Exosome-like Nanoparticles, High in Trans-δ-Viniferin Derivatives, Produced from Grape Cell Cultures: Preparation, Characterization, and Anticancer Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Isolation of ENs
2.3. Scanning Electron Microscopy
2.4. Nanoparticle Tracking Analysis
2.5. Isolation of RNA and miRNA, cDNA Synthesis, and PCR Analysis
2.6. Isolation and Analysis of GCEN Protein Cargo
2.6.1. Protein Extraction Procedure
2.6.2. Mass Spectrometry and Protein Identification
2.6.3. Western Blot Analysis
2.7. Analysis of Secondary Compounds
2.7.1. Chemicals
2.7.2. Extraction
2.7.3. Analytical Chromatography and Mass Spectrometry
2.8. In Vitro Experiments
2.8.1. GCEN Uptake by MDA-MB-231 Cells
2.8.2. Cytotoxicity Assays
2.8.3. Caspase 3/7 Activity
2.8.4. Annexin V-AF 488/PI Staining
2.8.5. Cell Cycle Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Characterization of ENs
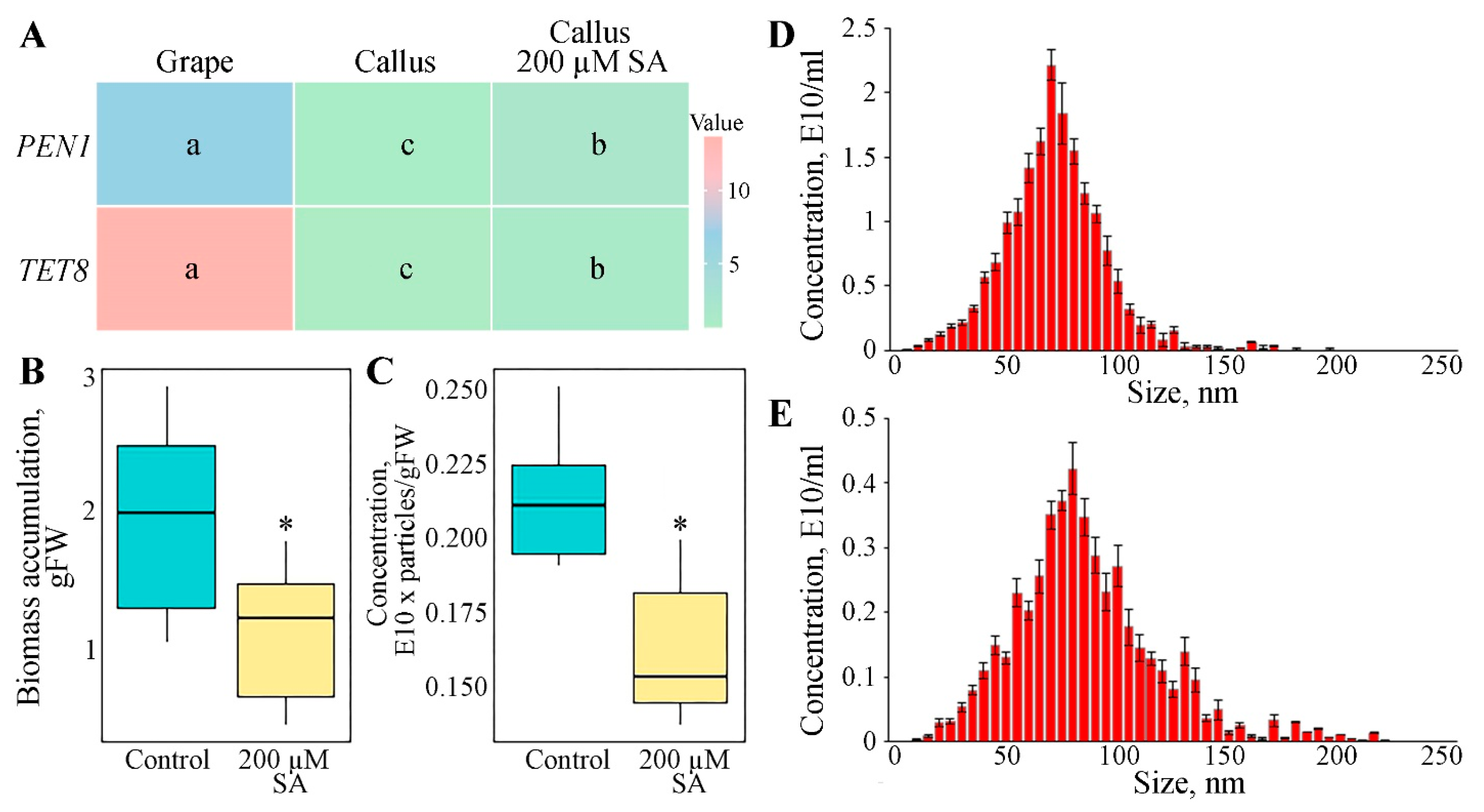
3.2. Expression of Genes Involved in GCEN Biogenesis
3.3. Impact of Salicylic Acid on GCENs Biogenesis
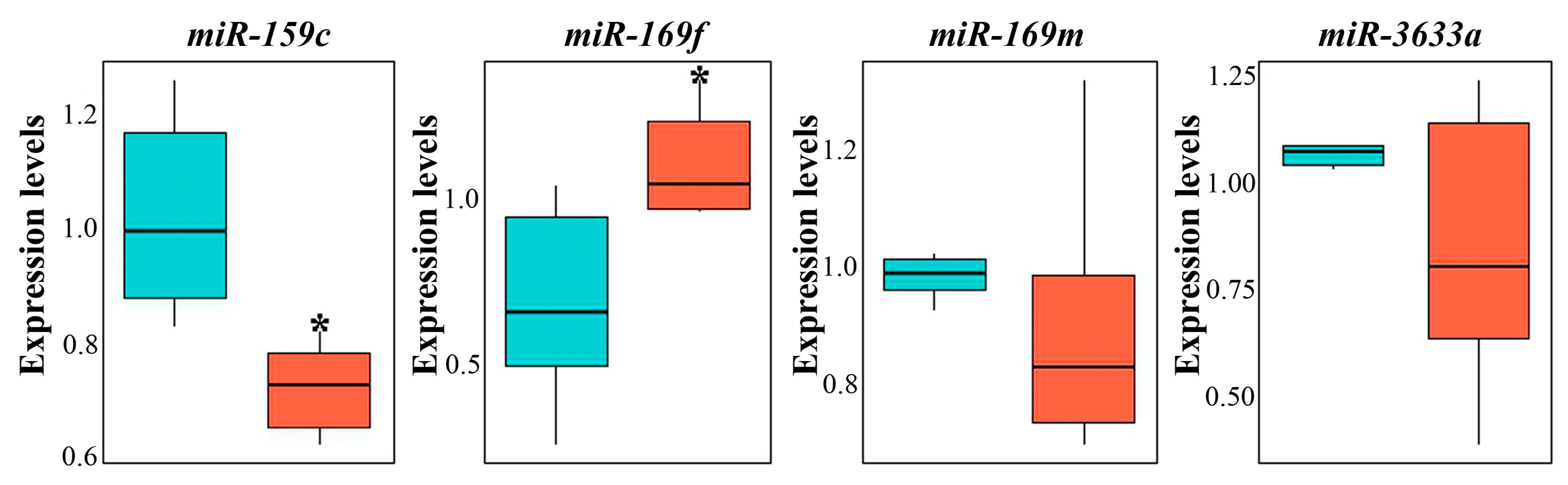
3.4. miRNA Composition of GCENs
3.5. Protein Composition of GCENs
| Protein | Functions | Occurrence in Plant-Derived ENs |
|---|---|---|
| Stress responses | ||
| Heat shock 70 kDa protein | A molecular chaperone that plays a crucial role in maintaining cellular homeostasis and enhancing plant stress tolerance by assisting in the proper folding of newly synthesized proteins, preventing the aggregation of misfolded proteins, and aiding in the refolding or degradation of damaged proteins. | Citrus fruit juice [83], Arabidopsis thaliana leaf apoplastic fluid [2], juice from ginger roots [84], tomato fruits [85], broccoli roots [86], grape berries [24], A. thaliana cell culture [44], Triticum aestivum grass juice [76] |
| GDSL esterase/lipase | Participates in the metabolism of lipids by hydrolyzing ester bonds in fatty acids and contributes to plant health by modulating lipid signaling and maintaining membrane integrity. | A. thaliana leaf apoplastic fluid [2], sunflower extracellular fluid [87], juice from ginger roots [84] |
| Isoflavone reductase-like protein | Involved in the biosynthesis of isoflavonoids, which are important for plant defense mechanisms against pathogens and environmental stress. | Citrus fruit juice [83], A. thaliana leaf apoplastic fluid [2] |
| Abscisic stress-ripening protein 2 | Plays a role in plant response to abscisic acid, which regulates stress responses and ripening processes. | A. thaliana leaf apoplastic fluid [2] |
| Class III chitinase | Breaks down chitin, a major component of fungal cell walls, providing a defense against fungal pathogens. Plays a morphogenetic role during apical growth, cell division, and differentiation. | A. thaliana leaf apoplastic fluid [2], sunflower extracellular fluid [87], tomato roots [88] |
| Class IV chitinase | Tomato roots [88] | |
| Chitinase 5 | Tomato roots [88] | |
| Acidic endochitinase | Tomato roots [88] | |
| Endochitinase EP3 | Tomato roots [88] | |
| Peroxidase | Catalyzes the breakdown of hydrogen peroxide, a reactive oxygen species, thus protecting cells from oxidative damage and playing roles in lignin formation and pathogen defense. | Citrus fruit juice [83]; sunflower extracellular fluid [87], grapefruit juice [89] |
| Peroxidase 4 | Sunflower extracellular fluid [87] | |
| Cationic peroxidase 1 | Sunflower extracellular fluid [87], tomato roots [88] | |
| Glutaredoxin-dependent peroxiredoxin | Reduces peroxides and protects cells from oxidative damage by using glutaredoxin as an electron donor. | - |
| Universal stress protein PHOS34 | Involved in plant stress responses, helping to improve tolerance to various environmental stresses. | - |
| Retrotrans_gag domain-containing protein | Functions as part of retrotransposons, which can influence gene expression and genome stability. | - |
| Peptidyl-prolyl cis-trans isomerase | Catalyzes the isomerization of peptide bonds at proline residues, aiding in protein folding and function. | A. thaliana leaf apoplastic fluid [2], grape berries [24], citrus fruit juice [83] |
| 5-methyltetrahydropteroyltriglutamate-homocysteine S-methyltransferase | Involved in the biosynthesis of methionine, which is crucial for protein synthesis and plays a significant role in the plant’s response to abiotic stresses. | A. thaliana leaf apoplastic fluid [2], olive vegetation water [90], tomato fruits [91], citrus fruit juice [83] |
