Abstract
An intriguing aspect of restrictive cardiomyopathies (RCM) is the microbiome role in the natural history of the disease. These cardiomyopathies are often difficult to diagnose and so result in significant morbidity and mortality. The human microbiome, composed of billions of microorganisms, influences various physiological and pathological processes, including cardiovascular health. Studies have shown that gut dysbiosis, an imbalance in the composition of intestinal bacteria, can contribute to systemic inflammation, a key factor in many cardiovascular conditions. An increase in gut permeability, frequently caused by dysbiosis, allows bacterial endotoxins to enter the bloodstream, activating inflammatory pathways that exacerbate cardiac dysfunction. Recent reports highlight the potential role of microbiome in amyloidogenesis, as certain bacteria produce proteins that accelerate the formation of amyloid fibrils. Concurrently, advancements in amyloidosis treatments have sparked renewed hopes, marking a promising era for managing these kinds of diseases. These findings suggest that the gut–heart axis may be a potential factor in the development and progression of cardiovascular disease like RCM, opening new paths for therapeutic intervention. The aim of this review is to provide a detailed overview of the gut–heart axis, focusing on RCM.
1. Introduction
Microbial–Myocardial Axis
Cardiovascular diseases (CVD), such as coronary artery disease (CAD), stroke, heart failure (HF) and arterial hypertension, have become the leading global cause of death [1]. CVD development and prognosis is driven by well-known risk factors, including obesity, arterial hypertension, diabetes, elevated low-density lipoprotein cholesterol (LDL), smoking, and a sedentary lifestyle [2]. However, restrictive cardiomyopathies (RCM) have a genetic component and are still poorly understood. RCM are characterized by diastolic dysfunction of a non-dilated ventricle caused by abnormal tissue that replaces the normal heart muscle. The gut microbiome (GM) has been recognized as a significant modulator of systemic inflammation, metabolism, and immune responses [3] and the gut–heart axis has emerged as a key area of interest in understanding the complex relationship between GM and CVD.
A microbiome is a collection of symbiotic microorganisms. It is estimated that the human body contains between 1013 and 1014 microbial cells, with most of these microorganisms residing in the gastrointestinal tract [4]. GM performs various functions that support host health, such as fermenting indigestible dietary fibers, modulating immunity and the synthesis of vitamins, and maintaining the gut barrier [5]. Research has focused on the relationship between GM and CVD, along with its associated risk factors. Advancements in metagenomics and metabolomics have enabled the identification of specific GM metabolites that contribute to the development of various cardiovascular conditions [6], suggesting that the gut microbiome could be a risk factor for CVD.
The imbalance of GM populations (dysbiosis) alters the production of key microbial metabolites such as short-chain fatty acids (SCFAs) and trimethylamine N-oxide (TMAO) [7], which is associated with CVD risk [8,9,10]. Elevated TMAO levels have been linked to all-cause mortality in cardiac patients [8].
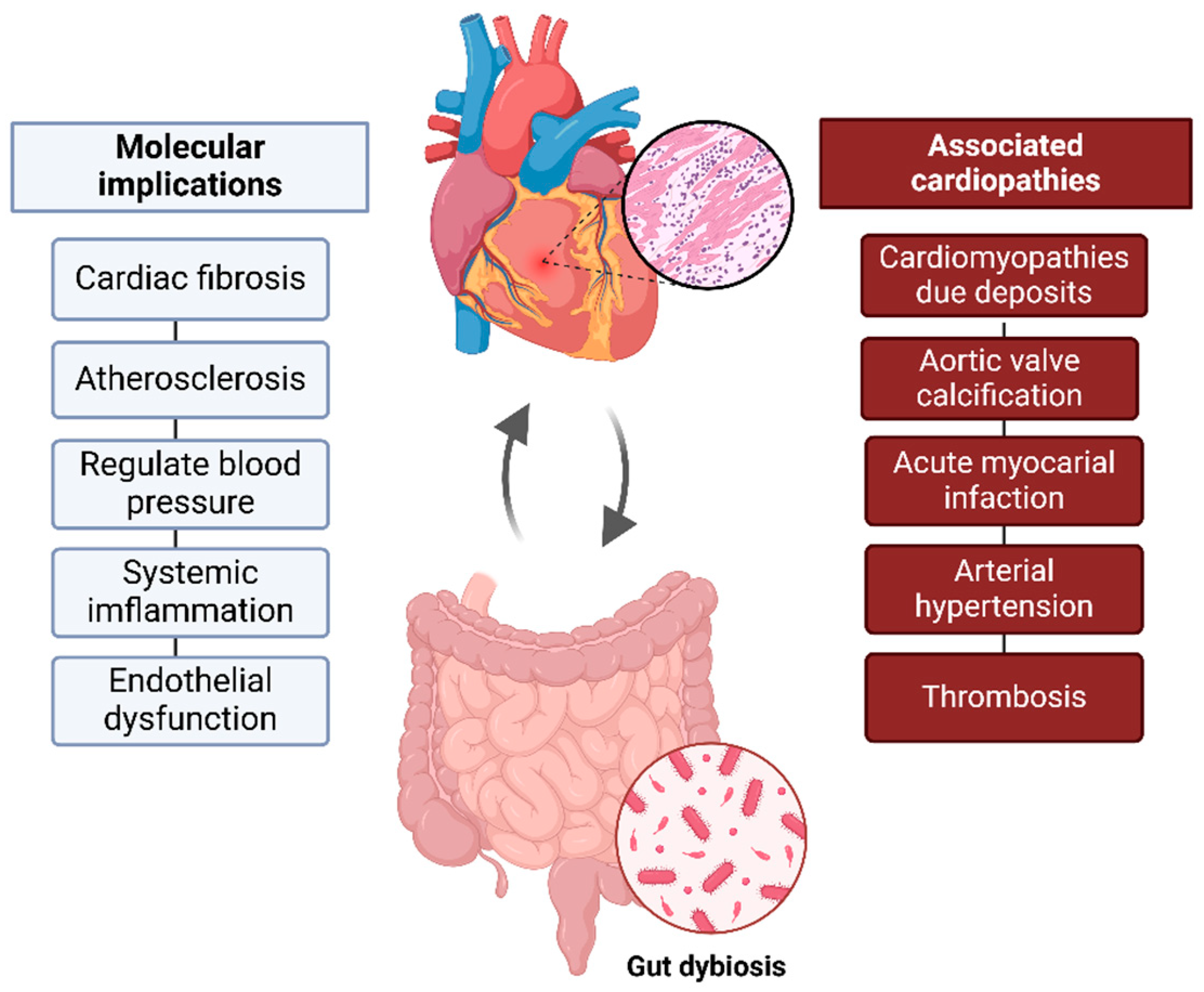
The GM SCFAs (produced by carbohydrate fermentation), such as acetate, propionate, and butyrate, support cellular homeostasis, acting as second messengers and providing anti-inflammatory effects [11]. This dynamic relationship highlights how gut health significantly impacts cardiovascular well-being, underscoring the crucial role of a healthy microbiome overall health (Figure 1).
