In Vitro Models of Cardiovascular Calcification
Abstract
:1. Introduction
2. Major Types of Calcifications in the Cardiovascular System
2.1. Intimal Calcification
2.2. Medial Calcification
2.3. Calciphylaxis
2.4. Heart Valve Calcification
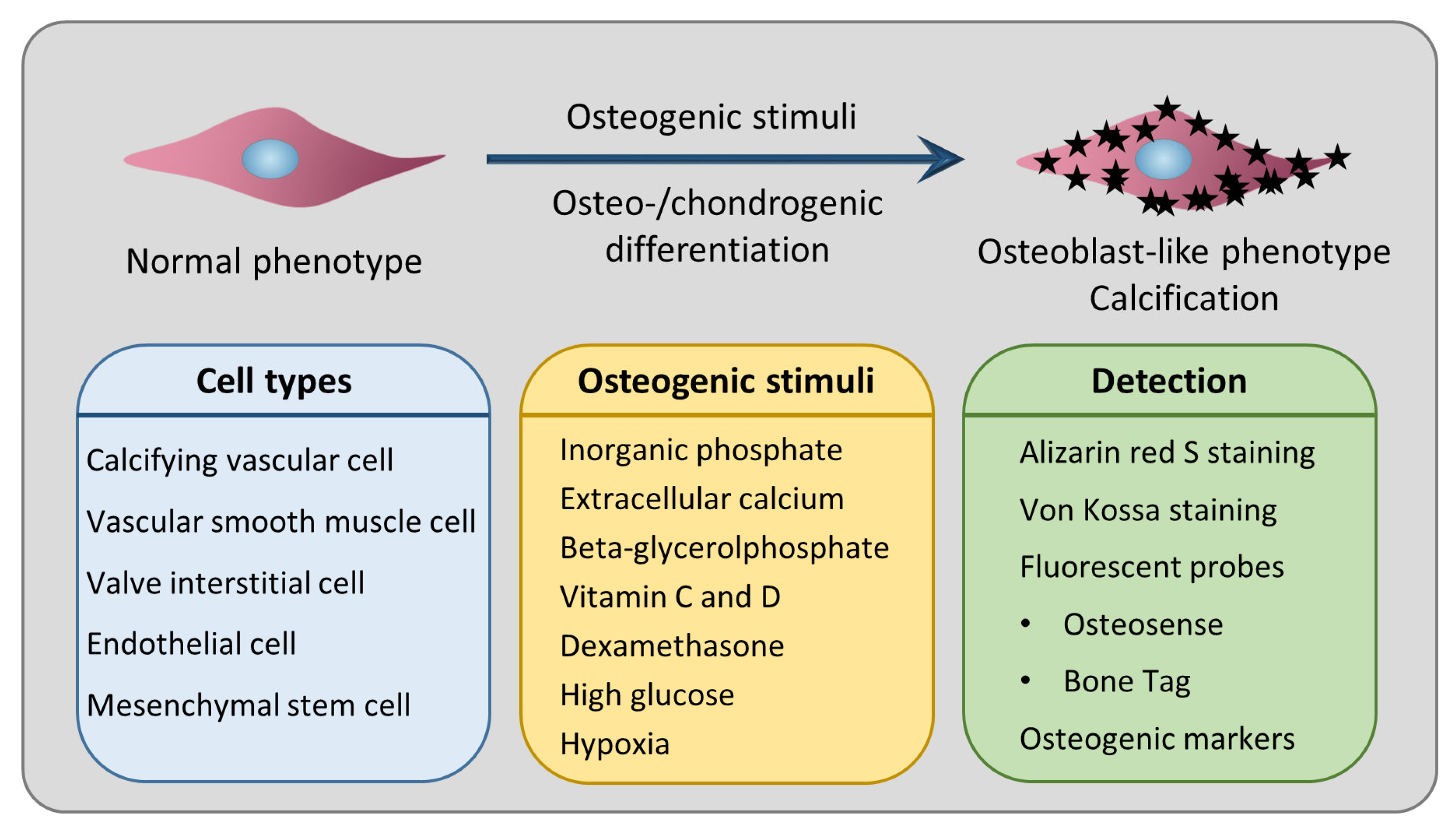
3. Osteo-/Chondrogenic Differentiation as the Underlying Cellular Mechanism of Calcification
4. Cell Types Used in In Vitro Models of Cardiovascular Calcification
4.1. Calcifying Vascular Cells (CVCs)
4.2. Vascular Smooth Muscle Cells (VSMCs)
4.3. Valve interstitial Cells (VICs)
4.4. Endothelial Cells (ECs)
4.5. EC–VSMC Co-Culture Systems
4.6. Mesenchymal Stem Cells (MSCs)
| Cell Type | Origin | Major Finding | Reference |
|---|---|---|---|
| Calcifying vascular cell | Human and bovine aorta | Spontaneous increase in osteogenic markers (ALP, OCN) and calcification under in vitro culture conditions. | [69] |
| Vascular smooth muscle cell | Human aorta | Calcification and gain of osteoblast markers (Runx2, OCN) and loss of smooth muscle markers in response to high Pi. | [34,101] |
| Calcification in response to high Ca. Synergistic effect of Ca on Pi-induced calcification. | [35] | ||
| Upregulation of osteogenic markers (Runx2, Sox9, ALP, OCN) and calcification in response to hypoxia (1% O2). | [48] | ||
| Hypoxia and a hypoxia-mimetic drug, Daprodustat, enhance Pi-induced calcification. | [47,102,103] | ||
| Bovine aorta | Calcification and increased ALP, osteopontin, and Runx2 in response to BGP-containing osteogenic medium. | [41,104] | |
| Calcification and increased ALP and osteopontin in response to active vitamin D3. | [45] | ||
| Calcification and gain of osteoblast markers (Runx2, OCN) in response to DEX. | [105] | ||
| Increased osteoblast markers (Runx2, OCN, ALP, and bone morphogenetic protein) in response to high glucose. | [43] | ||
| Mouse aorta | The role of ER stress/ATF4 in calcification. | [77] | |
| GATA6 accelerates VSMC calcification. | [78] | ||
| Matrix metalloproteinase 3 deletion attenuates osteogenic differentiation. | [79] | ||
| Valve interstitial cell | Bovine aortic valve | Calcification of VICs in response to endotoxin and phosphate. | [83] |
| Human aortic valve | Characterization of calcifying VICs from aortic valves. | [82] | |
| Hypoxia and a hypoxia-mimetic drug, Daprodustat, enhance Pi-induced calcification. | [103] | ||
| Endothelial cell | Human aorta | Inflammation promotes EC calcification. | [89] |
| Ovine mitral valve | Contribution of valve ECs to valve calcification. | [90] | |
| Mesenchymal stem cell | Human aorta | Potential role of MSCs in vascular calcification. | [73,98] |
| Mouse embryo | Msx2 regulates osteogenic differentiation of MSCs. | [99] |
5. Ex Vivo Models of Cardiovascular Calcification
5.1. Aorta Organ Culture Model
5.2. Valve Leaflet Organ Culture Model
6. Most Frequently Used Inducers of In Vitro Calcification and Their Mechanism of Action
6.1. Inorganic Phosphate (Pi)
6.2. Extracellular Calcium
6.3. β-Glycerolphosphate (BGP)
6.4. Ascorbic Acid (AA)
6.5. Vitamin D
6.6. Dexamethasone (DEX)
6.7. High Glucose
6.8. Hypoxia
7. Staining Techniques to Detect Calcification In Vitro
7.1. Alizarin Red S (ARS) Staining
7.2. Von Kossa Staining
7.3. Fluorescently Labeled Probes
8. Using In Vitro Calcification Methods in Drug Discovery
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rogers, M.A.; Aikawa, E. Cardiovascular calcification: Artificial intelligence and big data accelerate mechanistic discovery. Nat. Rev. Cardiol. 2019, 16, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, F.; Buers, I.; Nitschke, Y. Hereditary Disorders of Cardiovascular Calcification. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Virmani, R.; Joner, M.; Sakakura, K. Recent highlights of ATVB: Calcification. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.D.; Goettsch, C.; Rogers, M.A.; Aikawa, E. Revisiting cardiovascular calcification: A multifaceted disease requiring a multidisciplinary approach. Semin. Cell Dev. Biol. 2015, 46, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M. Vascular calcification mechanisms. J. Am. Soc. Nephrol. 2004, 15, 2959–2964. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Tintut, Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 715–723. [Google Scholar] [CrossRef]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Tóth, A.; Balogh, E.; Jeney, V. Regulation of vascular calcification by reactive oxygen species. Antioxidants 2020, 9, 963. [Google Scholar] [CrossRef]
