Analysis of Brain Age Gap across Subject Cohorts and Prediction Model Architectures
Abstract
1. Introduction
2. Materials and Methods
2.1. Datasets
2.1.1. Multi-Site Train Dataset
2.1.2. Diseased Datasets
2.1.3. T1-Weighted Image Preprocessing
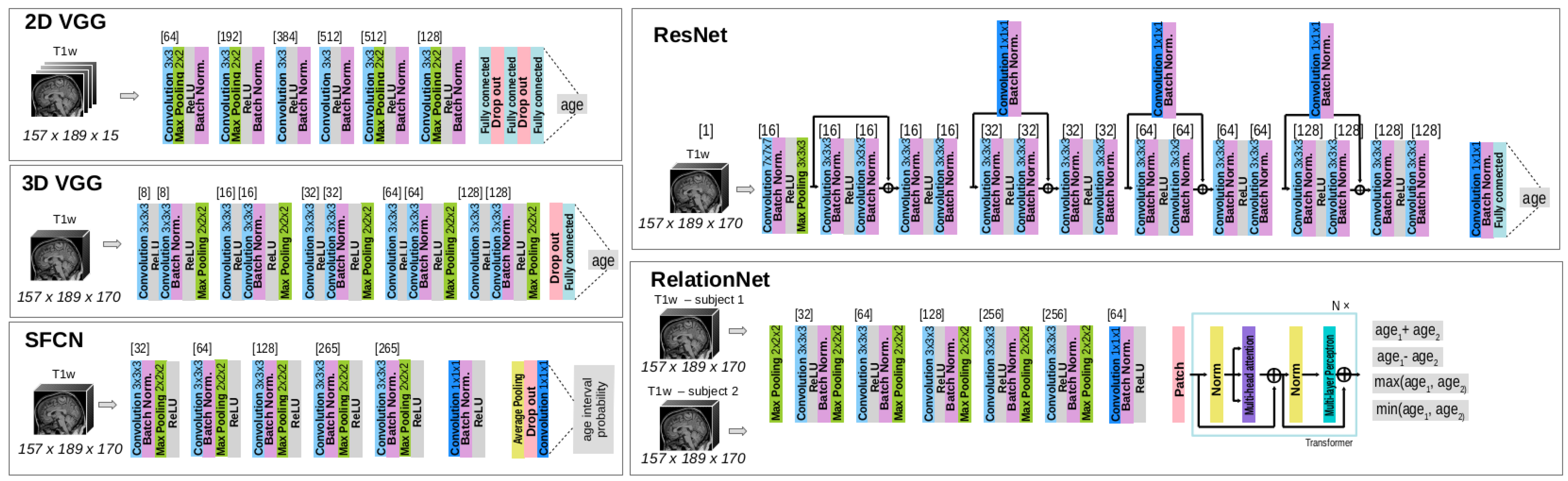
2.2. Model Architectures
2.3. Model Setup and Evaluation Protocols
2.3.1. Model Training
2.3.2. Evaluation
2.3.3. Statistical Analysis
3. Results
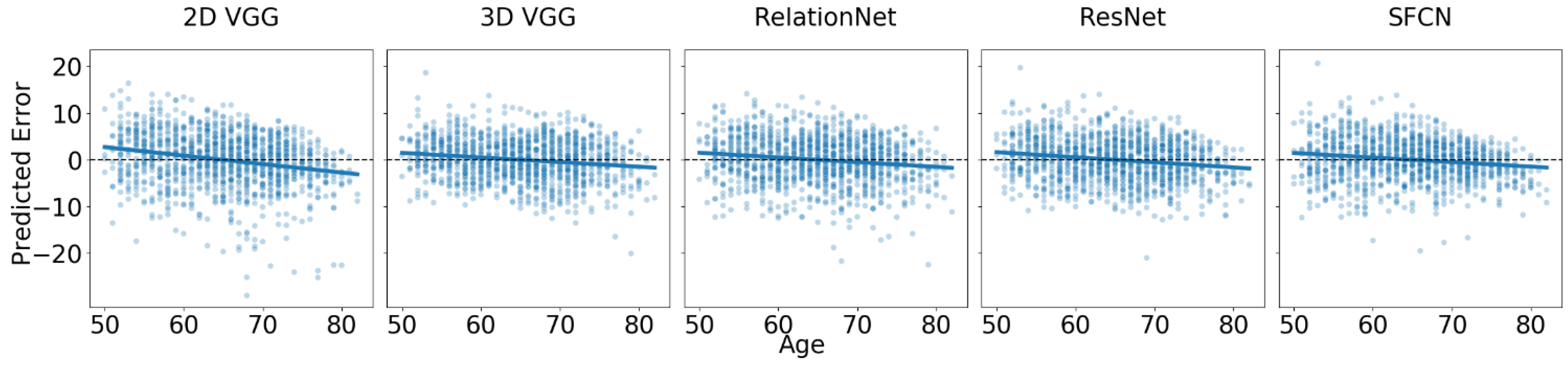
3.1. Baseline Performance on Dataset of Healthy Cohorts
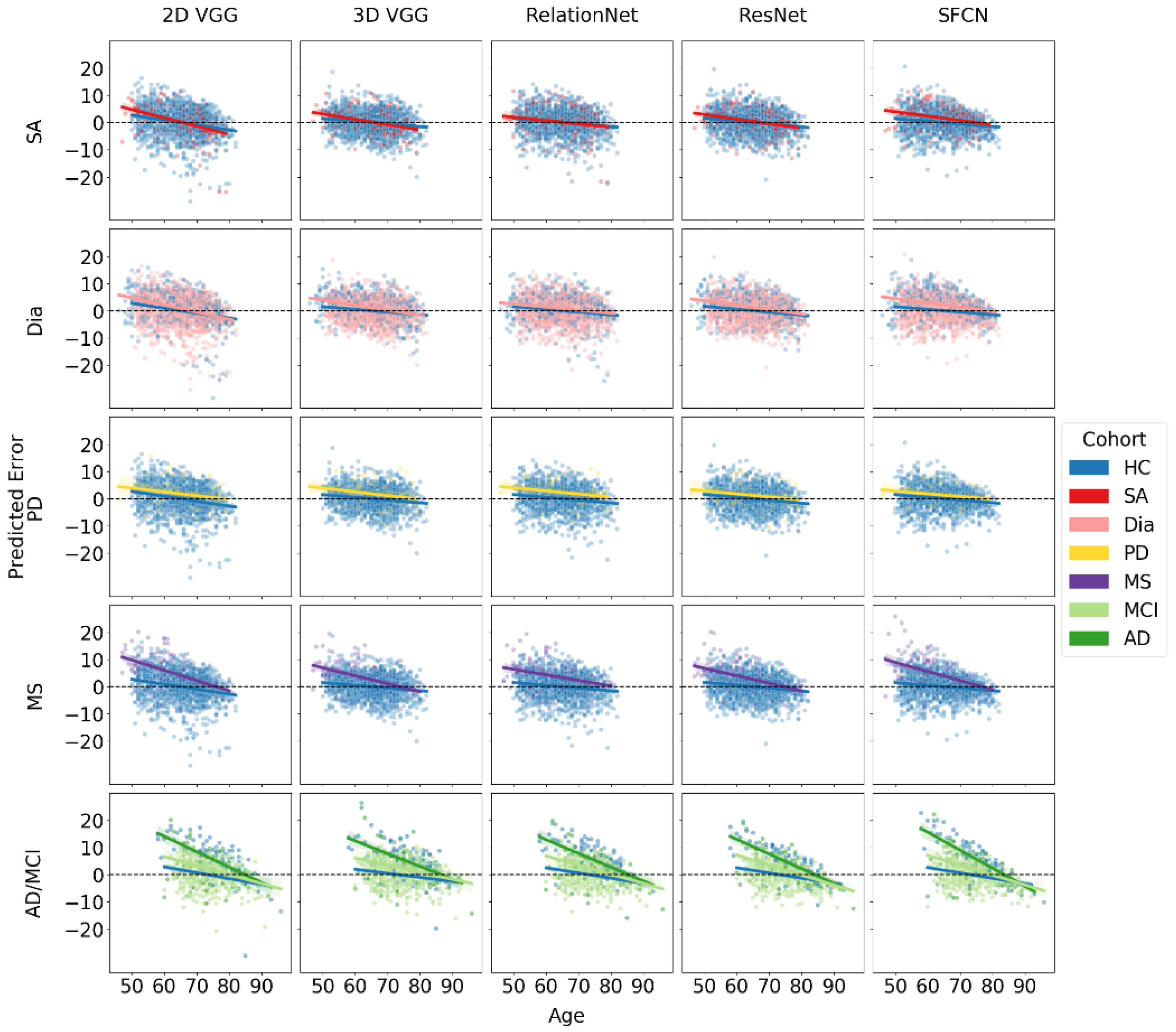
3.2. Brain Age Gap for Diseased Subgroups
4. Discussion
4.1. Brain Age Gap across Subject Cohorts
4.2. Brain Age Gap across Different Model Architectures
4.3. Limitations
4.4. Future Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T1w | T1-weighted |
| MR | Magnetic Resonance |
| BAG | Brain Age Gap |
| HC | Healthy Controls |
| SA | Sleep Apnea |
| Dia | Diabetes |
| MS | Multiple Sclerosis |
| PD | Parkinson’s Disease |
| MCI | Mild Cognitive Impairment |
| AD | Alzheimer’s Dementia |
| MNI | Montreal Neurological Institute |
| KLD | Kullback–Leibler Divergence |
| SGD | Stochastic Gradient Descent |
| BASE | Brain Age Standardized Evaluation |
| MAE | Mean Absolute Error |
| LMEMs | Linear Mixed-Effects Models |
| ANOVA | Analysis of Variance |
| EMM | Estimated Marginal Means |
References
- Sajedi, H.; Pardakhti, N. Age Prediction Based on Brain MRI Image: A Survey. J. Med. Syst. 2019, 43, 279. [Google Scholar] [CrossRef]
- Cole, J.H.; Franke, K.; Cherbuin, N. Quantification of the Biological Age of the Brain Using Neuroimaging. In Biomarkers of Human Aging; Healthy Ageing and Longevity; Moskalev, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 293–328. [Google Scholar] [CrossRef]
- Bashyam, V.M.; Erus, G.; Doshi, J.; Habes, M.; Nasrallah, I.M.; Truelove-Hill, M.; Srinivasan, D.; Mamourian, L.; Pomponio, R.; Fan, Y.; et al. MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14468 individuals worldwide. Brain 2020, 143, 2312–2324. [Google Scholar] [CrossRef]
- Beheshti, I.; Mishra, S.; Sone, D.; Khanna, P.; Matsuda, H. T1-weighted MRI-driven Brain Age Estimation in Alzheimer’s Disease and Parkinson’s Disease. Aging Dis. 2020, 11, 618–628. [Google Scholar] [CrossRef]
- Charissé, D.; Erus, G.; Pomponio, R.; Gorges, M.; Schmidt, N.; Schneider, C.; Liepelt-Scarfone, I.; Riedel, O.; Reetz, K.; Schulz, J.B.; et al. Brain age and Alzheimer’s-like atrophy are domain-specific predictors of cognitive impairment in Parkinson’s disease. Neurobiol. Aging 2022, 109, 31–42. [Google Scholar] [CrossRef]