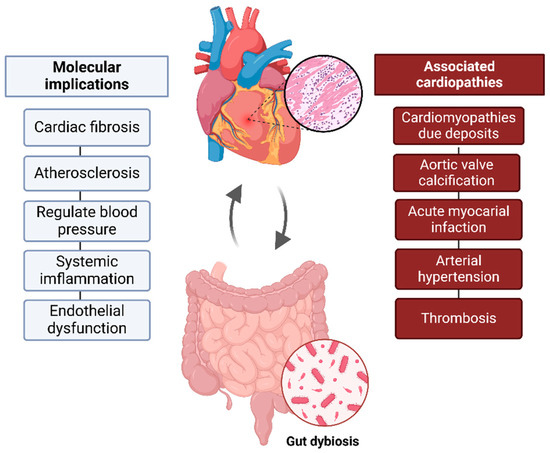
Figure 1.
Gut–heart axis. Bidirectional connection between gut microbiota and cardiovascular health. Gut dysbiosis, can contribute to inflammation, atherosclerosis, and cardiovascular changes, influencing the development of pathological conditions such as RCM and acute myocardial infarction, among others.
Additionally, it has been demonstrated that the lipopolysaccharides (LPS) trigger signal transduction via nuclear factor kappa B (NF-κB), activating the toll-like receptor 4 (TLR4) pathway [12], leading to chronic inflammation immune responses.
CVD and gastrointestinal diseases are prevalent health issues in the general population. They are interconnected through a shared pathogenic mechanism that underlines their relationship [13]. Several gastrointestinal diseases have been linked to CVD, including rhythm disorders such as atrial fibrillation (AF), HF, and coronary atherosclerosis.
Inflammatory bowel diseases (IBD), including ulcerative colitis and Crohn’s disease, may contribute to CAD development and progression. IBD has been associated with a risk of hospitalization due to HF [14], stroke [15,16], and CAD events [15,16]. This relationship is thought to be driven by endothelial dysfunction [17].
Additionally, Helicobacter pylori (H. pylori) has been associated with CVD for its pro-atherogenic mechanisms [18], increasing LDL levels [19], disrupting glucose and lipids metabolism [20], and promoting atherosclerosis [21].
Finally, gastroesophageal reflux disease (GERD) has been related with an increased AF risk [22] and it remains an independent risk factor for developing AF [23].
The comprehension of these correlations underscores the relevance of a multidisciplinary approach to address the overlapping pathophysiology of these conditions and enhance patient outcomes. Recent evidence suggests that GM dysbiosis may be a key factor in these associations, offering new perspectives for understanding the gut–heart axis.
2. Restrictive Cardiomyopathies
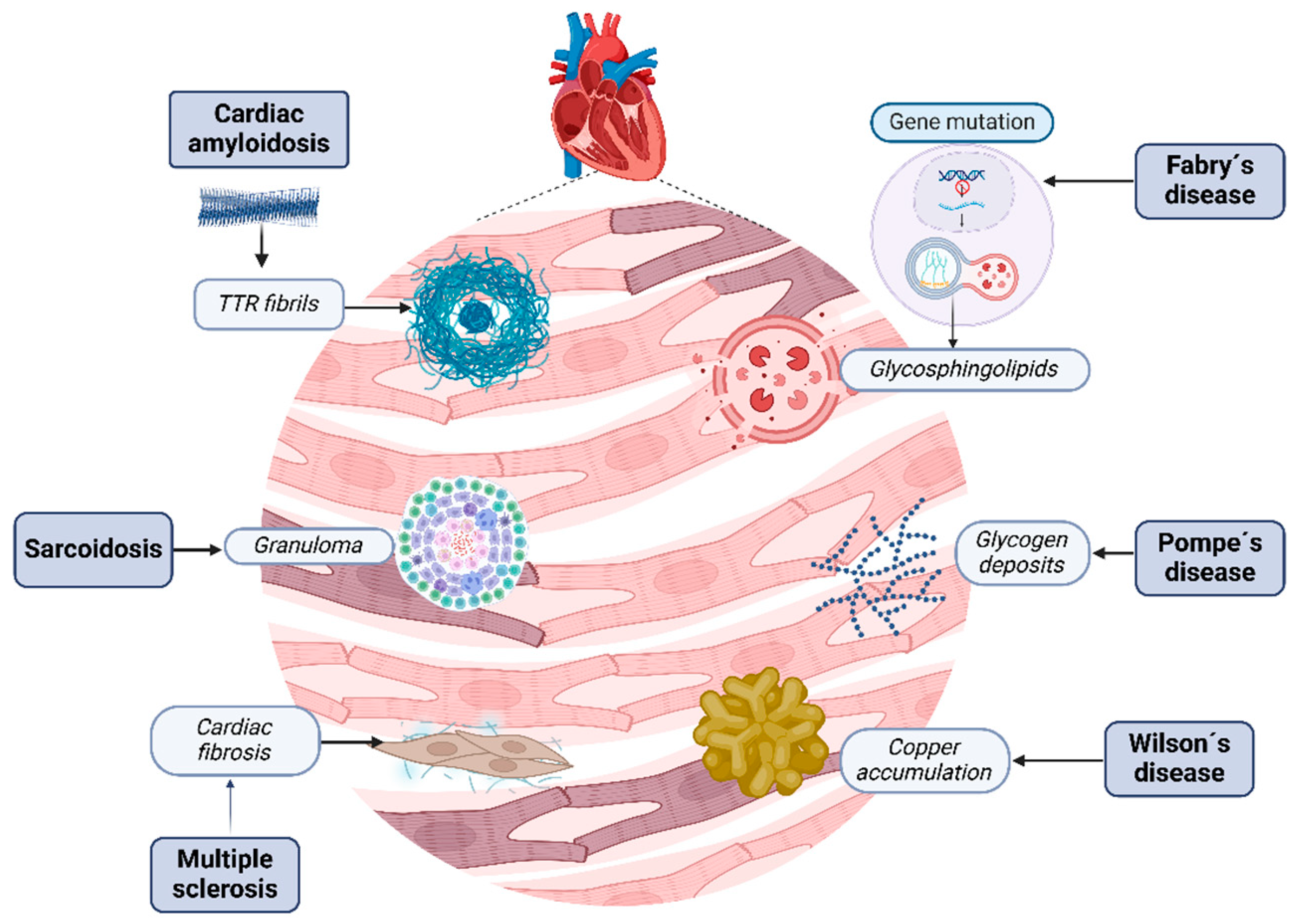
RCM are a heterogeneous group of cardiovascular disorders characterized by infiltrative diseases such as amyloidosis, storage disorders like hemochromatosis, and non-infiltrative diseases like systemic sclerosis and diabetic cardiomyopathy, involving the accumulation of abnormal substances within the myocardial tissue, leading to structural and functional disruption [24] (Figure 2).
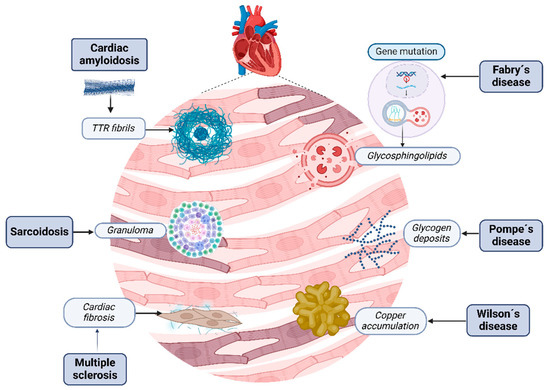
Figure 2.