- Mackey, R.H.; Venkitachalam, L.; Sutton-Tyrrell, K. Calcifications, arterial stiffness and atherosclerosis. Adv. Cardiol. 2007, 44, 234–244. [Google Scholar] [CrossRef]
- Onnis, C.; Virmani, R.; Kawai, K.; Nardi, V.; Lerman, A.; Cademartiri, F.; Scicolone, R.; Boi, A.; Congiu, T.; Faa, G.; et al. Coronary Artery Calcification: Current Concepts and Clinical Implications. Circulation 2024, 149, 251–266. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Theroux, P. Pathophysiology of coronary artery disease. Circulation 2005, 111, 3481–3488. [Google Scholar] [CrossRef] [PubMed]
- Jinnouchi, H.; Sato, Y.; Sakamoto, A.; Cornelissen, A.; Mori, M.; Kawakami, R.; Gadhoke, N.V.; Kolodgie, F.D.; Virmani, R.; Finn, A.V. Calcium deposition within coronary atherosclerotic lesion: Implications for plaque stability. Atherosclerosis 2020, 306, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Sakakura, K.; Yahagi, K.; Joner, M.; Virmani, R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler. Thromb. Vasc. Biol. 2014, 34, 724–736. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Madhavan, M.V.; Tarigopula, M.; Mintz, G.S.; Maehara, A.; Stone, G.W.; Généreux, P. Coronary artery calcification: Pathogenesis and prognostic implications. J. Am. Coll. Cardiol. 2014, 63, 1703–1714. [Google Scholar] [CrossRef]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St Hilaire, C.; Shanahan, C. Medial vascular calcification revisited: Review and perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef]
- Johnson, R.C.; Leopold, J.A.; Loscalzo, J. Vascular calcification: Pathobiological mechanisms and clinical implications. Circ. Res. 2006, 99, 1044–1059. [Google Scholar] [CrossRef]
- Lanzer, P.; Hannan, F.M.; Lanzer, J.D.; Janzen, J.; Raggi, P.; Furniss, D.; Schuchardt, M.; Thakker, R.; Fok, P.-W.; Saez-Rodriguez, J.; et al. Medial Arterial Calcification: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1145–1165. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Goettsch, C. Cardiovascular Calcification Heterogeneity in Chronic Kidney Disease. Circ. Res. 2023, 132, 993–1012. [Google Scholar] [CrossRef]
- Shanahan, C.M.; Cary, N.R.; Salisbury, J.R.; Proudfoot, D.; Weissberg, P.L.; Edmonds, M.E. Medial localization of mineralization-regulating proteins in association with Mönckeberg’s sclerosis: Evidence for smooth muscle cell-mediated vascular calcification. Circulation 1999, 100, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Rementer, C.; Giachelli, C.M. Vascular calcification: An update on mechanisms and challenges in treatment. Calcif. Tissue Int. 2013, 93, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Dao, H.H.; Essalihi, R.; Bouvet, C.; Moreau, P. Evolution and modulation of age-related medial elastocalcinosis: Impact on large artery stiffness and isolated systolic hypertension. Cardiovasc. Res. 2005, 66, 307–317. [Google Scholar] [CrossRef]
- Gallo Marin, B.; Aghagoli, G.; Hu, S.L.; Massoud, C.M.; Robinson-Bostom, L. Calciphylaxis and Kidney Disease: A Review. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2023, 81, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Nigwekar, S.U.; Thadhani, R.; Brandenburg, V.M. Calciphylaxis. N. Engl. J. Med. 2018, 378, 1704–1714. [Google Scholar] [CrossRef] [PubMed]
- Moncla, L.-H.M.; Briend, M.; Bossé, Y.; Mathieu, P. Calcific aortic valve disease: Mechanisms, prevention and treatment. Nat. Rev. Cardiol. 2023, 20, 546–559. [Google Scholar] [CrossRef] [PubMed]
- Salhiyyah, K.; Yacoub, M.H.; Chester, A.H. Cellular mechanisms in mitral valve disease. J. Cardiovasc. Transl. Res. 2011, 4, 702–709. [Google Scholar] [CrossRef]
- Rajamannan, N.M.; Subramaniam, M.; Rickard, D.; Stock, S.R.; Donovan, J.; Springett, M.; Orszulak, T.; Fullerton, D.A.; Tajik, A.J.; Bonow, R.O.; et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 2003, 107, 2181–2184. [Google Scholar] [CrossRef]