- Chen, C.-L.; Cheng, S.-Y.; Montaser-Kouhsari, L.; Wu, W.-C.; Hsu, Y.-C.; Tai, C.-H.; Tseng, W.-Y.I.; Kuo, M.-C.; Wu, R.-M. Advanced brain aging in Parkinson’s disease with cognitive impairment. NPJ Park. Dis. 2024, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Gaser, C. Longitudinal changes in individual BrainAGE in healthy aging, mild cognitive impairment, and Alzheimer’s disease. GeroPsych J. Gerontopsychol. Geriatr. Psychiatry 2012, 25, 235–245. [Google Scholar] [CrossRef]
- Lee, J.; Burkett, B.J.; Min, H.K.; Senjem, M.L.; Lundt, E.S.; Botha, H.; Graff-Radford, J.; Barnard, L.R.; Gunter, J.L.; Schwarz, C.G.; et al. Deep learning-based brain age prediction in normal aging and dementia. Nat. Aging 2022, 2, 412–424. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, Z.; Guan, H.; Wu, Z.; Zhu, H.; Jiang, J.; Wen, W.; Tao, D.; Liu, T. Brain Age Estimation From MRI Using Cascade Networks With Ranking Loss. IEEE Trans. Med. Imaging 2021, 40, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Høgestøl, E.A.; Kaufmann, T.; Nygaard, G.O.; Beyer, M.K.; Sowa, P.; Nordvik, J.E.; Kolskår, K.; Richard, G.; Andreassen, O.A.; Harbo, H.F.; et al. Cross-Sectional and Longitudinal MRI Brain Scans Reveal Accelerated Brain Aging in Multiple Sclerosis. Front. Neurol. 2019, 10, 450. [Google Scholar] [CrossRef]
- Brier, M.R.; Li, Z.; Ly, M.; Karim, H.T.; Liang, L.; Du, W.; McCarthy, J.E.; Cross, A.H.; Benzinger, T.L.S.; Naismith, R.T.; et al. “Brain age” predicts disability accumulation in multiple sclerosis. Ann. Clin. Transl. Neurol. 2023, 10, 990–1001. [Google Scholar] [CrossRef]
- Cole, J.H.; Raffel, J.; Friede, T.; Eshaghi, A.; Brownlee, W.J.; Chard, D.; Stefano, N.D.; Enzinger, C.; Pirpamer, L.; Filippi, M.; et al. Longitudinal Assessment of Multiple Sclerosis with the Brain-Age Paradigm. Ann. Neurol. 2020, 88, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Franke, K.; Gaser, C.; Manor, B.; Novak, V. Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front. Aging Neurosci. 2013, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Paixao, L.; Oliva, J.T.; Goparaju, B.; Carvalho, D.Z.; van Leeuwen, K.G.; Akeju, O.; Thomas, R.J.; Cash, S.S.; Bianchi, M.T.; et al. Brain age from the electroencephalogram of sleep. Neurobiol. Aging 2019, 74, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kolbeinsson, A.; Filippi, S.; Panagakis, Y.; Matthews, P.M.; Elliott, P.; Dehghan, A.; Tzoulaki, I. Accelerated MRI-predicted brain ageing and its associations with cardiometabolic and brain disorders. Sci. Rep. 2020, 10, 19940. [Google Scholar] [CrossRef]