Restrictive cardiomyopathies. Several kinds of molecules and changes can contribute to heart tissue damage; each disease has its own physiopathology that induces the development of these conglomerates. Cardiac amyloidosis disrupts myocardial tissue with misfolding amyloid fibrils. Fabry’s disease is characterized by the accumulation of globotriaosylceramide due to enzymatic deficiency. Sarcoidosis is characterized by the creation of granulomas, Wilson’s disease by excessive iron in cardiomyocytes due to dysregulated iron metabolism, and, finally, Pompe’s disease by excess glycogen within lysosomes due to enzymatic deficiency. Alternatively, sclerosis is characterized by injury, fibroblast activation, and excessive collagen deposition, resulting in tissue fibrosis.
In people under 30 years, RCM are primarily linked to genetic disorders causing increased fibrosis or abnormal deposition of substances like iron, proteins, or glycogen. In older adults, common causes include cardiac amyloidosis, iron overload, radiation-induced heart disease, and sarcoidosis [25]. These disorders impair the heart’s normal structure and function, which frequently results in arrhythmias like AF, thromboembolic problems, sudden cardiac death, and heart failure with preserved ejection fraction (HFpEF), which is the most prevalent manifestation [25,26].
RCM are typically diagnosed through a combination of clinical, laboratory tests, and imaging studies like echocardiography and cardiac magnetic resonance that are more valuable in identifying specific causes or narrowing down the differential diagnosis. Myocardial biopsy is a valuable but invasive diagnostic tool, typically reserved for cases with high clinical suspicion or inconclusive noninvasive test results [27].
Among RCM, cardiac amyloidosis is the most common, with a 5-year survival rate of 55% in adults, where HF accounts for 42% of fatalities [28], so timely identification is crucial for starting targeted treatments and enhancing patient prognosis. These cardiac disorders frequently provide significant insights through their clinical manifestations; in addition, diagnostic imaging and genetics studies have significantly improved the recognition and knowledge of these disorders (Table 1).

Table 1.
Restrictive cardiomyopathies, their etiology, infiltrative substance, principal clinical manifestations, and current treatment. Adapted and modified from [26].
Finally, understanding the interplay between the microbiota and myocardial health could open new avenues for therapeutic strategies in these diseases.
2.1. Is There a Relationship Between the Gut–Liver–Heart Axis and Restrictive Cardiomyopathies?
The association between the GM and liver is a complex interplay where microbiota significantly influences liver health and disease [23]. Liver diseases such as non-alcoholic fatty liver diseases (NAFLD), non-alcoholic steatohepatitis (NASH) [29,30], autoimmune liver diseases [31], and cirrhosis [32] are strongly associated with GM dysbiosis; in addition, a significant correlation with CVD has been demonstrated. NAFLD has been linked to an increased risk of CAD, HF, stroke, and arrhythmias [33].