- Mohler, E.R., 3rd; Gannon, F.; Reynolds, C.; Zimmerman, R.; Keane, M.G.; Kaplan, F.S. Bone formation and inflammation in cardiac valves. Circulation 2001, 103, 1522–1528. [Google Scholar] [CrossRef]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of “degenerative” valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef]
- Olsson, M.; Dalsgaard, C.J.; Haegerstrand, A.; Rosenqvist, M.; Rydén, L.; Nilsson, J. Accumulation of T lymphocytes and expression of interleukin-2 receptors in nonrheumatic stenotic aortic valves. J. Am. Coll. Cardiol. 1994, 23, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Thyberg, J.; Nilsson, J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.D.; Reichenbach, D.D.; Marcovina, S.M.; Kuusisto, J.; Alpers, C.E.; Otto, C.M. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of “degenerative” valvular aortic stenosis. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 307, F891–F900. [Google Scholar] [CrossRef]
- Yang, H.; Curinga, G.; Giachelli, C.M. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. 2004, 66, 2293–2299. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.-Y.; Giachelli, C.M. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis 2008, 199, 271–277. [Google Scholar] [CrossRef]
- Aikawa, E.; Nahrendorf, M.; Figueiredo, J.L.; Swirski, F.K.; Shtatland, T.; Kohler, R.H.; Jaffer, F.A.; Aikawa, M.; Weissleder, R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007, 116, 2841–2850. [Google Scholar] [CrossRef]
- Shioi, A.; Katagi, M.; Okuno, Y.; Mori, K.; Jono, S.; Koyama, H.; Nishizawa, Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: Roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ. Res. 2002, 91, 9–16. [Google Scholar] [CrossRef]
- Zickler, D.; Luecht, C.; Willy, K.; Chen, L.; Witowski, J.; Girndt, M.; Fiedler, R.; Storr, M.; Kamhieh-Milz, J.; Schoon, J.; et al. Tumour necrosis factor-alpha in uraemic serum promotes osteoblastic transition and calcification of vascular smooth muscle cells via extracellular signal-regulated kinases and activator protein 1/c-FOS-mediated induction of interleukin 6 expression. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2018, 33, 574–585. [Google Scholar] [CrossRef]
- Parhami, F.; Morrow, A.D.; Balucan, J.; Leitinger, N.; Watson, A.D.; Tintut, Y.; Berliner, J.A.; Demer, L.L. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 680–687. [Google Scholar] [CrossRef]
- Bear, M.; Butcher, M.; Shaughnessy, S.G. Oxidized low-density lipoprotein acts synergistically with beta-glycerophosphate to induce osteoblast differentiation in primary cultures of vascular smooth muscle cells. J. Cell. Biochem. 2008, 105, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Atkins, S.K.; Zheng, K.H.; Singh, S.A.; Chelvanambi, S.; Pham, T.H.; Kuraoka, S.; Stroes, E.S.G.; Aikawa, M.; Aikawa, E. Lipoprotein(a) Induces Vesicular Cardiovascular Calcification Revealed With Single-Extracellular Vesicle Analysis. Front. Cardiovasc. Med. 2022, 9, 778919. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.X.; Duan, D.; O’Neill, K.D.; Moe, S.M. High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2006, 21, 3435–3442. [Google Scholar] [CrossRef]
- Tanikawa, T.; Okada, Y.; Tanikawa, R.; Tanaka, Y. Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK. J. Vasc. Res. 2009, 46, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Jono, S.; Nishizawa, Y.; Shioi, A.; Morii, H. 1,25-Dihydroxyvitamin D3 increases in vitro vascular calcification by modulating secretion of endogenous parathyroid hormone-related peptide. Circulation 1998, 98, 1302–1306. [Google Scholar] [CrossRef]
- Delgado-Marin, M.; Sánchez-Esteban, S.; Cook-Calvete, A.; Jorquera-Ortega, S.; Zaragoza, C.; Saura, M. Indoxyl Sulfate-Induced Valve Endothelial Cell Endothelial-to-Mesenchymal Transition and Calcification in an Integrin-Linked Kinase-Dependent Manner. Cells 2024, 13, 481. [Google Scholar] [CrossRef]
- Mokas, S.; Larivière, R.; Lamalice, L.; Gobeil, S.; Cornfield, D.N.; Agharazii, M.; Richard, D.E. Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int. 2016, 90, 598–609. [Google Scholar] [CrossRef]
- Balogh, E.; Tóth, A.; Méhes, G.; Trencsényi, G.; Paragh, G.; Jeney, V. Hypoxia Triggers Osteochondrogenic Differentiation of Vascular Smooth Muscle Cells in an HIF-1 (Hypoxia-Inducible Factor 1)-Dependent and Reactive Oxygen Species-Dependent Manner. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1088–1099. [Google Scholar] [CrossRef]