| Usp domain-containing protein | Associated with stress responses, helping plants survive adverse conditions by stabilizing cellular proteins and membranes. | - |
| Annexin D1 and D2 | Bind to phospholipids in a calcium-dependent manner, playing roles in membrane-related processes such as signal transduction and stress responses. | A. thaliana leaf apoplastic fluid [2], citrus fruit juice [83] |
| Cell wall remodeling and carbohydrate metabolism | ||
| Endoglucanase 1 | Breaks down cellulose into glucose units, aiding in cell wall remodeling and degradation. | Tomato roots [88] |
| Fructose-bisphosphate aldolase | Catalyzes a key step in glycolysis and gluconeogenesis, converting fructose-bisphosphate to glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. | Grape berries [24], kiwi fruit pollen [92], fresh tea flowers [93], sunflower extracellular fluid [87] |
| Alpha-mannosidase | Involved in the modification and degradation of glycoproteins by cleaving mannose residues. | Sunflower extracellular fluid [87] |
| Putative polygalacturonase | Degrades pectin in the plant cell wall, facilitating cell wall modification during growth and development. | A. thaliana leaf apoplastic fluid [2], Craterostigma plantagineum cell suspension medium [27], grapefruit juice [85] |
| Beta-galactosidase | Hydrolyzes beta-galactosides into monosaccharides, involved in cell wall remodeling and carbohydrate metabolism. | A. thaliana leaf apoplastic fluids [2], C. plantagineum cell suspension medium [30], grapefruit juice [89], sunflower extracellular fluid [87] |
| Epidermis-specific secreted glycoprotein EP1 | Plays a role in epidermal cell differentiation and defense against pathogens. | Tomato roots [88] |
| UDP-arabinopyranose mutase | Catalyzes the conversion of UDP-arabinopyranose to UDP-arabinofuranose, important for cell wall polysaccharide biosynthesis. | C. plantagineum cell suspension medium [30], citrus fruit juice [83], broccoli roots [86] |
| UTP-glucose-1-phosphate uridylyltransferase | Converts glucose-1-phosphate to UDP-glucose, a precursor for the synthesis of polysaccharides and glycoproteins. | Citrus fruit juice [83] |
| Primary and Secondary Metabolic Pathways | ||
| Sucrose synthase 2 | Catalyzes the reversible conversion of sucrose and UDP to UDP-glucose and fructose, playing a key role in sucrose metabolism and partitioning. | Citrus fruit juice [83], sunflower extracellular fluid [87], grapefruit juice [89] |
| Phosphoenolpyruvate carboxylase, housekeeping isozyme | Catalyzes the fixation of CO2 into oxaloacetate, a key step in photosynthesis and various metabolic pathways. | Citrus fruit juice [83], sunflower extracellular fluid [87], tomato fruits [91], grapefruit juice [89] |
| Phosphopyruvate hydratase (Enolase) | Catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate in glycolysis. | Citrus fruit juice [83] |
| Purple acid phosphatase | Hydrolyzes phosphate esters and anhydrides under acidic conditions, involved in phosphorus metabolism and mobilization. | Tea flowers [93], sunflower extracellular fluid [87] |
| Glyceraldehyde-3-phosphate dehydrogenase | Catalyzes a key step in glycolysis, converting glyceraldehyde-3-phosphate to 1,3-bisphosphoglycerate. | Olive vegetation water [90], citrus fruit juice [83], bitter melon juice [94], tea flowers [93] |
| Glyco_hydro_32C domain-containing protein | Likely involved in the hydrolysis of glycosidic bonds in complex carbohydrates. | - |
| Berberine bridge enzyme-like 2 | Involved in the biosynthesis of alkaloids, which play roles in plant defense and secondary metabolism. | Olive vegetation water [90], grape berries [24] |
3.6. Composition of Secondary Metabolites in GCENs
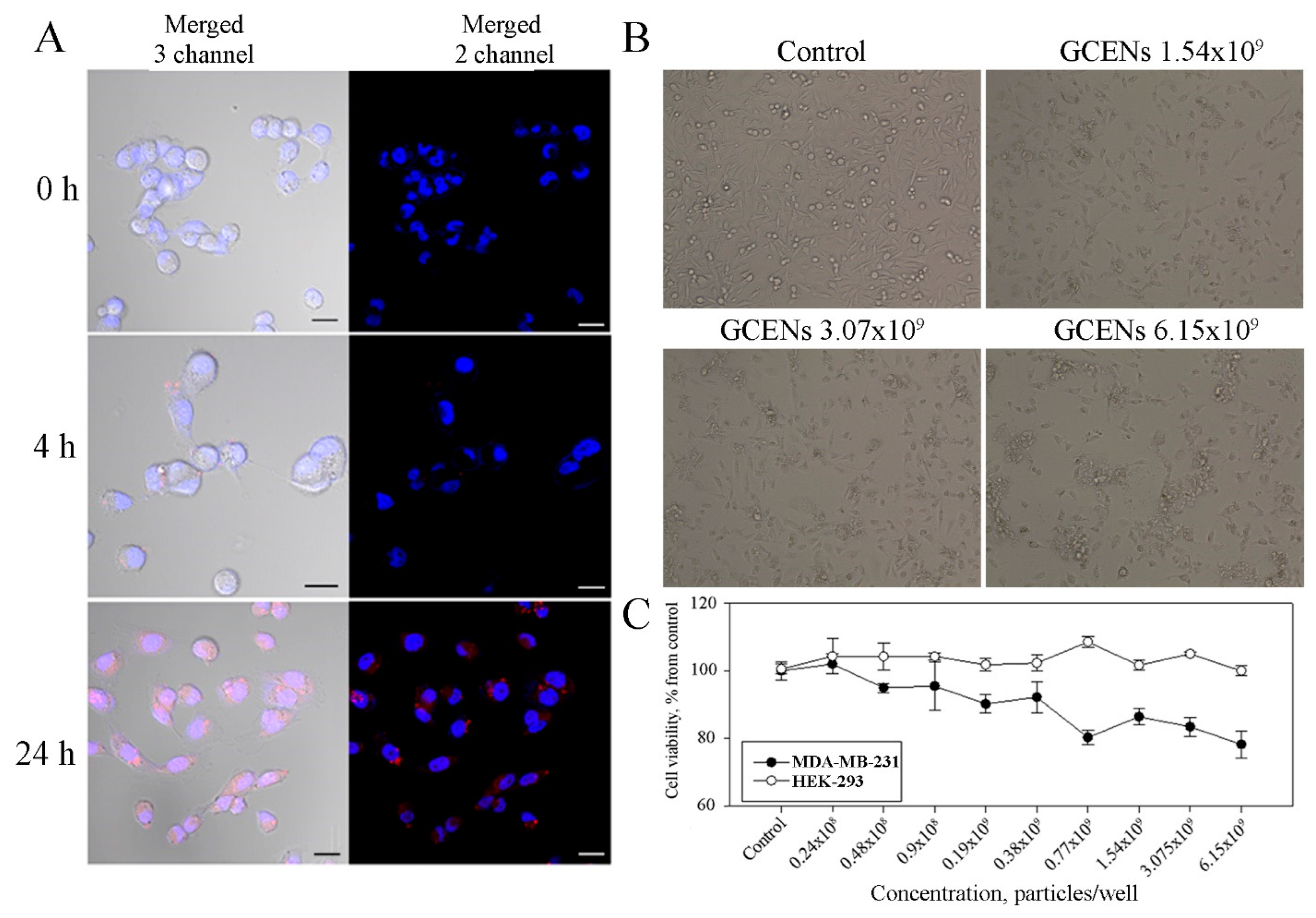
3.7. Uptake of GCENs by Human Cells and Their Cytotoxic Effects
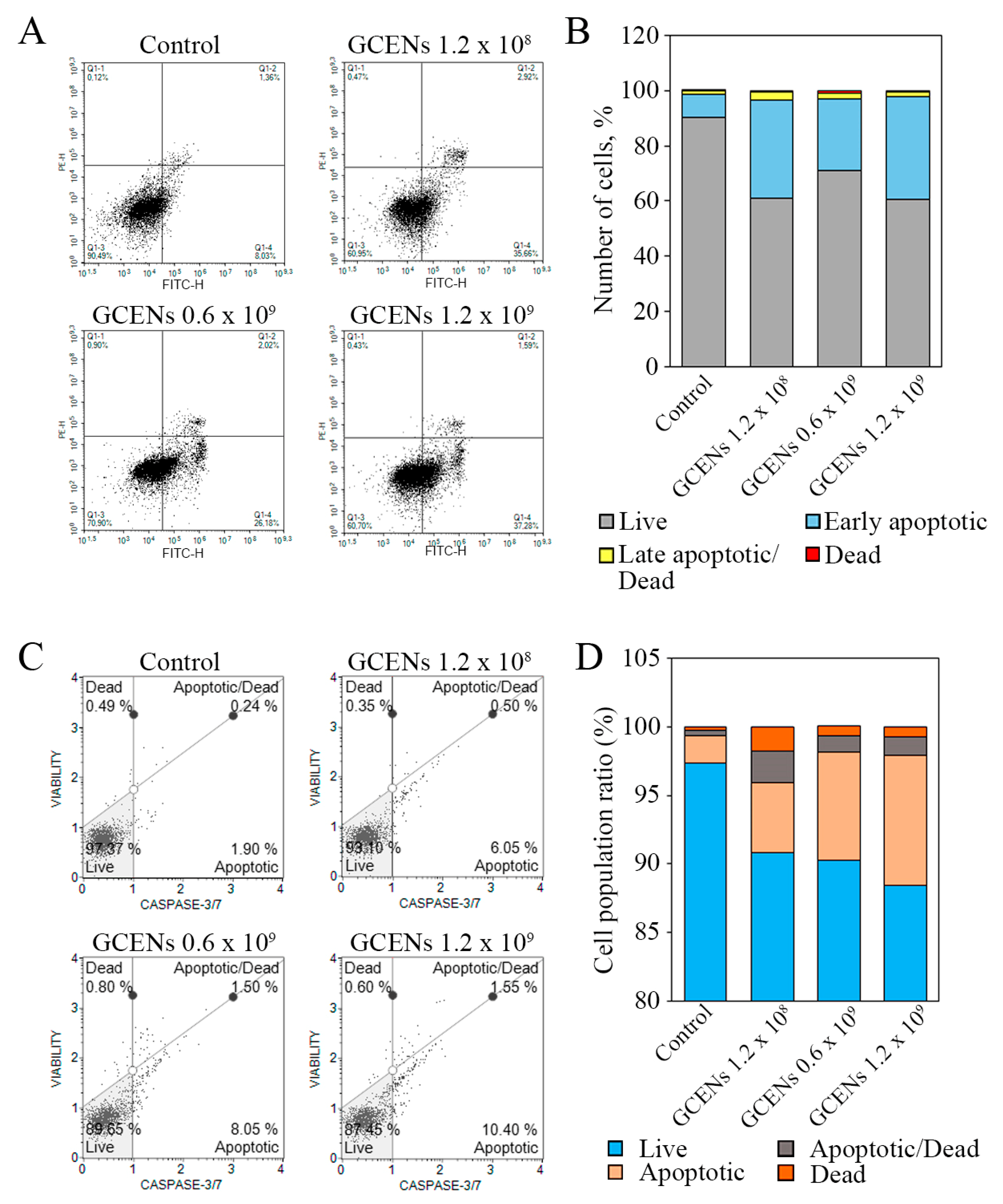
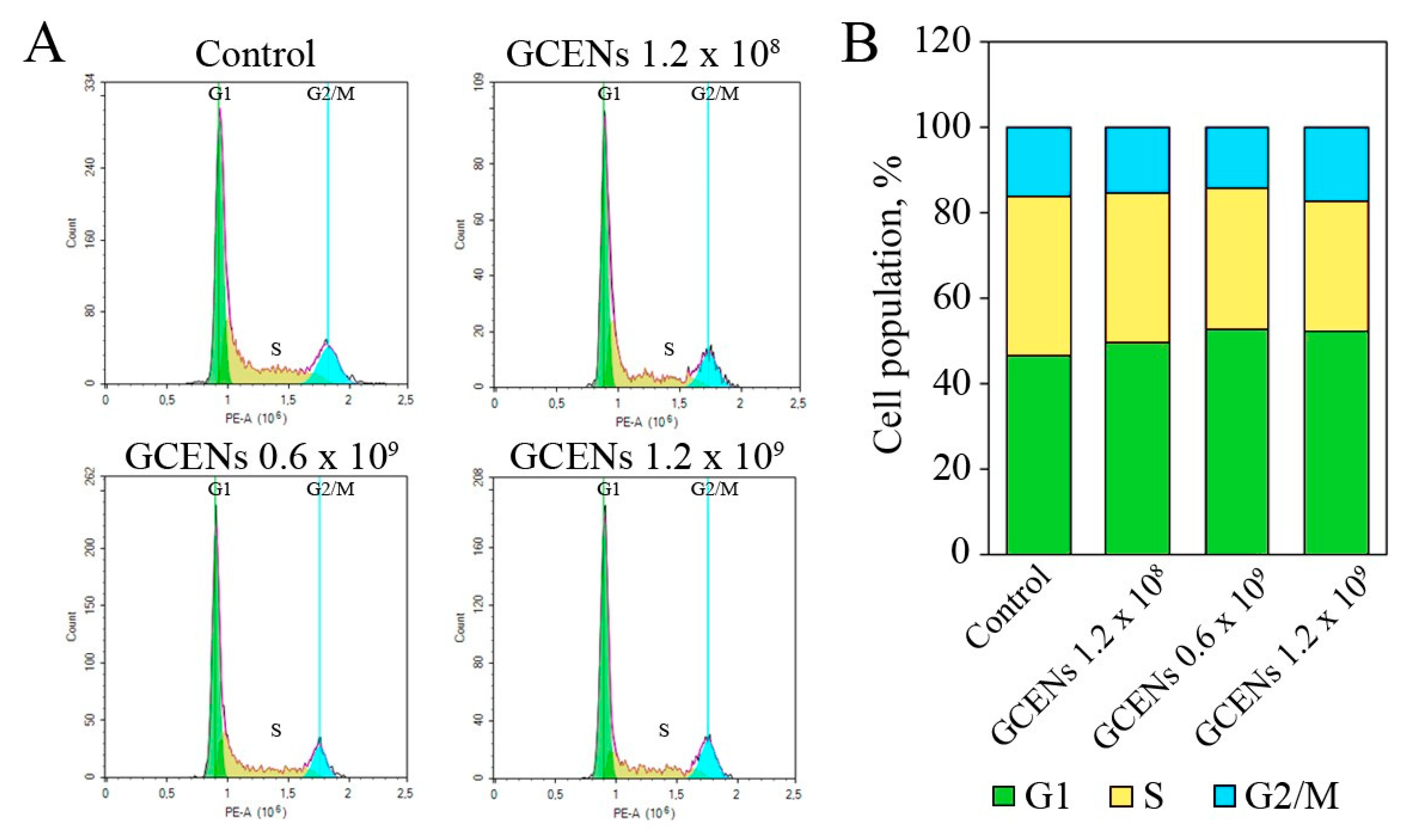
3.8. Apoptotic and Cytostatic Effects of GCENs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhu, Y.; Lin, Z.; Lei, J.; Li, L.; Zhu, W.; Wu, D. Evidence of cross-kingdom gene regulation by plant microRNAs and possible reasons for inconsistencies. J. Agric. Food Chem. 2024, 72, 4564–4573. [Google Scholar] [CrossRef]
- Liu, T.; Xu, L.G.; Duan, C.G. The trans-kingdom communication of noncoding RNAs in plant-environment interactions. Plant Genome 2023, 16, e20289. [Google Scholar] [CrossRef]