- Cole, J.H. Multimodality neuroimaging brain-age in UK biobank: Relationship to biomedical, lifestyle, and cognitive factors. Neurobiol. Aging 2020, 92, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Chin Fatt, C.R.; Minhajuddin, A.; Mayes, T.L.; Berry, J.D.; Trivedi, M.H. Accelerated brain aging in individuals with diabetes: Association with poor glycemic control and increased all-cause mortality. Psychoneuroendocrinology 2022, 145, 105921. [Google Scholar] [CrossRef]
- Zhang, T.; Shaw, M.; Cherbuin, N. Association between Type 2 Diabetes Mellitus and Brain Atrophy: A Meta-Analysis. Diabetes Metab. J. 2022, 46, 781–802. [Google Scholar] [CrossRef]
- Wang, D.Q.; Wang, L.; Wei, M.M.; Xia, X.S.; Tian, X.L.; Cui, X.H.; Li, X. Relationship Between Type 2 Diabetes and White Matter Hyperintensity: A Systematic Review. Front. Endocrinol. 2020, 11, 595962. [Google Scholar] [CrossRef]
- Weihs, A.; Frenzel, S.; Wittfeld, K.; Obst, A.; Stubbe, B.; Habes, M.; Szentkirályi, A.; Berger, K.; Fietze, I.; Penzel, T.; et al. Associations between sleep apnea and advanced brain aging in a large-scale population study. Sleep 2021, 44, zsaa204. [Google Scholar] [CrossRef]
- Bittner, N.; Jockwitz, C.; Franke, K.; Gaser, C.; Moebus, S.; Bayen, U.J.; Amunts, K.; Caspers, S. When your brain looks older than expected: Combined lifestyle risk and BrainAGE. Brain Struct. Funct. 2021, 226, 621–645. [Google Scholar] [CrossRef]
- Dular, L.; Špiclin, Ž. BASE: Brain Age Standardized Evaluation. NeuroImage 2024, 285, 120469. [Google Scholar] [CrossRef] [PubMed]
- Baecker, L.; Dafflon, J.; da Costa, P.F.; Garcia-Dias, R.; Vieira, S.; Scarpazza, C.; Calhoun, V.D.; Sato, J.R.; Mechelli, A.; Pinaya, W.H.L. Brain age prediction: A comparison between machine learning models using region- and voxel-based morphometric data. Hum. Brain Mapp. 2021, 42, 2332–2346. [Google Scholar] [CrossRef]
- Beheshti, I.; Ganaie, M.A.; Paliwal, V.; Rastogi, A.; Razzak, I.; Tanveer, M. Predicting Brain Age Using Machine Learning Algorithms: A Comprehensive Evaluation. IEEE J. Biomed. Health Infor. 2022, 26, 1432–1440. [Google Scholar] [CrossRef]