- Schinke, T.; Amendt, C.; Trindl, A.; Pöschke, O.; Müller-Esterl, W.; Jahnen-Dechent, W. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J. Biol. Chem. 1996, 271, 20789–20796. [Google Scholar] [CrossRef]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef]
- Orriss, I.R.; Arnett, T.R.; Russell, R.G.G. Pyrophosphate: A key inhibitor of mineralisation. Curr. Opin. Pharmacol. 2016, 28, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ter Braake, A.D.; Tinnemans, P.T.; Shanahan, C.M.; Hoenderop, J.G.J.; de Baaij, J.H.F. Magnesium prevents vascular calcification in vitro by inhibition of hydroxyapatite crystal formation. Sci. Rep. 2018, 8, 2069. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Jeney, V.; Arosio, P.; Poli, M.; Antal-Szalma, P.; Agarwal, A.; Balla, G.; Balla, J. Ferritin prevents calcification and osteoblastic differentiation of vascular smooth muscle cells. J. Am. Soc. Nephrol. 2009, 20, 1254–1263. [Google Scholar] [CrossRef]
- Balogh, E.; Chowdhury, A.; Ababneh, H.; Csiki, D.M.; Tóth, A.; Jeney, V. Heme-Mediated Activation of the Nrf2/HO-1 Axis Attenuates Calcification of Valve Interstitial Cells. Biomedicines 2021, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-S.; Cheng, S.-L.; Pingsterhaus, J.M.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J. Clin. Investig. 2005, 115, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-S.; Al Aly, Z.; Lai, C.-F.; Cheng, S.-L.; Cai, J.; Huang, E.; Behrmann, A.; Towler, D.A. Vascular Bmp Msx2 Wnt signaling and oxidative stress in arterial calcification. Ann. N. Y. Acad. Sci. 2007, 1117, 40–50. [Google Scholar] [CrossRef]
- Kanno, Y.; Into, T.; Lowenstein, C.J.; Matsushita, K. Nitric oxide regulates vascular calcification by interfering with TGF-signalling. Cardiovasc. Res. 2008, 77, 221–230. [Google Scholar] [CrossRef]
- Huang, A.; Li, L.; Liu, X.; Lian, Q.; Guo, G.; Xu, T.; Lu, X.; Ma, L.; Ma, H.; Yu, Y.; et al. Hedgehog signaling is a potential therapeutic target for vascular calcification. Gene 2023, 872, 147457. [Google Scholar] [CrossRef]
- Jono, S.; Nishizawa, Y.; Shioi, A.; Morii, H. Parathyroid hormone-related peptide as a local regulator of vascular calcification. Its inhibitory action on in vitro calcification by bovine vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1135–1142. [Google Scholar] [CrossRef]
- Jimbo, R.; Kawakami-Mori, F.; Mu, S.; Hirohama, D.; Majtan, B.; Shimizu, Y.; Yatomi, Y.; Fukumoto, S.; Fujita, T.; Shimosawa, T. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney Int. 2014, 85, 1103–1111. [Google Scholar] [CrossRef]
- Shimizu, T.; Tanaka, T.; Iso, T.; Doi, H.; Sato, H.; Kawai-Kowase, K.; Arai, M.; Kurabayashi, M. Notch signaling induces osteogenic differentiation and mineralization of vascular smooth muscle cells: Role of Msx2 gene induction via Notch-RBP-Jk signaling. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Szabó, L.; Balogh, N.; Tóth, A.; Angyal, Á.; Gönczi, M.; Csiki, D.M.; Tóth, C.; Balatoni, I.; Jeney, V.; Csernoch, L.; et al. The mechanosensitive Piezo1 channels contribute to the arterial medial calcification. Front. Physiol. 2022, 13, 1037230. [Google Scholar] [CrossRef] [PubMed]
- Speer, M.Y.; Yang, H.-Y.; Brabb, T.; Leaf, E.; Look, A.; Lin, W.-L.; Frutkin, A.; Dichek, D.; Giachelli, C.M. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ. Res. 2009, 104, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Sallam, T.; Cheng, H.; Demer, L.L.; Tintut, Y. Regulatory circuits controlling vascular cell calcification. Cell. Mol. Life Sci. 2013, 70, 3187–3197. [Google Scholar] [CrossRef]
- Sage, A.P.; Tintut, Y.; Demer, L.L. Regulatory mechanisms in vascular calcification. Nat. Rev. Cardiol. 2010, 7, 528–536. [Google Scholar] [CrossRef]
- Sutton, N.R.; Malhotra, R.; St Hilaire, C.; Aikawa, E.; Blumenthal, R.S.; Gackenbach, G.; Goyal, P.; Johnson, A.; Nigwekar, S.U.; Shanahan, C.M.; et al. Molecular Mechanisms of Vascular Health: Insights From Vascular Aging and Calcification. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 15–29. [Google Scholar] [CrossRef]
- Shanahan, C.M. Mechanisms of vascular calcification in CKD—Evidence for premature ageing? Nat. Rev. Nephrol. 2013, 9, 661–670. [Google Scholar] [CrossRef]