- Mahanty, B.; Mishra, R.; Joshi, R.K. Cross-kingdom small RNA communication between plants and fungal phytopathogens-recent updates and prospects for future agriculture. RNA Biol. 2023, 20, 109–119. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, K.; Hu, H.; Xing, X.; Huang, X.; Gao, H. Extracellular vesicles: Their functions in plant-pathogen interactions. Mol. Plant Pathol. 2022, 23, 760–771. [Google Scholar] [CrossRef]
- Liu, G.; Kang, G.; Wang, S.; Huang, Y.; Cai, Q. Extracellular vesicles: Emerging players in plant defense against pathogens. Front. Plant Sci. 2021, 12, 757925. [Google Scholar] [CrossRef] [PubMed]
- Shkryl, Y.; Tsydeneshieva, Z.; Degtyarenko, A.; Yugay, Y.; Balabanova, L.; Rusapetova, T.; Bulgakov, V. Plant exosomal vesicles: Perspective information nanocarriers in biomedicine. Appl. Sci. 2022, 12, 8262. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, e12283. [Google Scholar] [CrossRef]
- Fuloria, S.; Sekar, M.; Khattulanuar, F.S.; Gan, S.H.; Rani, N.N.I.M.; Ravi, S.; Subramaniyan, V.; Jeyabalan, S.; Begum, M.Y.; Chidambaram, K.; et al. Chemistry, biosynthesis and pharmacology of viniferin: Potential resveratrol-derived molecules for new drug discovery, development and therapy. Molecules 2022, 27, 5072. [Google Scholar] [CrossRef]
- Colin, D.; Gimazane, A.; Lizard, G.; Izard, J.C.; Solary, E.; Latruffe, N.; Delmas, D. Effects of resveratrol analogs on cell cycle progression, cell cycle associated proteins and 5-fluoro-uracil sensitivity in human derived colon cancer cells. Int. J. Cancer 2009, 124, 2780–2788. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Gromek, S.; Niesen, D.; Seeram, N.P.; Henry, G.E. Resveratrol oligomers isolated from Carex species inhibit growth of human colon tumorigenic cells mediated by cell cycle arrest. J. Agric. Food Chem. 2011, 59, 8632–8638. [Google Scholar] [CrossRef] [PubMed]
- Nivelle, L.; Hubert, J.; Courot, E.; Jeandet, P.; Aziz, A.; Nuzillard, J.M.; Renault, J.H.; Clément, C.; Martiny, L.; Delmas, D.; et al. Anti-cancer activity of resveratrol and derivatives produced by grapevine cell suspensions in a 14 L stirred bioreactor. Molecules 2017, 22, 474. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhang, Y.; Su, L.; Chen, D.; Lou, S.; Luo, X.; Wang, L.; Tang, R.; Zhang, L.; Tian, X. The mechanism of trans-δ-viniferin inhibiting the proliferation of lung cancer cells A549 by targeting the mitochondria. Front. Pharmacol. 2023, 14, 1190127. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Marcourt, L.; Quiros-Guerrero, L.M.; Luscher, A.; Schnee, S.; Michellod, E.; Ducret, V.; Kohler, T.; Perron, K.; Wolfender, J.L.; et al. Chiral separation of stilbene dimers generated by biotransformation for absolute configuration determination and antibacterial evaluation. Front. Chem. 2022, 10, 912396. [Google Scholar] [CrossRef] [PubMed]
- Zwygart, A.C.; Medaglia, C.; Huber, R.; Poli, R.; Marcourt, L.; Schnee, S.; Michellod, E.; Mazel-Sanchez, B.; Constant, S.; Huang, S.; et al. Antiviral properties of trans-δ-viniferin derivatives against enveloped viruses. Biomed. Pharmacother. 2023, 163, 114825. [Google Scholar] [CrossRef]
- Kong, Q.; Ren, X.; Hu, R.; Yin, X.; Jiang, G.; Pan, Y. Isolation and purification of two antioxidant isomers of resveratrol dimer from the wine grape by counter-current chromatography. J. Sep. Sci. 2016, 39, 2374–2379. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.R.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.; et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef]
- Lavate, M.A.; Karpe, S.T.; Biradar, S.N.; Bhandare, M.R. Solid lipid nanoparticles: A promising drug delivery method to get greater bioavailability: A review. World J. Biol. Pharm. Health Sci. 2023, 14, 072–080. [Google Scholar] [CrossRef]
- Varia, J.K.; Dodiya, S.S.; Sawant, K.K. Cyclosporine A loaded solid lipid nanoparticles: Optimization of formulation, process variable and characterization. Curr. Drug Deliv. 2008, 5, 64–69. [Google Scholar] [CrossRef]
- Peixoto, F.S.; Dias, P.M.; Ramaldes, G.A.; Vilela, J.M.C.; Andrade, M.S.; Cunha, A.S. Atomic force microscopy applied to the characterization of solid lipid nanoparticles. Microsc. Microanal. 2005, 11, 52–55. [Google Scholar] [CrossRef]
- Chaudhari, P.M.; Ghodake, M.V. Development and optimization of solid lipid nanoparticle for topical delivery. J. Drug Deliv. Ther. 2019, 9, 105–121. [Google Scholar] [CrossRef]
- Teng, Y.; He, J.; Zhong, Q.; Zhang, Y.; Lu, Z.; Guan, T.; Pan, Y.; Luo, X.; Feng, W.; Ou, C. Grape exosome-like nanoparticles: A potential therapeutic strategy for vascular calcification. Front. Pharmacol. 2022, 13, 1025768. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Farheen, J.; Iqbal, M.Z.; Lu, Y.; Tang, Z.; Kong, X. Vitis vinifera Kyoho-derived exosome-like nanoparticles-based drug delivery and therapeutic modalities for breast cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 92, 105332. [Google Scholar] [CrossRef]