The GM plays an essential role in modulating bile acid (BA) metabolism and SCFA production and its dysbiosis contributes to liver inflammation and fibrosis, which may exacerbate cardiovascular dysfunction [34,35]. Understanding gut–liver–heart axis provides a framework for exploring how the GM and liver metabolism influence cardiovascular health.
Finally, cirrhotic cardiomyopathy is a well-recognized cardiac dysfunction associated with liver cirrhosis. It is characterized by impaired cardiac contractility, diastolic dysfunction, and electrophysiological abnormalities, occurring independently of preexisting cardiac diseases [36,37]. NAFLD is associated with an increased risk of cardiomyopathy, it can lead to left ventricular diastolic dysfunction and hypertrophy, contributing to congestive HF [38,39]. The GM can modulate these metabolic pathways, potentially influencing the development of cardiomyopathy through systemic inflammation and metabolic dysregulation.
The liver plays a significant role in cardiovascular health. When it comes to RCM, their relationship to liver pathology remains poorly understood and understudied. Although some evidence suggests that liver diseases may contribute to myocardial fibrosis and diastolic dysfunction, specific data linking liver diseases to the RCM development/progression are scarce. This documented data missing emphasizes the need for further research to focus the liver–heart axis in RCM.
2.2. Amyloidosis
Amyloidosis is a systemic disease that covers a group of disorders characterized by the bowel movement of misfolded fibrillar proteins in the extracellular space [40,41]. Over 98% of cardiac amyloidosis are due to two main types: light chain cardiac amyloidosis (AL) and transthyretin (TTR) which at the same time is subdivided in two entities, hereditary and wild-type [42]. Hereditary-TTR amyloidosis (h-ATTR) affects younger ages, with an episode of cardiomyopathy, polyneuropathy, or mixed depending on the TTR gene mutation [41]. Meanwhile, wild-type TTR amyloidosis (wt-ATTR) most frequently affects elderly men, who begin with RCM.
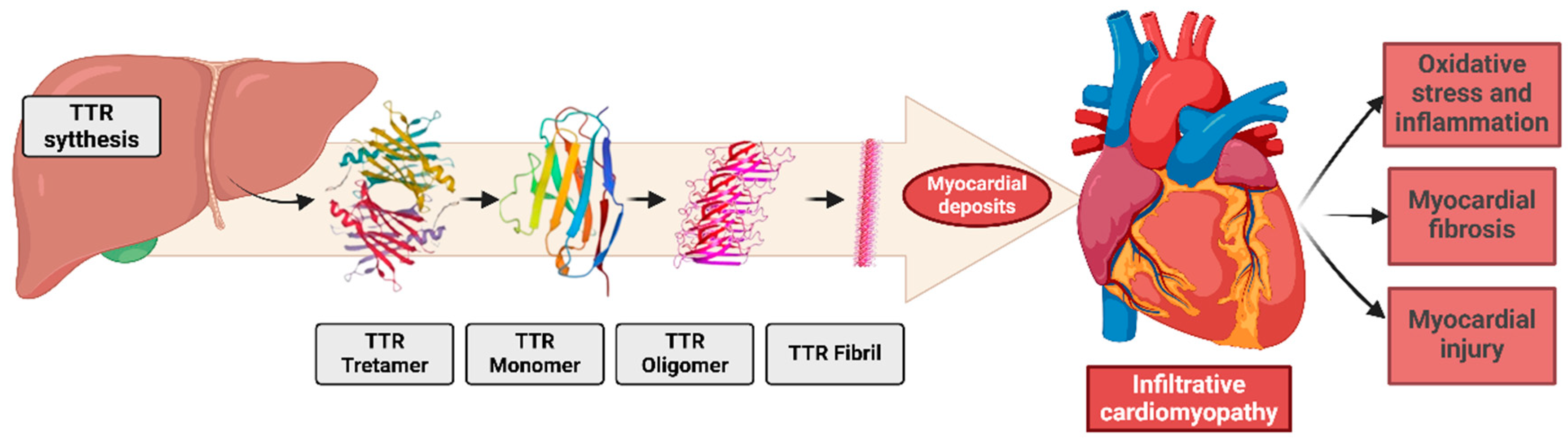
Pathophysiology involves the misfolding of the tetrameric protein transthyretin, synthesized and secreted by the liver. Transthyretin is dissociated from tetramers into dimers and monomers, forming amorphous aggregates that deposit extracellularly, especially in the myocardium [43]. These deposits disrupt cardiac physiology, contributing to HFpEF, left ventricular hypertrophy, arrhythmias [44], and systemic manifestations such as carpal tunnel syndrome and lumbar canal stenosis [40,42] (Figure 3).
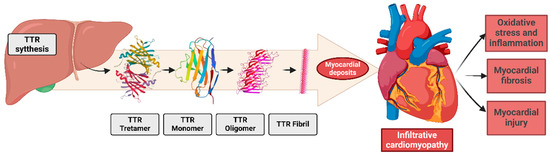
Figure 3.
The pathogenesis of transthyretin (TTR) amyloid fibril deposition involves the misfolding and aggregation of TTR monomers into insoluble amyloid fibrils, which accumulate in the extracellular matrix. Additionally, these deposits cause molecular changes in the heart and in all cardiac systems.