- Gheorghe, S.R.; Crăciun, A.M.; Ilyés, T.; Tisa, I.B.; Sur, L.; Lupan, I.; Samasca, G.; Silaghi, C.N. Converging Mechanisms of Vascular and Cartilaginous Calcification. Biology 2024, 13, 565. [Google Scholar] [CrossRef]
- Watson, K.E.; Boström, K.; Ravindranath, R.; Lam, T.; Norton, B.; Demer, L.L. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J. Clin. Investig. 1994, 93, 2106–2113. [Google Scholar] [CrossRef]
- Radcliff, K.; Tang, T.-B.; Lim, J.; Zhang, Z.; Abedin, M.; Demer, L.L.; Tintut, Y. Insulin-like growth factor-I regulates proliferation and osteoblastic differentiation of calcifying vascular cells via extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase pathways. Circ. Res. 2005, 96, 398–400. [Google Scholar] [CrossRef]
- Zebboudj, A.F.; Shin, V.; Boström, K. Matrix GLA protein and BMP-2 regulate osteoinduction in calcifying vascular cells. J. Cell. Biochem. 2003, 90, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Tintut, Y.; Alfonso, Z.; Saini, T.; Radcliff, K.; Watson, K.; Boström, K.; Demer, L.L. Multilineage potential of cells from the artery wall. Circulation 2003, 108, 2505–2510. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Dweck, M.R.; Narula, N.; Pisapia, D.; Narula, J.; Strauss, H.W. Coronary Artery Calcification From Mechanism to Molecular Imaging. JACC Cardiovasc. Imaging 2017, 10, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Dart, A.M.; Kingwell, B.A. Pulse pressure—A review of mechanisms and clinical relevance. J. Am. Coll. Cardiol. 2001, 37, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Ceccherini, E.; Cecchettini, A.; Gisone, I.; Persiani, E.; Morales, M.A.; Vozzi, F. Vascular Calcification: In Vitro Models under the Magnifying Glass. Biomedicines 2022, 10, 2491. [Google Scholar] [CrossRef]
- Furmanik, M.; Shanahan, C.M. ER stress regulates alkaline phosphatase gene expression in vascular smooth muscle cells via an ATF4-dependent mechanism. BMC Res. Notes 2018, 11, 483. [Google Scholar] [CrossRef]
- Li, X.; Liu, A.; Xie, C.; Chen, Y.; Zeng, K.; Xie, C.; Zhang, Z.; Luo, P.; Huang, H. The transcription factor GATA6 accelerates vascular smooth muscle cell senescence-related arterial calcification by counteracting the role of anti-aging factor SIRT6 and impeding DNA damage repair. Kidney Int. 2024, 105, 115–131. [Google Scholar] [CrossRef]
- Xie, Y.; Lin, T.; Jin, Y.; Berezowitz, A.G.; Wang, X.-L.; Lu, J.; Cai, Y.; Guzman, R.J. Smooth muscle cell-specific matrix metalloproteinase 3 deletion reduces osteogenic transformation and medial artery calcification. Cardiovasc. Res. 2024, 120, 658–670. [Google Scholar] [CrossRef]
- Hinton, R.B.; Yutzey, K.E. Heart valve structure and function in development and disease. Annu. Rev. Physiol. 2011, 73, 29–46. [Google Scholar] [CrossRef]
- Johnson, C.M.; Hanson, M.N.; Helgeson, S.C. Porcine cardiac valvular subendothelial cells in culture: Cell isolation and growth characteristics. J. Mol. Cell. Cardiol. 1987, 19, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Mohler, E.R., 3rd; Chawla, M.K.; Chang, A.W.; Vyavahare, N.; Levy, R.J.; Graham, L.; Gannon, F.H. Identification and characterization of calcifying valve cells from human and canine aortic valves. J. Heart Valve Dis. 1999, 8, 254–260. [Google Scholar] [PubMed]
- Rattazzi, M.; Iop, L.; Faggin, E.; Bertacco, E.; Zoppellaro, G.; Baesso, I.; Puato, M.; Torregrossa, G.; Fadini, G.P.; Agostini, C.; et al. Clones of interstitial cells from bovine aortic valve exhibit different calcifying potential when exposed to endotoxin and phosphate. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Y.; Jones, P.L.; Lafferty, R.P.; Safer, D.; Levy, R.J. Thymosin beta4 regulation, expression and function in aortic valve interstitial cells. J. Heart Valve Dis. 2002, 11, 726–735. [Google Scholar]
- El Husseini, D.; Boulanger, M.-C.; Mahmut, A.; Bouchareb, R.; Laflamme, M.-H.; Fournier, D.; Pibarot, P.; Bossé, Y.; Mathieu, P. P2Y2 receptor represses IL-6 expression by valve interstitial cells through Akt: Implication for calcific aortic valve disease. J. Mol. Cell. Cardiol. 2014, 72, 146–156. [Google Scholar] [CrossRef]