- Di Raimo, R.; Mizzoni, D.; Spada, M.; Dolo, V.; Fais, S.; Logozzi, M. Oral treatment with plant-derived exosomes restores redox balance in H2O2-treated mice. Antioxidants 2023, 12, 1169. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bermúdez, P.; Blesa, J.; Soriano, J.M.; Marcilla, A. Extracellular vesicles in food: Experimental evidence of their secretion in grape fruits. Eur. J. Pharm. Sci. 2017, 98, 40–50. [Google Scholar] [CrossRef]
- Anusree, K.P.; Ashin, M.; Anusha, R.; Sijisha, K.S.; Priya, S. Optimization of the filtration and centrifugation steps for the isolation of exosome-like nanoparticles (ELNs) from grapes. Sep. Sci. Technol. 2024, 59, 365–371. [Google Scholar] [CrossRef]
- Woith, E.; Guerriero, G.; Hausman, J.F.; Renaut, J.; Leclercq, C.C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M.F. Plant extracellular vesicles and nanovesicles: Focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int. J. Mol. Sci. 2021, 22, 3719. [Google Scholar] [CrossRef]
- Kim, W.S.; Ha, J.-H.; Jeong, S.-H.; Lee, J.-I.; Lee, B.-W.; Jeong, Y.J.; Kim, C.Y.; Park, J.-Y.; Ryu, Y.B.; Kwon, H.-J.; et al. Immunological effects of Aster yomena callus-derived extracellular vesicles as potential therapeutic agents against allergic asthma. Cells 2022, 11, 2805. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.-G.; Choi, S.-Y.; Kim, H.; Choi, E.-J.; Lee, E.-J.; Park, P.-J.; Ko, J.; Kim, K.P.; Baek, H.S. Panax ginseng-derived extracellular vesicles facilitate anti-senescence effects in human skin cells: An eco-friendly and sustainable way to use ginseng substances. Cells 2021, 10, 486. [Google Scholar] [CrossRef]
- Subha, D.; Harshnii, K.; Madhikiruba, K.G.; Nandhini, M.; Tamilselvi, K.S. Nanovesicles: An updated overview. Plant Nano Biol. 2023, 3, 100022. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Ochoa-Villarreal, M.; Howat, S.; Hong, S.; Jang, M.O.; Jin, Y.W.; Lee, E.K.; Loake, G.J. Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-derived exosome-like nanovesicles: Current progress and prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef]
- Feng, J.; Xiu, Q.; Huang, Y.; Troyer, Z.; Li, B.; Zheng, L. Plant-derived vesicle-like nanoparticles as promising biotherapeutic tools: Present and future. Adv. Mater. 2023, 35, e2207826. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-derived extracellular vesicles and their exciting potential as the future of next-generation drug delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef]
- Cong, M.; Tan, S.; Li, S.; Gao, L.; Huang, L.; Zhang, H.G.; Qiao, H. Technology insight: Plant-derived vesicles-How far from the clinical biotherapeutics and therapeutic drug carriers? Adv. Drug Deliv. Rev. 2022, 182, 114108. [Google Scholar] [CrossRef]
- Ambrosone, A.; Barbulova, A.; Cappetta, E.; Cillo, F.; De Palma, M.; Ruocco, M.; Pocsfalvi, G. Plant extracellular vesicles: Current landscape and future directions. Plants 2023, 12, 4141. [Google Scholar] [CrossRef]
- Kocholata, M.; Prusova, M.; Auer Malinska, H.; Maly, J.; Janouskova, O. Comparison of two isolation methods of tobacco-derived extracellular vesicles, their characterization and uptake by plant and rat cells. Sci. Rep. 2022, 12, 19896. [Google Scholar] [CrossRef] [PubMed]
- Boccia, E.; Alfieri, M.; Belvedere, R.; Santoro, V.; Colella, M.; Del Gaudio, P.; Moros, M.; Dal Piaz, F.; Petrella, A.; Leone, A.; et al. Plant hairy roots for the production of extracellular vesicles with antitumor bioactivity. Commun. Biol. 2022, 5, 848. [Google Scholar] [CrossRef] [PubMed]
- Vestuto, V.; Conte, M.; Vietri, M.; Mensitieri, F.; Santoro, V.; Di Muro, A.; Alfieri, M.; Moros, M.; Miranda, M.R.; Amante, C.; et al. Multiomic profiling and neuroprotective bioactivity of Salvia hairy root-derived extracellular vesicles in a cellular model of Parkinson’s disease. Int. J. Nanomed. 2024, 19, 9373–9393. [Google Scholar] [CrossRef] [PubMed]
- Yugay, Y.; Tsydeneshieva, Z.; Rusapetova, T.; Grischenko, O.; Mironova, A.; Bulgakov, D.; Silant’ev, V.; Tchernoded, G.; Bulgakov, V.; Shkryl, Y. Isolation and characterization of extracellular vesicles from Arabidopsis thaliana cell culture and investigation of the specificities of their biogenesis. Plants 2023, 12, 3604. [Google Scholar] [CrossRef] [PubMed]