Gut Dysbiosis, Inflammation, and Protein Misfolding in Cardiac Amyloidosis
It has been reported that GM dysbiosis induces inflammation, which leads to degenerative diseases and different associated comorbidities such as amyloidosis [45]. In amyloidosis, bacterial components such as LPS and metabolites, formed by bacteria such as SCFAs, and TMAO have been associated with amyloid deposits and pro-inflammatory cytokines (e.g., interleukin-1β (IL-1β) and IL-6) [46] (Table 2). Peterle et al. conducted an experimental study observing that the serine protease protein secreted by bacteria of the phyla Firmicutes, specifically by the species Bacillus subtilis, participates in the production of amyloidogenic fragments together with TTR [46] (Table 2). Although the mechanism by which amyloidosis fibers are generated and accumulate is not entirely clear, an association between a poor prognosis of TTR amyloidosis and GM dysbiosis has been found [47]. In another study, significant differences were found in gut microbial communities between a healthy control group and AL patients that showed an abundance of Actinobacteria and Verrucomicrobiota and a decreased presence of Bacteroidota [48]. Bifidobacterium, a genus of Actinobacteria, has recently shown evidence of its ability to modulate host immunity by increasing immunoglobulins and inducing or reducing pro- or anti-inflammatory cytokines [48] (Table 2). Hu et al. observed in TTR amyloidosis that Bifidobacterium and Eubacterium were significantly decreased, while Parabacteroides was more abundant. The diminished probiotics were negatively associated with TTR cardiac amyloidosis through metabolites like GABA and taurine [49] (Table 2). Evidence proposes that GM dysbiosis may be a relevant factor in human AL progression. Additionally, in a group of mice treated with antibiotics, the authors observed that their use significantly reduces the deposits of β-amyloid protein, concluding that the changes generated in the microbiota decrease the β-amyloid protein accumulation, showing a positive change and disease improvement [25].

Table 2.
Gut–heart axis. Findings of cardiomyopathy and its relationship with gut microbiota.
Notably, doxycycline, a drug used in amyloidosis treatment, modifies the gut microbiota. This alteration may influence the natural disease history, suggesting that GM could play a role in disease progression. In detail, evidence suggests that GM could trigger inflammation, protein misfolding, and tissue deposition and could have potential therapeutic targets.
2.3. Fabry’s Disease
Fabry disease (FD) is a rare X-linked lysosomal storage disorder caused by mutations in the GLA gene, which encodes the enzyme α-galactosidase A (α-Gal A) [63]. This enzymatic deficiency leads to the accumulation of glycosphingolipids, primarily globotriaosylceramide (Gb3), and its deacylated form, globotriaosylsphingosine (lyso-Gb3), within lysosomes across various tissues and organs [48,49,50]. This accumulation results in a multisystemic disease characterized by progressive renal failure, cardiomyopathy, and cerebrovascular complications, significantly impacting life expectancy [64,65]. Clinically, FD presents with a range of different symptoms. Cardiac involvement is common and can lead to ventricular hypertrophy, fibrosis, HF, and arrhythmias [66,67,68].
Metabolic Alterations Associated with Gut Dysbiosis in Fabry’s Diseases
A murine FD model demonstrated that the disease is associated with early GM compositional and functional dysbiosis. This dysbiosis is linked to alterations in fecal SCFAs levels, which may contribute to symptoms such as diarrhea and visceral hypersensitivity, as well as impaired communication along the gut–brain axis [55]. Additionally, lyso-Gb3, a metabolite accumulated in FD, has been shown to modulate the gut microbiota by increasing the biofilm-forming capacity of certain bacteria, such as Bacteroides fragilis, and altering the SCFAs production, especially decreasing butyrate levels [56] (Table 2). These changes in GM composition and metabolic profile could potentially exacerbate gastrointestinal symptoms in FD patients.
Furthermore, clinical observations have suggested that dietary interventions, such as a low-fermentable oligosaccharides, disaccharides, monosaccharides and polyols diet (FODMAP), may alleviate gastrointestinal symptoms in FD by reducing dysbiosis and improving gut health [69,70] (Table 2). Murine models and clinical observations underscore the significant role of GM dysbiosis in FD. This highlights the potential role of microbiota-targeted therapies in FD managing related gastrointestinal manifestations and systemic inflammation. Evidence regarding Fabry’s disease associated with this cardiac presentation is rare, probably due to a low incidence. More research is required in this regard.
2.4. Wilson’s Disease (Hemochromatosis)
Wilson’s disease (WD) is an autosomal recessive genetic disorder characterized by impaired copper metabolism due to mutations in the ATP7B gene, which encodes a copper-transporting ATPase [71]. This defect leads to copper accumulation primarily in the liver and subsequently in other organs, including the brain and cornea. The ATP7B protein is crucial for incorporating copper into ceruloplasmin and promoting its excretion into bile. Mutations in this protein result in defective copper excretion, leading to copper accumulation in hepatocytes, this excess generates reactive oxygen species (ROS) [71,72,73]. Cardiac involvement in WD is a recognized but less commonly discussed WD manifestation, primarily characterized by copper accumulation leading to left ventricular remodeling, hypertrophy, diastolic dysfunction, and, less frequently, systolic dysfunction [74,75].
Iron Metabolism and Microbial Composition in Animal Models
A study analyzed fecal iron content and microbiota in mice with mutations in the iron regulatory protein 2 (Irp2) and the hereditary hemochromatosis gene (Hfe). Irp2-/-mice showed elevated iron and other minerals in their feces compared to wild-type and Hfe-/-mice [58]. Significant differences in the bacterial populations were observed, with certain lactic acid bacteria being more abundant in specific mouse strains. Iron depletion through repeated venesection is the primary treatment for hemochromatosis, an iron-overload disorder. During treatment, increased iron absorption occurs in the gastrointestinal tract to compensate for the loss. This study shows that decreased iron availability in the colon, after venesection, leads to changes in GM composition, a phenomenon not previously studied in hemochromatosis patients [57]. These findings suggest genetic mutations in iron metabolism and changes in GM composition, which may have clinical implications for human disease progression and this myocardiopathy presentation. In addition, research in this field would bring us closer to promoting gut microbiota as a determinant of disease progression.
2.5. Cardiac Systemic Sclerosis
Systemic sclerosis (SS) is a complex autoimmune disorder characterized by fibrosis of the skin and internal organs, vasculopathy, and immune dysregulation [76,77]. The SS pathophysiology involves a multifaceted interplay between genetic predisposition, environmental triggers, and immunity abnormalities. This leads to endothelial cell injury, fibroblast activation, and excessive collagen deposition, resulting in tissue fibrosis and organ dysfunction [78,79].