- Jiang, H.; Li, L.; Zhang, L.; Zang, G.; Sun, Z.; Wang, Z. Role of endothelial cells in vascular calcification. Front. Cardiovasc. Med. 2022, 9, 895005. [Google Scholar] [CrossRef]
- Yao, Y.; Jumabay, M.; Ly, A.; Radparvar, M.; Cubberly, M.R.; Boström, K.I. A role for the endothelium in vascular calcification. Circ. Res. 2013, 113, 495–504. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Wu, X.; Zhang, L.; Cai, X.; Ji, J.; Chen, S.; Vera, A.; Boström, K.I.; Yao, Y. CDK1 inhibition reduces osteogenesis in endothelial cells in vascular calcification. JCI Insight 2024, 9, e176065. [Google Scholar]
- Sánchez-Duffhues, G.; García de Vinuesa, A.; van de Pol, V.; Geerts, M.E.; de Vries, M.R.; Janson, S.G.; van Dam, H.; Lindeman, J.H.; Goumans, M.-J.; Ten Dijke, P. Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J. Pathol. 2019, 247, 333–346. [Google Scholar] [CrossRef]
- Wylie-Sears, J.; Aikawa, E.; Levine, R.A.; Yang, J.-H.; Bischoff, J. Mitral valve endothelial cells with osteogenic differentiation potential. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 598–607. [Google Scholar] [CrossRef]
- Fan, L.; Yao, D.; Fan, Z.; Zhang, T.; Shen, Q.; Tong, F.; Qian, X.; Xu, L.; Jiang, C.; Dong, N. Beyond VICs: Shedding light on the overlooked VECs in calcific aortic valve disease. Biomed. Pharmacother. 2024, 178, 117143. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Yuan, Z.; Li, F.; Cai, Z. Oxidative stress and valvular endothelial cells in aortic valve calcification. Biomed. Pharmacother. 2023, 163, 114775. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Barbero, N.; Gutiérrez-Muñoz, C.; Blanco-Colio, L.M. Cellular Crosstalk between Endothelial and Smooth Muscle Cells in Vascular Wall Remodeling. Int. J. Mol. Sci. 2021, 22, 7284. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Shan, S.-K.; Xu, F.; Zhong, J.-Y.; Wu, F.; Duan, J.-Y.; Guo, B.; Li, F.-X.-Z.; Wang, Y.; Zheng, M.-H.; et al. The crosstalk between endothelial cells and vascular smooth muscle cells aggravates high phosphorus-induced arterial calcification. Cell Death Dis. 2022, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- Ceccherini, E.; Persiani, E.; Cabiati, M.; Guiducci, L.; Del Ry, S.; Gisone, I.; Falleni, A.; Cecchettini, A.; Vozzi, F. A Dynamic Cellular Model as an Emerging Platform to Reproduce the Complexity of Human Vascular Calcification In Vitro. Int. J. Mol. Sci. 2024, 25, 7427. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Abedin, M.; Tintut, Y.; Demer, L.L. Mesenchymal stem cells and the artery wall. Circ. Res. 2004, 95, 671–676. [Google Scholar] [CrossRef]
- Cheng, S.-L.; Shao, J.-S.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J. Biol. Chem. 2003, 278, 45969–45977. [Google Scholar] [CrossRef]
- Balogh, E.; Tolnai, E.; Nagy, B.; Nagy, B.; Balla, G.; Balla, J.; Jeney, V. Iron overload inhibits osteogenic commitment and differentiation of mesenchymal stem cells via the induction of ferritin. Biochim. Biophys. Acta 2016, 1862, 1640–1649. [Google Scholar] [CrossRef]
- Steitz, S.A.; Speer, M.Y.; Curinga, G.; Yang, H.Y.; Haynes, P.; Aebersold, R.; Schinke, T.; Karsenty, G.; Giachelli, C.M. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001, 89, 1147–1154. [Google Scholar] [CrossRef]
- Tóth, A.; Csiki, D.M.; Nagy, B.; Balogh, E.; Lente, G.; Ababneh, H.; Szöőr, Á.; Jeney, V. Daprodustat Accelerates High Phosphate-Induced Calcification Through the Activation of HIF-1 Signaling. Front. Pharmacol. 2022, 13, 798053. [Google Scholar] [CrossRef] [PubMed]
- Csiki, D.M.; Ababneh, H.; Tóth, A.; Lente, G.; Szöőr, Á.; Tóth, A.; Fillér, C.; Juhász, T.; Nagy, B.; Balogh, E.; et al. Hypoxia-inducible factor activation promotes osteogenic transition of valve interstitial cells and accelerates aortic valve calcification in a mice model of chronic kidney disease. Front. Cardiovasc. Med. 2023, 10, 1168339. [Google Scholar] [CrossRef]
- Shioi, A.; Nishizawa, Y.; Jono, S.; Koyama, H.; Hosoi, M.; Morii, H. Beta-glycerophosphate accelerates calcification in cultured bovine vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Shioi, A.; Jono, S.; Nishizawa, Y.; Morii, H. Dexamethasone enhances In vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Diglio, C.A.; Grammas, P.; Giacomelli, F.; Wiener, J. Angiogenesis in rat aorta ring explant cultures. Lab. Invest. 1989, 60, 523–531. [Google Scholar] [PubMed]