- Makhazen, D.S.; Veremeichik, G.N.; Shkryl, Y.N.; Bulgakov, V.P.; Zhuravlev, Y.N. RNA inhibition of the JAZ9 gene increases the production of resveratrol in grape cell cultures. Plant Cell Tissue Organ Cult. 2021, 147, 611–618. [Google Scholar] [CrossRef]
- Yugay, Y.; Rusapetova, T.; Mashtalyar, D.; Grigorchuk, V.; Vasyutkina, E.; Kudinova, O.; Zenkina, K.; Trifuntova, I.; Karabtsov, A.; Ivanov, V.; et al. Biomimetic synthesis of functional silver nanoparticles using hairy roots of Panax ginseng for wheat pathogenic fungi treatment. Colloids Surf. B Biointerfaces 2021, 207, 112031. [Google Scholar] [CrossRef]
- Shkryl, Y.; Yugay, Y.; Avramenko, T.; Grigorchuk, V.; Gorpenchenko, T.; Grischenko, O.; Bulgakov, V. CRISPR/Cas9-mediated knockout of HOS1 reveals its role in the regulation of secondary metabolism in Arabidopsis thaliana. Plants 2021, 10, 104. [Google Scholar] [CrossRef]
- Yang, L.H.; Wang, S.L.; Tang, L.L.; Liu, B.; Ye, W.L.; Wang, L.L.; Wang, Z.Y.; Zhou, M.T.; Chen, B.C. Universal stem-loop primer method for screening and quantification of microRNA. PLoS ONE 2014, 9, e115293. [Google Scholar] [CrossRef]
- Taverna, S.; Amodeo, V.; Saieva, L.; Russo, A.; Giallombardo, M.; De Leo, G.; Alessandro, R. Exosomal shuttling of miR-126 in endothelial cells modulates adhesive and migratory abilities of chronic myelogenous leukemia cells. Mol. Cancer 2014, 13, 169. [Google Scholar] [CrossRef]
- Mao, X.; Li, T.; Qi, W.; Miao, Z.; Zhu, L.; Zhang, C.; Jin, H.; Pan, H.; Wang, D. Advances in the study of plant-derived extracellular vesicles in the skeletal muscle system. Pharmacol. Res. 2024, 204, 107202. [Google Scholar] [CrossRef]
- Fisher, W.S.; Tchounwou, C.; Wei, S.; Roberts, L.; Ewert, K.K.; Safinya, C.R. Exosomes are secreted at similar densities by M21 and PC3 human cancer cells and show paclitaxel solubility. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183841. [Google Scholar] [CrossRef] [PubMed]
- Zabeo, D.; Cvjetkovic, A.; Lässer, C.; Schorb, M.; Lötvall, J.; Höög, J.L. Exosomes purified from a single cell type have diverse morphology. J. Extracell. Vesicles 2017, 6, 1329476. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Xiao, Q.; Wang, X.; Zhu, J.; Li, J.; Liang, X.; Peng, Y.; Wu, C.; Lu, R.; Pan, Y.; et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021, 21, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Takahashi, Y.; Nishikawa, M.; Sano, K.; Morishita, M.; Charoenviriyakul, C.; Saji, H.; Takakura, Y. Role of phosphatidylserine-derived negative surface charges in the recognition and uptake of intravenously injected B16BL6-derived exosomes by macrophages. J. Pharm. Sci. 2017, 106, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.J.; Wang, N.; Bao, J.J.; Zhu, H.X.; Wang, L.J.; Chen, X.Y. Lipidomic analysis reveals the importance of GIPCs in Arabidopsis leaf extracellular vesicles. Mol. Plant 2020, 13, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Jang, J.; Jeong, H.; Jang, E.; Kim, E.; Yoon, Y.; Jang, S.; Jeong, H.S.; Jang, G. Isolation of high-purity and high-stability exosomes from ginseng. Front. Plant Sci. 2023, 13, 1064412. [Google Scholar] [CrossRef]
- Viršilė, A.; Samuolienė, G.; Laužikė, K.; Šipailaitė, E.; Balion, Z.; Jekabsone, A. Species-specific plant-derived nanoparticle characteristics. Plants 2022, 11, 3139. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Ban, J.J.; Lee, M.; Im, W.; Kim, M. Low pH increases the yield of exosome isolation. Biochem. Biophys. Res. Commun. 2015, 461, 76–79. [Google Scholar] [CrossRef]
- Robatzek, S. Vesicle trafficking in plant immune responses. Cell. Microbiol. 2007, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Jimenez, S.; Hashimoto, K.; Santana, O.; Aguirre, J.; Kuchitsu, K.; Cárdenas, L. Emerging roles of tetraspanins in plant inter-cellular and inter-kingdom communication. Plant Signal. Behav. 2019, 14, e1581559. [Google Scholar] [CrossRef]
- Liu, N.; Hou, L.; Chen, X.; Bao, J.; Chen, F.; Cai, W.; Zhu, H.; Wang, L.; Chen, X. Arabidopsis TETRASPANIN8 mediates exosome secretion and glycosyl inositol phosphoceramide sorting and trafficking. Plant Cell 2024, 36, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qiao, Q.; Sun, Y.; Xu, Y.; Shu, H.; Zhang, Z.; Liu, F.; Wang, H.; Ye, W.; Dong, S.; et al. Divergent sequences of tetraspanins enable plants to specifically recognize microbe-derived extracellular vesicles. Nat. Commun. 2023, 14, 4877. [Google Scholar] [CrossRef]