The SS process begins with vascular injury, usually due to autoimmune attacks on endothelial cells, which leads to structural vascular changes such as capillary loss and arteriolar stenosis [80,81,82]. Myocardial fibrosis is a hallmark of cardiac involvement in cardiac systemic sclerosis (SSc), often resulting from chronic inflammation and endothelial damage. This fibrosis can lead to a range of cardiac complications including arrhythmias, HF, and conduction abnormalities [59,83,84].
Dysbiosis in Cardiac Systemic Sclerosis: TMAO Participation
In a study investigating the gut microbiota, adults patients completed surveys on their gastrointestinal symptoms and diet. The results showed that more severe gastrointestinal symptoms were linked to decreased microbial diversity and changes in microbial composition, with increased abundance of pathobionts like Klebsiella and Enterococcus [85] (Table 2). This points to the relevance of further research, especially through controlled trials, to better understand how diet and microbiota influence disease progression and symptoms management.
Intestinal dysbiosis is common in systemic sclerosis, as documented by [86,87], but its role in microvascular injury and fibrosis remains unclear. GM produces trimethylamine (TMA), which is converted by flavin-containing monooxygenase (FMO3) into TMAO. One study shows that TMAO reprograms skin fibroblasts, vascular endothelial cells, and adipocytic progenitor cells into myofibroblasts via the TMAO receptor protein pERK. The findings suggest that the FMO3-TMAO-pERK axis connects GM to vascular remodeling and fibrosis in SSc, offering a potential therapeutic target [60]. Other studies evidenced that the TMAO concentration is increased in SS, especially in patients with advanced organ involvement [61] (Table 2). Similarly, a recent study documented elevated TMAO circulating levels, regardless of comorbidities such as age, sex, renal function, diabetes, and CVD [62]. These findings underscore the microbiota’s influence on cardiac systemic sclerosis; given that TMAO, a metabolite produce by gut microbiota, is a potentially modifiable factor, further research is needed to explore its relationship with SS severity and progression.
2.6. Sarcoidosis
Sarcoidosis is an inflammatory multi-systemic disease of unknown etiology that is characterized by the formation of granulomas in different organs; pulmonary complaints are the most common [88,89,90], except for the Japanese population, in whom cardiac presentation is the most common [91]. In addition, recent studies indicate that cardiac sarcoidosis (CS) is more prevalent than previously thought [51,91].
Microbiota in Sarcoidosis: Immune Dysregulation and Therapeutic Prospects
The cause of sarcoidosis has not been discovered; it is believed that it involves some genetic predispositions in conjunction with environmental triggers. This rare disease can resemble or coexist with other autoimmune disorders, with different immunosuppressive treatment, supporting an autoimmune key role [92]. In addition, evidence suggests that certain microorganisms, such as Cutibacterium acnes (C. Acnes) and Mycobacterium tuberculosis may be implicated in sarcoidosis development [91,93] (Table 2), proposing microbiota as a potential therapeutic and prognosis target. Granulomatous inflammation is believed to result from a dysregulated immune response to unidentified environmental antigens in genetically predisposed individuals. CS is relatively uncommon but can present with a variety of manifestations. The most frequent symptoms include arrhythmias, such as tachycardia or heart block. Less commonly, it may lead to pericardial effusion, where fluid accumulates around the heart, or the formation of myocardial granulomas, which can result in cardiac fibrosis and impaired heart function [52,53]. These different clinical manifestations highlight the complexity involved in sarcoidosis.
The main studies associated with sarcoidosis are focused on the respiratory tract, due to its high prevalence [94]. For example, one study that researched the associations between GM and chronic respiratory diseases found three genetically predicted taxes, such as Methanobacteria, order Methanobacteriales, class Methanobacteriaceae, that were significantly associated with sarcoidosis [95] (Table 2). Focusing on cardiovascular systems, a study examining formalin-fixed paraffin-embedded myocardial tissues from patients with CS myocarditis or other cardiomyopathies immunohistochemistry with C. Acnes specific monoclonal antibody was used to identify bacterial presence. C. acnes was observed in 63% of cardiac sarcoidosis samples with granulomas [96] (Table 2). These findings suggest that P. acnes may contribute to granuloma formation in CS patients. Alternatively, a prospective, multicenter, randomized, open-label, controlled clinical trial, named JACNES in Japan, focused on evaluating the clinical response of CS patients to antibiotic therapy. This study randomly assigned patients to receive either standard corticosteroid therapy combined with antibiotics effective against C. acnes, or corticosteroid therapy alone [54]. The study results are currently under investigation. These findings may improve the treatment of CS patients. The GM role in sarcoidosis appears to be significant, certain microorganisms could contributegranulomatous and deposits. Dysbiosis may exacerbate disease progression by participating in cardiac and respiratory presentations. The evidence suggests that the microbiota plays a role in the pathophysiology of cardiac sarcoidosis, and its modification through the proper use of antibiotics could reduce disease progression, improving drugs that regulate the microbiota, inflammation, and immunity
2.7. Pompe’s Disease
Pompe disease (PD) or glycogenosis type II is a rare, chronic, and muscle-weakening, often fatal neuromuscular disease, caused by a partial or total deficiency of acid alpha-glucosidase (GAA), a key enzyme for glycogen catabolism. Glycogen, an intracellular polymer of glucose residue bonds and branching points connected by α-1,6 bonds [97]. PD has very low prevalence; there is not enough evidence related to the microbiome associated with this disease.