- Akiyoshi, T.; Ota, H.; Iijima, K.; Son, B.-K.; Kahyo, T.; Setou, M.; Ogawa, S.; Ouchi, Y.; Akishita, M. A novel organ culture model of aorta for vascular calcification. Atherosclerosis 2016, 244, 51–58. [Google Scholar] [CrossRef]
- Watts, L.K.; Duffy, P.; Field, R.B.; Stafford, E.G.; O’Brien, M.F. Establishment of a viable homograft cardiac valve bank: A rapid method of determining homograft viability. Ann. Thorac. Surg. 1976, 21, 230–236. [Google Scholar] [CrossRef]
- Lester, W.M.; Gotlieb, A.I. In vitro repair of the wounded porcine mitral valve. Circ. Res. 1988, 62, 833–845. [Google Scholar] [CrossRef]
- Chester, A.H.; Sarathchandra, P.; McCormack, A.; Yacoub, M.H. Organ Culture Model of Aortic Valve Calcification. Front. Cardiovasc. Med. 2021, 8, 734692. [Google Scholar] [CrossRef]
- Lacerda, C.M.R.; Kisiday, J.; Johnson, B.; Orton, E.C. Local serotonin mediates cyclic strain-induced phenotype transformation, matrix degradation, and glycosaminoglycan synthesis in cultured sheep mitral valves. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1983–H1990. [Google Scholar] [CrossRef] [PubMed]
- Lester, W.M.; Damji, A.A.; Tanaka, M.; Gedeon, I. Bovine mitral valve organ culture: Role of interstitial cells in repair of valvular injury. J. Mol. Cell. Cardiol. 1992, 24, 43–53. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.-Y.; Giachelli, C.M. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ. Res. 2006, 98, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Crouthamel, M.H.; Lau, W.L.; Leaf, E.M.; Chavkin, N.W.; Wallingford, M.C.; Peterson, D.F.; Li, X.; Liu, Y.; Chin, M.T.; Levi, M.; et al. Sodium-dependent phosphate cotransporters and phosphate-induced calcification of vascular smooth muscle cells: Redundant roles for PiT-1 and PiT-2. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2625–2632. [Google Scholar] [CrossRef]
- Reynolds, J.L.; Joannides, A.J.; Skepper, J.N.; Mcnair, R.; Schurgers, L.J.; Proudfoot, D.; Jahnen-Dechent, W.; Weissberg, P.L.; Shanahan, C.M. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol. 2004, 15, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Alesutan, I.; Moritz, F.; Haider, T.; Shouxuan, S.; Gollmann-Tepeköylü, C.; Holfeld, J.; Pieske, B.; Lang, F.; Eckardt, K.-U.; Heinzmann, S.S.; et al. Impact of β-glycerophosphate on the bioenergetic profile of vascular smooth muscle cells. J. Mol. Med. 2020, 98, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, P.; Volpi, E.; Brenna, I.; Arnaboldi, L.; Neri, L.; Brancaccio, D.; Cozzolino, M. Combined effects of ascorbic acid and phosphate on rat VSMC osteoblastic differentiation. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2012, 27, 122–127. [Google Scholar] [CrossRef]
- Ivanov, V.; Ivanova, S.; Niedzwiecki, A.; Rath, M. Vitamin C inhibits the calcification process in human vascular smooth muscle cells. Am. J. Cardiovasc. Dis. 2020, 10, 108–116. [Google Scholar]
- Arakawa, E.; Hasegawa, K.; Irie, J.; Ide, S.; Ushiki, J.; Yamaguchi, K.; Oda, S.; Matsuda, Y. L-ascorbic acid stimulates expression of smooth muscle-specific markers in smooth muscle cells both in vitro and in vivo. J. Cardiovasc. Pharmacol. 2003, 42, 745–751. [Google Scholar] [CrossRef]
- Aguirre, R.; May, J.M. Inflammation in the vascular bed: Importance of vitamin C. Pharmacol. Ther. 2008, 119, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Cardús, A.; Parisi, E.; Gallego, C.; Aldea, M.; Fernández, E.; Valdivielso, J.M. 1,25-Dihydroxyvitamin D3 stimulates vascular smooth muscle cell proliferation through a VEGF-mediated pathway. Kidney Int. 2006, 69, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Rebsamen, M.C.; Sun, J.; Norman, A.W.; Liao, J.K. 1α,25-dihydroxyvitamin D3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circ. Res. 2002, 91, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Rajasree, S.; Umashankar, P.R.; Lal, A.V.; Sarma, P.S.; Kartha, C.C. 1,25-dihydroxyvitamin D3 receptor is upregulated in aortic smooth muscle cells during hypervitaminosis D. Life Sci. 2002, 70, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Han, M.-S.; Che, X.; Cho, G.; Park, H.-R.; Lim, K.-E.; Park, N.-R.; Jin, J.-S.; Jung, Y.-K.; Jeong, J.-H.; Lee, I.-K.; et al. Functional cooperation between vitamin D receptor and Runx2 in vitamin D-induced vascular calcification. PLoS ONE 2013, 8, e83584. [Google Scholar] [CrossRef] [PubMed]