- Ding, X.; Wang, S.; Cui, X.; Zhong, H.; Zou, H.; Zhao, P.; Guo, Z.; Chen, H.; Li, C.; Zhu, L.; et al. LKS4-mediated SYP121 phosphorylation participates in light-induced stomatal opening in Arabidopsis. Curr. Biol. 2024, 34, 3102–3115.e6. [Google Scholar] [CrossRef] [PubMed]
- Assaad, F.F.; Qiu, J.L.; Youngs, H.; Ehrhardt, D.; Zimmerli, L.; Kalde, M.; Wanner, G.; Peck, S.C.; Edwards, H.; Ramonell, K.; et al. The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol. Biol. Cell 2004, 15, 5118–5129. [Google Scholar] [CrossRef]
- Xiao, J.; Feng, S.; Wang, X.; Long, K.; Luo, Y.; Wang, Y.; Ma, J.; Tang, Q.; Jin, L.; Li, X.; et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ 2018, 6, e5186. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant extracellular vesicles contain diverse small RNA species and are enriched in 10- to 17-nucleotide “tiny” RNAs. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef]
- Hansen, L.L.; Nielsen, M.E. Plant exosomes: Using an unconventional exit to prevent pathogen entry? J. Exp. Bot. 2017, 69, 59–68. [Google Scholar] [CrossRef]
- Urzì, O.; Gasparro, R.; Ganji, N.R.; Alessandro, R.; Raimondo, S. Plant-RNA in extracellular vesicles: The secret of cross-kingdom communication. Membranes 2022, 12, 352. [Google Scholar] [CrossRef]
- Timms, K.; Holder, B.; Day, A.; Mclaughlin, J.; Forbes, K.A.; Westwood, M. Watermelon-derived extracellular vesicles influence human ex vivo placental cell behavior by altering intestinal secretions. Mol. Nutr. Food Res. 2022, 66, e2200013. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, P.; Wang, X.; Sun, D.; Li, Z.; Zhang, Y.; Liu, Y.; Wu, C.; Zhu, J.; Li, J. Detection of dietetically absorbed maize-derived microRNAs in pigs. Sci. Rep. 2017, 7, 645. [Google Scholar] [CrossRef]
- Subha, D.; AnuKiruthika, R.; Sreeraj, H.; Kirubakaran, S.; Madhikiruba, K.G.; Tamilselvi, K.S. Plant exosomes: Nano conveyors of pathogen resistance. Discov. Nano 2023, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Şahin, F.; Koçak, P.; Güneş, M.Y.; Özkan, İ.; Yıldırım, E.; Kala, E.Y. In vitro wound healing activity of wheat-derived nanovesicles. Appl. Biochem. Biotechnol. 2019, 188, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, R.; Wang, A.; Li, Y.; Zhang, M.; Kim, J.; Zhu, Y.; Wang, Q.; Zhang, Y.; Wei, Y.; et al. Panax notoginseng-derived exosome-like nanoparticles attenuate ischemia reperfusion injury via altering microglia polarization. J. Nanobiotechnol. 2023, 21, 416. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, A. A review on plant annexins: The calcium binding proteins with multifaceted roles in plant growth, development and stress tolerance. S. Afr. J. Bot. 2023, 162, 108–114. [Google Scholar] [CrossRef]
- Clark, G.B.; Morgan, R.O.; Fernandez, M.-P.; Roux, S.J. Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol. 2012, 196, 695–712. [Google Scholar] [CrossRef]
- Man, F.; Meng, C.; Liu, Y.; Wang, Y.; Zhou, Y.; Ma, J.; Lu, R. The study of ginger-derived extracellular vesicles as a natural nanoscale drug carrier and their intestinal absorption in rats. AAPS PharmSciTech 2021, 22, 206. [Google Scholar] [CrossRef]
- Sanderson, R.D.; Bandari, S.K.; Vlodavsky, I. Proteases and glycosidases on the surface of exosomes: Newly discovered mechanisms for extracellular remodeling. Matrix Biol. 2019, 75–76, 160–169. [Google Scholar] [CrossRef]
- Perrot, T.; Pauly, M.; Ramírez, V. Emerging roles of β-glucanases in plant development and adaptative responses. Plants 2022, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Turiák, L.; Ambrosone, A.; Del Gaudio, P.; Puska, G.; Fiume, I.; Silvestre, T.; Vékey, K. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J. Plant Physiol. 2018, 229, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef] [PubMed]
- Bokka, R.; Ramos, A.P.; Fiume, I.; Manno, M.; Raccosta, S.; Turiák, L.; Sugár, S.; Adamo, G.; Csizmadia, T.; Pocsfalvi, G. Biomanufacturing of tomato-derived nanovesicles. Foods 2020, 9, 1852. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.D.C.; García-Gomez, P.; Yepes-Molina, L.; Guarnizo, A.L.; Teruel, J.A.; Carvajal, M. Plasma membrane aquaporins mediates vesicle stability in broccoli. PLoS ONE 2018, 13, e0192422. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Pinedo, M.; San Clemente, H.; Balliau, T.; Jamet, E.; de la Canal, L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef]