Fecal Microbiota Transplantation in Pompe Disease: A Case Study with Gastrointestinal Infection and Dilated Cardiomyopathy
Currently, one clinical case of fecal microbiota transplantation (FMT) has been reported in an infant with PD with chronic gastrointestinal infection by Clostridium difficile (C. difficile) and dilated cardiomyopathy, with multiple hospitalizations and the administration of multiple doses and types of antibiotics without response to treatment, who responded favorably after FMT [98]. A 21-month-old Hispanic girl, born full-term via cesarean, was diagnosed with PD at 4 months of age. Her condition was complicated by dilated cardiomyopathy, ventilator dependence, and failure to thrive, requiring a gastrojejunostomy feeding tube. At 9 months, she developed recurrent foul-smelling diarrhea, fever, and abdominal discomfort while on antibiotics for presumed aspiration pneumonia. C. difficile infection was confirmed by PCR. Despite multiple treatments, she experienced recurrent C. difficile infection episodes with significant symptoms and hospitalizations. After 12 months of recurrent C. difficile infection and failed antibiotic therapies, FMT was proposed, with her mother screened as the donor. She did not experience any side effects [98]. Controlled trials found FMT to be highly effective and safe in adults, achieving up to 90% success and outperforming antibiotics [99,100]. This report highlights that FMT can be a safe and effective treatment option for the medically complex. This suggests that the microbiota may be a potential determinant for the adjuvant treatment of medical conditions in which there is little response to such treatments.
3. Dysbiosis: Prevention Strategies in Heart Failure
Recent evidence suggests that HF is linked to disrupted intestinal epithelial function, likely due to reduced blood flow and ischemia [101,102]. This leads to increased bowel wall thickness and gut permeability [103], resulting in the translocation of bacteria and their wall products into the circulation with inflammatory activation and modulating metabolites that can have both beneficial and harmful effects on the CVD development [103]. Harmful metabolites such as TMAO contribute to atherosclerosis, thrombosis, and are linked to cardiovascular event; on the other hand, beneficial metabolites like SCFAs can help improve blood pressure and support myocardial repair, being closely linked to dietary fiber intake.
Gut dysbiosis has been suggested as a pathogenic factor in various diseases, including HF development, supporting the “gut hypothesis” for the condition [104]. Luedde et al. observed in patients with stable HF an overgrowth of pathogenic bacteria such as Campylobacter, Shigella, Salmonella, Yersinia, and species of Candida [105]. In addition, there has been a reported depletion of bacteria that are known to produce SCFAs in HF patients in comparison to control subjects; in addition, HF patients were observed to have a lower intake of dietary fiber [106]. Dysbiosis is associated with HF in the reduction in bacteria that produce metabolites for cardiovascular homeostasis.
Gut Microbiota as a Target in Heart Failure
Dietary changes, probiotics, prebiotics, and FMT [107,108] are therapeutic approaches currently used to enhance gut bacterial health; specific interventions to improve gut dysbiosis and improve cardiovascular health may still be some time away [109]. Probiotics have shown limited benefit in reducing myocardial hypertrophy in animal studies [110]; they have been linked to improved left ventricular ejection fraction [111]. The gut–heart study, currently underway, is investigating the effects of rifaximin, the probiotics yeast Saccharomyces boulardii (S. boulardii), and a no-treatment control in HF [112]. Additionally, a recent study investigated the impact of lactic-fermented bee pollen probiotics on GM [113]. The results showed an increased abundance of beneficial bacteria, Lactobacillus spp., and Bifidobacterium spp. [113], providing valuable insights into potential roles in HF management. FMT has shown success in treating gut dysbiosis but has variability in patient response [114]. Additionally, it carries a potential risk of transmitting harmful infections to the new host [115].
Indole-3-propionic acid (IPA) is a metabolite produced by GM from dietary tryptophan. IPA plays a key role in preserving mucosal homeostasis and supporting gut barrier function by binding to its receptors [116]. IPA has been reported to be reduced in patients and a mouse model [117]. IPA supplementation attenuates diastolic dysfunction, metabolic remodeling, oxidative stress, inflammation, GM dysbiosis, and gut barrier damage in a mouse HF model [117]. IPA supplementation could offer a therapeutic and prevention strategy for HF.
It has been proposed that polyphenols have beneficial effects on GM. Polyphenols and their derivatives present an opportunity to prevent and treat CVD by promoting gut eubiosis [118]. A recent study by the Optimal Nutraceutical Supplementation in Heart Failure (ONUS-HF) group confirmed the potential benefits of combining natural products, including apple-derived phlorizin, Vitis vinifera extracts, bergamot polyphenols, and Olea europaea derivatives, in patients at an early HF stage [119].
These findings highlight the relevance of gut health in CVD and suggest that targeting GM may become an important strategy for treating HF in the future. However, research on preventive measures and targeted interventions on GM in the context of RCM remains limited. Expanding research in this area may provide valuable insights into novel therapeutic approaches that can address unmet needs in managing complex cardiac diseases.
4. Drugs’ Impact on Gut Microbiota and Cardiovascular Health
GM influences drugs’ metabolism through well-established pharmacokinetic pathways, including microbial enzymes that convert drug molecules. GM interferes with pharmacokinetics and pharmacodynamics [120,121], but at the same time medication can alter GM [122,123]. Several human studies have reported an association between specific drug use and changes in microbial composition and function [122,124,125,126].
Many commonly used cardiovascular drugs showed strong interaction with GM, including, aspirin, digoxin, sodium-glucose cotransporter-2 (SGLT2), calcium channel blockers, betablockers, renin-angiotensin system inhibitors, statins, warfarin, clopidogrel, heparin, amiodarone, and antiplatelets.
Aspirin metabolism is influenced by GM [127], but its use significantly alters GM composition with Bacteroides and Ruminococcaceae [128]. GM significantly affects the digoxin bioavailability [129]. Early research showed that gastrointestinal Eubacterium lenta (E. lentum) produces an inactive metabolite of digoxin [130] and intestinal digoxin inactivation is one of the clearest associations between cardiovascular medicine and GM. Regarding the angiotensin-converting-enzyme inhibitors, a study with enalapril did not find an alteration of GM species, but reduced plasma levels of TMAO were documented [131]. These data suggest a potential enalapril role in modulating GM production of this harmful metabolite. Calcium channel blockers have demonstrated significant interactions with GM.