- Kaufmann, T.; van der Meer, D.; Doan, N.T.; Schwarz, E.; Lund, M.J.; Agartz, I.; Alnæs, D.; Barch, D.M.; Baur-Streubel, R.; Bertolino, A.; et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat. Neurosci. 2019, 22, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Antonopoulos, G.; Hoffstaedter, F.; Caspers, J.; Eickhoff, S.B.; Patil, K.R. Brain-age prediction: A systematic comparison of machine learning workflows. NeuroImage 2023, 270, 119947. [Google Scholar] [CrossRef]
- Shafto, M.A.; Tyler, L.K.; Dixon, M.; Taylor, J.R.; Rowe, J.B.; Cusack, R.; Calder, A.J.; Marslen-Wilson, W.D.; Duncan, J.; Dalgleish, T.; et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: A cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Williams, N.; Cusack, R.; Auer, T.; Shafto, M.A.; Dixon, M.; Tyler, L.K.; Cam-CAN; Henson, R.N. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: Structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. NeuroImage 2017, 144, 262–269. [Google Scholar] [CrossRef]
- Souza, R.; Lucena, O.; Garrafa, J.; Gobbi, D.; Saluzzi, M.; Appenzeller, S.; Rittner, L.; Frayne, R.; Lotufo, R. An open, multi-vendor, multi-field-strength brain MR dataset and analysis of publicly available skull stripping methods agreement. NeuroImage 2018, 170, 482–494. [Google Scholar] [CrossRef]
- Marcus, D.S.; Fotenos, A.F.; Csernansky, J.G.; Morris, J.C.; Buckner, R.L. Open Access Series of Imaging Studies: Longitudinal MRI Data in Nondemented and Demented Older Adults. J. Cogn. Neurosci. 2010, 22, 2677–2684. [Google Scholar] [CrossRef]
- Miller, K.L.; Alfaro-Almagro, F.; Bangerter, N.K.; Thomas, D.L.; Yacoub, E.; Xu, J.; Bartsch, A.J.; Jbabdi, S.; Sotiropoulos, S.N.; Andersson, J.L.R.; et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. 2016, 19, 1523–1536. [Google Scholar] [CrossRef]
- Pucak, M.L.; Carroll, K.A.L.; Kerr, D.A.; Kaplin, A.L. Neuropsychiatric manifestations of depression in multiple sclerosis: Neuroinflammatory, neuroendocrine, and neurotrophic mechanisms in the pathogenesis of immune-mediated depression. Dialogues Clin. Neurosci. 2007, 9, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Chikatimalla, R.; Dasaradhan, T.; Koneti, J.; Cherukuri, S.; Kalluru, R.; Gadde, S. Depression in Parkinson’s Disease: A Narrative Review. Cureus 2022, 14, e27750. [Google Scholar] [CrossRef]