- De Palma, M.; Ambrosone, A.; Leone, A.; Del Gaudio, P.; Ruocco, M.; Turiák, L.; Bokka, R.; Fiume, I.; Tucci, M.; Pocsfalvi, G. Plant roots release small extracellular vesicles with antifungal activity. Plants 2020, 9, 1777. [Google Scholar] [CrossRef]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Buratta, S.; Latella, R.; Chiaradia, E.; Salzano, A.M.; Tancini, B.; Pellegrino, R.M.; Urbanelli, L.; Cerrotti, G.; Calzoni, E.; Alabed, H.B.R.; et al. Characterization of nanovesicles isolated from olive vegetation water. Foods 2024, 13, 835. [Google Scholar] [CrossRef]
- Huang, Z.; Nielsen, S.D.-H.; Whitehead, B.; Nejsum, P.; Corredig, M.; Rasmussen, M.K. Importance of isolation method on characteristics and bioactivity of extracellular vesicles from tomatoes. J. Food Compos. Anal. 2024, 129, 106064. [Google Scholar] [CrossRef]
- Suanno, C.; Tonoli, E.; Fornari, E.; Savoca, M.P.; Aloisi, I.; Parrotta, L.; Faleri, C.; Cai, G.; Coveney, C.; Boocock, D.J.; et al. Small extracellular vesicles released from germinated kiwi pollen (pollensomes) present characteristics similar to mammalian exosomes and carry a plant homolog of ALIX. Front. Plant Sci. 2023, 14, 1090026. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Q.; Liang, Y.; Zu, M.; Chen, N.; Canup, B.S.B.; Luo, L.; Wang, C.; Zeng, L.; Xiao, B. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharm. Sin. B 2022, 12, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luo, Q.; Chen, X.; Chen, F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J. Nanobiotechnol. 2021, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Mulinacci, N.; Innocenti, M.; Santamaria, A.R.; la Marca, G.; Pasqua, G. High-performance liquid chromatography/electrospray ionization tandem mass spectrometric investigation of stilbenoids in cell cultures of Vitis vinifera L., cv. Malvasia. Rapid Commun. Mass Spectrom. 2010, 24, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Veremeichik, G.N.; Grigorchuk, V.P.; Shkryl, Y.N.; Bulgakov, D.V.; Silantieva, S.A.; Bulgakov, V.P. Induction of resveratrol biosynthesis in Vitis amurensis cells by heterologous expression of the Arabidopsis constitutively active, Ca2+-independent form of the AtCPK1 gene. Process Biochem. 2017, 54, 144–155. [Google Scholar] [CrossRef]
- Huber, R.; Marcourt, L.; Héritier, M.; Luscher, A.; Guebey, L.; Schnee, S.; Michellod, E.; Guerrier, S.; Wolfender, J.L.; Scapozza, L.; et al. Generation of potent antibacterial compounds through enzymatic and chemical modifications of the trans-δ-viniferin scaffold. Sci. Rep. 2023, 13, 15986. [Google Scholar] [CrossRef]
- Kim, J.; Zhu, Y.; Chen, S.; Wang, D.; Zhang, S.; Xia, J.; Li, S.; Qiu, Q.; Lee, H.; Wang, J. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J. Nanobiotechnol. 2023, 21, 253. [Google Scholar] [CrossRef]
- Dolma, L.; Damodaran, A.; Panonnummal, R.; Nair, S.C. Exosomes isolated from citrus lemon: A promising candidate for the treatment of Alzheimer’s disease. Ther. Deliv. 2024, 15, 1–13. [Google Scholar] [CrossRef]
- Bruno, S.P.; Paolini, A.; D’Oria, V.; Sarra, A.; Sennato, S.; Bordi, F.; Masotti, A. Extracellular vesicles derived from Citrus sinensis modulate inflammatory genes and tight junctions in a human model of intestinal epithelium. Front. Nutr. 2021, 8, 778998. [Google Scholar] [CrossRef]
- Zhu, H.; He, W. Ginger: A representative material of herb-derived exosome-like nanoparticles. Front. Nutr. 2023, 10, 1223349. [Google Scholar] [CrossRef] [PubMed]
- Tajik, T.; Baghaei, K.; Moghadam, V.E.; Farrokhi, N.; Salami, S.A. Extracellular vesicles of cannabis with high CBD content induce anticancer signaling in human hepatocellular carcinoma. Biomed. Pharmacother. 2022, 152, 113209. [Google Scholar] [CrossRef] [PubMed]
- Emmanuela, N.; Muhammad, D.R.; Iriawati; Wijaya, C.H.; Ratnadewi, Y.M.D.; Takemori, H.; Ana, I.D.; Yuniati, R.; Handayani, W.; Wungu, T.D.K.; et al. Isolation of plant-derived exosome-like nanoparticles (PDENs) from Solanum nigrum L. berries and their effect on interleukin-6 expression as a potential anti-inflammatory agent. PLoS ONE 2024, 19, e0296259. [Google Scholar] [CrossRef]
- Yin, L.; Yan, L.; Yu, Q.; Wang, J.; Liu, C.; Wang, L.; Zheng, L. Characterization of the microRNA profile of ginger exosome-like nanoparticles and their anti-inflammatory effects in intestinal caco-2 cells. J. Agric. Food Chem. 2022, 70, 4725–4734. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, W.L.; Ye, Y.Y.; Bai, X.C.; Liu, R.Q.; Chang, L.F.; Zhou, Q.; Sui, S.F. Cellular internalization of exosomes occurs through phagocytosis. Traffic 2010, 11, 675–687. [Google Scholar] [CrossRef]