- Shalhoub, V.; Conlon, D.; Tassinari, M.; Quinn, C.; Partridge, N.; Stein, G.S.; Lian, J.B. Glucocorticoids promote development of the osteoblast phenotype by selectively modulating expression of cell growth and differentiation-associated genes. J. Cell. Biochem. 1992, 50, 425–440. [Google Scholar] [CrossRef]
- Kirton, J.P.; Wilkinson, F.L.; Canfield, A.E.; Alexander, M.Y. Dexamethasone downregulates calcification-inhibitor molecules and accelerates osteogenic differentiation of vascular pericytes: Implications for vascular calcification. Circ. Res. 2006, 98, 1264–1272. [Google Scholar] [CrossRef]
- Stabley, J.N.; Towler, D.A. Arterial Calcification in Diabetes Mellitus: Preclinical Models and Translational Implications. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 205–217. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, J.; Yang, W.; Zheng, H.; Xue, S. High mobility group box 1 (HMGB1) mediates high-glucose-induced calcification in vascular smooth muscle cells of saphenous veins. Inflammation 2013, 36, 1592–1604. [Google Scholar] [CrossRef]
- Tóth, A.; Lente, G.; Csiki, D.M.; Balogh, E.; Szöőr, Á.; Nagy, B.J.; Jeney, V. Activation of PERK/eIF2α/ATF4/CHOP branch of endoplasmic reticulum stress response and cooperation between HIF-1α and ATF4 promotes Daprodustat-induced vascular calcification. Front. Pharmacol. 2024, 15, 1399248. [Google Scholar] [CrossRef]
- Bernar, A.; Gebetsberger, J.V.; Bauer, M.; Streif, W.; Schirmer, M. Optimization of the Alizarin Red S Assay by Enhancing Mineralization of Osteoblasts. Int. J. Mol. Sci. 2022, 24, 723. [Google Scholar] [CrossRef] [PubMed]
- Lievremont, M.; Potus, J.; Guillou, B. Use of alizarin red S for histochemical staining of Ca2+ in the mouse; some parameters of the chemical reaction in vitro. Cells Tissues Organs 1982, 114, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R. Von Kossa and his staining technique. Histochem. Cell Biol. 2021, 156, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, A.; Lenkinski, R.E.; Mahmood, A.; Jones, A.G.; Cantley, L.C.; Frangioni, J.V. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat. Biotechnol. 2001, 19, 1148–1154. [Google Scholar] [CrossRef]
- Greco, A.; Herrmann, J.; Babic, M.; Gummi, M.R.; van der Giet, M.; Tölle, M.; Schuchardt, M. Molecular Imaging and Quantification of Smooth Muscle Cell and Aortic Tissue Calcification In Vitro and Ex Vivo with a Fluorescent Hydroxyapatite-Specific Probe. Biomedicines 2022, 10, 2271. [Google Scholar] [CrossRef]
- Kovar, J.L.; Xu, X.; Draney, D.; Cupp, A.; Simpson, M.A.; Olive, D.M. Near-infrared-labeled tetracycline derivative is an effective marker of bone deposition in mice. Anal. Biochem. 2011, 416, 167–173. [Google Scholar] [CrossRef]
- Leenders, N.H.J.; Bos, C.; Hoekstra, T.; Schurgers, L.J.; Vervloet, M.G.; Hoenderop, J.G.J. Dietary magnesium supplementation inhibits abdominal vascular calcification in an experimental animal model of chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2022, 37, 1049–1058. [Google Scholar] [CrossRef]
- Bressendorff, I.; Hansen, D.; Schou, M.; Kragelund, C.; Svensson, M.; Hashemi, B.; Kristensen, T.; Vrist, M.H.; Borg, R.; Tougaard, B.; et al. The Effect of Magnesium Supplementation on Vascular Calcification in CKD: A Randomized Clinical Trial (MAGiCAL-CKD). J. Am. Soc. Nephrol. 2023, 34, 886–894. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, A.; Balogh, E.; Jeney, V. In Vitro Models of Cardiovascular Calcification. Biomedicines 2024, 12, 2155. https://doi.org/10.3390/biomedicines12092155
Tóth A, Balogh E, Jeney V. In Vitro Models of Cardiovascular Calcification. Biomedicines. 2024; 12(9):2155. https://doi.org/10.3390/biomedicines12092155
Chicago/Turabian StyleTóth, Andrea, Enikő Balogh, and Viktória Jeney. 2024. "In Vitro Models of Cardiovascular Calcification" Biomedicines 12, no. 9: 2155. https://doi.org/10.3390/biomedicines12092155
APA StyleTóth, A., Balogh, E., & Jeney, V. (2024). In Vitro Models of Cardiovascular Calcification. Biomedicines, 12(9), 2155. https://doi.org/10.3390/biomedicines12092155