- Ellis, K.A.; Bush, A.I.; Darby, D.; De Fazio, D.; Foster, J.; Hudson, P.; Lautenschlager, N.T.; Lenzo, N.; Martins, R.N.; Maruff, P.; et al. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: Methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. Int. Psychogeriatr. 2009, 21, 672–687. [Google Scholar] [CrossRef] [PubMed]
- Dular, L.; Pernuš, F.; Špiclin, Ž. Extensive T1-weighted MRI preprocessing improves generalizability of deep brain age prediction models. Comput. Biol. Med. 2024, 173, 108320. [Google Scholar] [CrossRef]
- Manjón, J.V.; Coupé, P.; Martí-Bonmatí, L.; Collins, D.L.; Robles, M. Adaptive non-local means denoising of MR images with spatially varying noise levels. J. Magn. Reson. Imaging 2010, 31, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Modat, M.; Cash, D.M.; Daga, P.; Winston, G.P.; Duncan, J.S.; Ourselin, S. Global image registration using a symmetric block-matching approach. J. Med. Imaging 2014, 1, 024003. [Google Scholar] [CrossRef] [PubMed]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef]
- Huang, T.; Chen, H.; Fujimoto, R.; Ito, K.; Wu, K.; Sato, K.; Taki, Y.; Fukuda, H.; Aoki, T. Age estimation from brain MRI images using deep learning. In Proceedings of the 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017), Melbourne, Australia, 18–21 April 2017; pp. 849–852. [Google Scholar] [CrossRef]
- Cole, J.H.; Poudel, R.P.K.; Tsagkrasoulis, D.; Caan, M.W.A.; Steves, C.; Spector, T.D.; Montana, G. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. NeuroImage 2017, 163, 115–124. [Google Scholar] [CrossRef]
- He, S.; Feng, Y.; Grant, P.E.; Ou, Y. Deep Relation Learning for Regression and Its Application to Brain Age Estimation. IEEE Trans. Med. Imaging 2022, 41, 2304–2317. [Google Scholar] [CrossRef]
- Hu, L.; Wan, Q.; Huang, L.; Tang, J.; Huang, S.; Chen, X.; Bai, X.; Kong, L.; Deng, J.; Liang, H.; et al. MRI-based brain age prediction model for children under 3 years old using deep residual network. Brain Struct. Funct. 2023, 228, 1771–1784. [Google Scholar] [CrossRef]
- Dartora, C.; Marseglia, A.; Mårtensson, G.; Rukh, G.; Dang, J.; Muehlboeck, J.S.; Wahlund, L.O.; Moreno, R.; Barroso, J.; Ferreira, D.; et al. A deep learning model for brain age prediction using minimally preprocessed T1w images as input. Front. Aging Neurosci. 2024, 15, 1303036. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Gong, W.; Beckmann, C.F.; Vedaldi, A.; Smith, S.M. Accurate brain age prediction with lightweight deep neural networks. Med. Image Anal. 2021, 68, 101871. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.R.; Chen, A.; Ramadan, R.; Le, T.T.; Ruparel, K.; Moore, T.M.; Satterthwaite, T.D.; Zhang, F.; Shou, H.; Gur, R.C.; et al. Pitfalls in brain age analyses. Hum. Brain Mapp. 2021, 42, 4092–4101. [Google Scholar] [CrossRef] [PubMed]