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. Phosphatidic acid in membrane rearrangements. FEBS Lett. 2019, 593, 2428–2451. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Liu, K. Plant-derived extracellular vesicles as oral drug delivery carriers. J. Control. Release 2022, 350, 389–400. [Google Scholar] [CrossRef]
- Mourdjeva, M.; Kyurkchiev, D.; Mandinova, A.; Altankova, I.; Kehayov, I.; Kyurkchiev, S. Dynamics of membrane translocation of phosphatidylserine during apoptosis detected by a monoclonal antibody. Apoptosis 2005, 10, 209–217. [Google Scholar] [CrossRef]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Anusha, R.; Ashin, M.; Priya, S. Ginger exosome-like nanoparticles (GELNs) induced apoptosis, cell cycle arrest, and anti-metastatic effects in triple-negative breast cancer MDA-MB-231 cells. Food Chem. Toxicol. 2023, 182, 114102. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef] [PubMed]
- Le, P.N.; McDermott, J.D.; Jimeno, A. Targeting the Wnt pathway in human cancers: Therapeutic targeting with a focus on OMP-54F28. Pharmacol. Ther. 2015, 146, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.; Koval, A.; Marcourt, L.; Héritier, M.; Schnee, S.; Michellod, E.; Scapozza, L.; Katanaev, V.L.; Wolfender, J.L.; Gindro, K.; et al. Chemoenzymatic synthesis of original stilbene dimers possessing Wnt inhibition activity in triple-negative breast cancer cells using the enzymatic secretome of Botrytis cinerea Pers. Front. Chem. 2022, 10, 881298. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lin, Z.J.; Chen, J.C.; Zheng, H.J.; Lai, Y.H.; Huang, H.C. α-Viniferin-induced apoptosis through downregulation of SIRT1 in non-small cell lung cancer cells. Pharmaceuticals 2023, 16, 727. [Google Scholar] [CrossRef]
- Chiou, W.-C.; Huang, C.; Lin, Z.-J.; Hong, L.-S.; Lai, Y.-H.; Chen, J.-C.; Huang, H.-C. α-Viniferin and ε-Viniferin inhibited TGF-β1-induced epithelial-mesenchymal transition, migration and invasion in lung cancer cells through downregulation of vimentin expression. Nutrients 2022, 14, 2294. [Google Scholar] [CrossRef]
- Aja, I.; Ruiz-Larrea, M.B.; Courtois, A.; Krisa, S.; Richard, T.; Ruiz-Sanz, J.-I. Screening of natural stilbene oligomers from Vitis vinifera for anticancer activity on human hepatocellular carcinoma cells. Antioxidants 2020, 9, 469. [Google Scholar] [CrossRef]


| Samples | Average Size, nm | Ζ-Potential, mV | Concentration, Particles/g FW * |
|---|---|---|---|
| GCENs | 78.8 ± 6.5 | −13.9 ± 1.3 | 1.2 × 1010 |
| GENs | 99.7 ± 8.3 | −30.3 ± 2.2 | 2.96 × 1010 |
| Compound | Trans-δ-Viniferin Diglycoside I | Trans-δ-Viniferin Diglycoside II | Trans-δ-Viniferin Glycoside I | Trans-δ-Viniferin Glycoside II | Cis-δ-Viniferin Glycoside | Trans-δ-Viniferin |
|---|---|---|---|---|---|---|
| No. of peak | 1 | 2 | 3 | 4 | 5 | 6 |
| Content | 178.2 ± 36.4 | 61.4 ± 8.5 | 49.7 ± 3.5 | 480.4 ± 37.3 | 27.0 ± 2.2 | 136.7 ± 9.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkryl, Y.; Tsydeneshieva, Z.; Menchinskaya, E.; Rusapetova, T.; Grishchenko, O.; Mironova, A.; Bulgakov, D.; Gorpenchenko, T.; Kazarin, V.; Tchernoded, G.; et al. Exosome-like Nanoparticles, High in Trans-δ-Viniferin Derivatives, Produced from Grape Cell Cultures: Preparation, Characterization, and Anticancer Properties. Biomedicines 2024, 12, 2142. https://doi.org/10.3390/biomedicines12092142
Shkryl Y, Tsydeneshieva Z, Menchinskaya E, Rusapetova T, Grishchenko O, Mironova A, Bulgakov D, Gorpenchenko T, Kazarin V, Tchernoded G, et al. Exosome-like Nanoparticles, High in Trans-δ-Viniferin Derivatives, Produced from Grape Cell Cultures: Preparation, Characterization, and Anticancer Properties. Biomedicines. 2024; 12(9):2142. https://doi.org/10.3390/biomedicines12092142
Chicago/Turabian StyleShkryl, Yury, Zhargalma Tsydeneshieva, Ekaterina Menchinskaya, Tatiana Rusapetova, Olga Grishchenko, Anastasia Mironova, Dmitry Bulgakov, Tatiana Gorpenchenko, Vitaly Kazarin, Galina Tchernoded, and et al. 2024. "Exosome-like Nanoparticles, High in Trans-δ-Viniferin Derivatives, Produced from Grape Cell Cultures: Preparation, Characterization, and Anticancer Properties" Biomedicines 12, no. 9: 2142. https://doi.org/10.3390/biomedicines12092142
APA StyleShkryl, Y., Tsydeneshieva, Z., Menchinskaya, E., Rusapetova, T., Grishchenko, O., Mironova, A., Bulgakov, D., Gorpenchenko, T., Kazarin, V., Tchernoded, G., Bulgakov, V., Aminin, D., & Yugay, Y. (2024). Exosome-like Nanoparticles, High in Trans-δ-Viniferin Derivatives, Produced from Grape Cell Cultures: Preparation, Characterization, and Anticancer Properties. Biomedicines, 12(9), 2142. https://doi.org/10.3390/biomedicines12092142







