 |
| sTNFRI/sTNFRII ratio | 7.2332 | 1.857 | 28.1700 | 0.004 |
| Performance Metrics | ||||
| Accuracy | 0.740 | Specificity | 0.870 | |
| AUC | 0.830 | Sensitivity | 0.520 | |
| Predictor (Parameter) 1 | Odds ratio | 95% Confidence Interval | p-Value | |
| Lower | Upper | |||
| CRP (mg/L) | 1.0084 | 1.0032 | 1.0140 | 0.002 |
| sTNFRI/sTNFRII ratio | 8.3405 | 2.4201 | 28.7450 | <0.001 |
| Performance Metrics | ||||
| Accuracy | 0.770 | Specificity | 0.900 | |
| AUC | 0.810 | Sensitivity | 0.590 | |
| Predictor (Parameter) 1 | Odds ratio | 95% Confidence Interval | p-Value | |
| Lower | Upper | |||
| sTNFRI/sTNFRII ratio | 3.9740 | 2.0700 | 7.6280 | <0.001 |
| Performance Metrics | ||||
| Accuracy | 0.680 | Specificity | 0.920 | |
| AUC | 0.740 | Sensitivity | 0.370 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farnesi-de-Assunção, T.S.; Oliveira-Scussel, A.C.d.M.; Rodrigues, W.F.; Matos, B.S.; da Silva, D.A.A.; de Andrade e Silva, L.E.; Mundim, F.V.; Helmo, F.R.; Bernardes e Borges, A.V.; Desidério, C.S.; et al. COVID-19 Inflammatory Syndrome: Lessons from TNFRI and CRP about the Risk of Death in Severe Disease. Biomedicines 2024, 12, 2138. https://doi.org/10.3390/biomedicines12092138
Farnesi-de-Assunção TS, Oliveira-Scussel ACdM, Rodrigues WF, Matos BS, da Silva DAA, de Andrade e Silva LE, Mundim FV, Helmo FR, Bernardes e Borges AV, Desidério CS, et al. COVID-19 Inflammatory Syndrome: Lessons from TNFRI and CRP about the Risk of Death in Severe Disease. Biomedicines. 2024; 12(9):2138. https://doi.org/10.3390/biomedicines12092138
Chicago/Turabian StyleFarnesi-de-Assunção, Thaís Soares, Ana Carolina de Morais Oliveira-Scussel, Wellington Francisco Rodrigues, Beatriz Sodré Matos, Djalma Alexandre Alves da Silva, Leonardo Eurípedes de Andrade e Silva, Fabiano Vilela Mundim, Fernanda Rodrigues Helmo, Anna Victória Bernardes e Borges, Chamberttan Souza Desidério, and et al. 2024. "COVID-19 Inflammatory Syndrome: Lessons from TNFRI and CRP about the Risk of Death in Severe Disease" Biomedicines 12, no. 9: 2138. https://doi.org/10.3390/biomedicines12092138