- de Lange, A.M.G.; Anatürk, M.; Rokicki, J.; Han, L.K.M.; Franke, K.; Alnæs, D.; Ebmeier, K.P.; Draganski, B.; Kaufmann, T.; Westlye, L.T.; et al. Mind the gap: Performance metric evaluation in brain-age prediction. Hum. Brain Mapp. 2022, 43, 3113–3129. [Google Scholar] [CrossRef]
- Karim, H.T.; Aizenstein, H.J.; Mizuno, A.; Ly, M.; Andreescu, C.; Wu, M.; Hong, C.H.; Roh, H.W.; Park, B.; Lee, H.; et al. Independent replication of advanced brain age in mild cognitive impairment and dementia: Detection of future cognitive dysfunction. Mol. Psychiatry 2022, 27, 5235–5243. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.J.; Cen, S.Y.; Jaberzadeh, A.; Zheng, L.; Hauser, S.L.; Pelletier, D. Contribution of normal aging to brain atrophy in MS. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e616. [Google Scholar] [CrossRef]
- Leng, Y.; McEvoy, C.T.; Allen, I.E.; Yaffe, K. Association of Sleep-Disordered Breathing with Cognitive Function and Risk of Cognitive Impairment: A Systematic Review and Meta-analysis. JAMA Neurol. 2017, 74, 1237–1245. [Google Scholar] [CrossRef]
| Multi-Site Dataset | N | M%/F% | Age Interval | |
|---|---|---|---|---|
| ABIDE I 1 | 161 | 88/12 | – | 25.7 ± 6.4 |
| ADNI 2,* | 248 | 51/49 | – | 76.2 ± 5.1 |
| Cam-CAN [27,28] 3 | 624 | 49/51 | – | 54.2 ± 18.4 |
| CC-359 [29] 4 | 349 | 49/51 | – | 53.5 ± 7.8 |
| FCON 1000 5 | 572 | 34/66 | – | 45.3 ± 18.9 |
| IXI 6 | 472 | 47/53 | – | 49.0 ± 16.2 |
| OASIS-2 [30] 7 | 78 | 28/72 | – | 75.6 ± 8.4 |
| Total | 2504 | 48/52 | – | 52.1 ± 19.1 |
| UK Biobank 1 | AIBL 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| HC | SA | Dia | MS | PD | HC | MCI | AD | |
| N | 1347 | 201 | 454 | 114 | 58 | 440 | 95 | 95 |
| sex M % | 45 | 81 | 65 | 26 | 36 | 43 | 54 | 44 |
| age | ||||||||
| age interval | [50.0, 82.0] | [47.0, 79.0] | [46.0, 81.0] | [47.0, 80.0] | [46.0,79.0] | [60.0, 92.0] | [60.0, 96.0] | [58.0, 93.0] |
| 2D VGG | 3D VGG | RelationNet | ResNet | SFCN | |
|---|---|---|---|---|---|
| Input Size | |||||
| * Batch Size | 32 | 16 | 16 | 16 | 8 |
| Loss Function | L1 | L1 | L1 | L1 | KLD |
| * Learning Rate (LR) | |||||
| LR Decay | 3% | 50% | 50% | 70% | |
| LR Decay Step | 1 | 1 | 35 | 30 | 30 |
| Weight Decay | 0 | 0 | 0 | ||
| Momentum | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| Epochs | 400 | 110 | 160 | 130 | 110 |
| Accuracy | Robustness | LMEM | ||
|---|---|---|---|---|
| ME (SD) | MAE (SD) | mMAE | Age Trend Slope Coefficient [CI] | |
| 2D VGG | 4.36 | |||
| 3D VGG | ||||
| RelationNet | 3.92 | |||
| ResNet | 3.78 | |||
| SFCN | 4.40 | |||
| Without Offset Corr. | With Offset Corr. | ||||||
|---|---|---|---|---|---|---|---|
| Accuracy | Robustness | Accuracy | Robustness | LMEM | |||
| ME (SD) | MAE (SD) | mMAE | ME (SD) | MAE (SD) | mMAE | Age Trend Estimate [CI] | |
| 2D VGG | 6.38 | 5.27 | |||||
| 3D VGG | 5.82 | 4.32 | |||||
| RelationNet | 6.13 | 4.22 | |||||
| ResNet | 4.38 | 3.94 | |||||
| SFCN | 5.39 | 4.38 | |||||
| Cohort | Metric | 2D VGG | 3D VGG | RelationNet | ResNet | SFCN |
|---|---|---|---|---|---|---|
| SA | BAG | |||||
| Age trend | ||||||
| Dia | BAG | |||||
| Age trend | ||||||
| MS | BAG | |||||
| Age trend | ||||||
| PD | BAG | |||||
| Age trend | ||||||
| MCI | BAG | |||||
| Age trend | ||||||
| AD | BAG | |||||
| Age trend |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dular, L.; Špiclin, Ž.; for the Alzheimer’s Disease Neuroimaging Initiative; the Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing. Analysis of Brain Age Gap across Subject Cohorts and Prediction Model Architectures. Biomedicines 2024, 12, 2139. https://doi.org/10.3390/biomedicines12092139
Dular L, Špiclin Ž, for the Alzheimer’s Disease Neuroimaging Initiative, the Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing. Analysis of Brain Age Gap across Subject Cohorts and Prediction Model Architectures. Biomedicines. 2024; 12(9):2139. https://doi.org/10.3390/biomedicines12092139
Chicago/Turabian StyleDular, Lara, Žiga Špiclin, for the Alzheimer’s Disease Neuroimaging Initiative, and the Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing. 2024. "Analysis of Brain Age Gap across Subject Cohorts and Prediction Model Architectures" Biomedicines 12, no. 9: 2139. https://doi.org/10.3390/biomedicines12092139
APA StyleDular, L., Špiclin, Ž., for the Alzheimer’s Disease Neuroimaging Initiative, & the Australian Imaging Biomarkers and Lifestyle Flagship Study of Ageing. (2024). Analysis of Brain Age Gap across Subject Cohorts and Prediction Model Architectures. Biomedicines, 12(9), 2139. https://doi.org/10.3390/biomedicines12092139