Amlodipine is partially metabolized by gut microbiota. In some studies, a 9% reduction in unchanged amlodipine has been observed during 24 h incubations with human faecalis, suggesting microbial involvement [132]. Beyond its pharmacological role, amlodipine exhibits antimicrobial properties, effectively inhibiting bacterial species such as Staphylococcus aureus, Vibrio cholerae, Shigella, and Salmonella [133].
Dapagliflozin is an SGLT2, used for glycemic control and blood pressure control [134] and has demonstrated its protective role in HF patients [135]. A recent animal study on HF documented that dapagliflozin treatment reduced inflammation, infarction area, and cardiac fibrosis in mice. Dapagliflozin decreased the ratio of Firmicutes/Bacteroidetes, which was increased in HF mice [136]. These findings suggest that dapagliflozin may modulate GM, contributing to HF treatment.
It has been reported that oral administration of vancomycin significantly impacts host GM diversity [137]. Some studies suggest that this GM population reduction in mice provided cardioprotective benefits, including smaller myocardial infarction size and lower circulating leptin levels in an ischemia/reperfusion mice model [138,139]. However, it has been reported that using an enteral-non-absorbable polymyxin B/tobramycin regimen induced decreased fecal endotoxin concentration [140]. Although antibiotics may provide some cardiovascular protection, their effects appear to be only for the treatment duration. Antibiotics often reduce the overall GM, removing harmful and beneficial bacteria.
These findings highlight the dynamic association between cardiovascular drugs, antibiotics, and GM and suggest that the modulation of the GM may become an integral part of cardiovascular treatment.
5. Conclusions
Although the actual evidence reported about GM and these diseases is scarce, the evidence so far on the role of the GM in RCM have highlighted the potential impact of gut bacteria on disease progression. Understanding how dysbiosis and microbial metabolites contribute to cardiac fibrosis and HF opens up new opportunities for targeted therapies and a deeper pathophysiological knowledge of these diseases. This review approaches the emerging role of the GM in RCM pathogenesis. Amyloidosis, FD, and CS demonstrate distinct microbial imbalances: increased Parabacteroides and reduced Bifidobacterium and Eubacterium in amyloidosis alongside Bacillus subtilis, producing amyloidogenic fragments as well as altered SCFAs production linked to Bacteroides fragilis in FD and the participation of C. acnes and Mycobacterium tuberculosis in CS, which may contribute to granuloma formation. In conclusion, our review contributes to filling this gap by synthesizing current knowledge about RCM and GM; advancing our understanding in this area may result in the non-invasive identification of biomarkers, enabling earlier detection and GM-targeted therapies that could complement current treatments and improve their efficacy. Delving into this field represents a unique opportunity to advance personalized medicine in cardiology.
To search for relevant information and scientific impact, a detailed search was carried out on recent reports on restrictive cardiomyopathy and its relationship with GM alterations.
We were able to corroborate that there are few clinical and scientific findings associated with intestinal dysbiosis that can indicate a relationship between the gut–heart axis; however, the obtained results suggest a close relationship between the presence of disease symptoms with GM dysbiosis and its implications in immune response.
Therefore, they lead us to discuss and investigate the field of RCM and the potential relationship it has with GM.
There is little evidence because there appears to be a low prevalence in these associated pathologies; however, this review expands the field of research between the gut–heart axis, knowing that intestinal dysbiosis has a relevant impact, mainly due to the disruption of permeability giving way to toxins. These toxins are mobilized through the bloodstream, targeting vital organs (e.g., heart) marking an important inflammation sign and activating various mechanisms that directly impact disease prognosis.
6. Perspectives
Research on the gut–heart axis offers promising opportunities to understand and treat cardiovascular diseases. Future studies should focus on identifying the fine mechanisms driving changes that influence inflammation and fibrosis in cardiovascular conditions. Metagenomics and machine learning can provide essential insights into personalized microbiota-based therapies. With precision microbiota approaches, we may uncover new therapeutic targets and preventive strategies for cardiovascular care.
Author Contributions
S.J.-A., I.I.L.-T., J.B.-D.l.S., and D.C.B.-V. contributed to the writing of the manuscript; E.A.B.-B., A.A., F.J.R.G. and M.M.A.-G. contributed to the reviewing and editing of the manuscript and figure design. All authors discussed and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This review was funded by the UNAM-DGAPA-PAPIIT, grant number IN212422 and CONAHCYT CBF2023-2024-734, which were awarded to M.M.A.-G.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Jaimez-Alvarado. S. was financially supported by the Nacional Program of Social Service in Health of 2023–2024 of the CIFRHS. López-Tenorio I.I was financially supported by the postdoctoral program of 2022–2025 of the CONAHCYT. Barragán-De los Santos J. and Bello-Vega D.C. thank the AFINES program at Facultad de Medicina UNAM.
Conflicts of Interest
The authors declare no conflicts of interest.
List of Abbreviations
| AF | Atrial fibrillation |
| AL | Light chain cardiac amyloidosis |
| α-Gal A | α-galactosidase A |
| BA | Bile acid |
| CAD | Coronary artery diseases |
| CS | Cardiac sclerosis |
| CVD | Cardiovascular diseases |
| FD | Fabry disease |
| FMO3 | Flavin-containing monooxygenase |
| FMT | Microbiota transplantation |
| GAA | Alpha-glucosidase |
| GERD | Gastroesophageal reflux disease |
| Gb3 | Globotriaosylceramide |
| GM | Gut microbiome |
| h-ATTR | Hereditary TTR amyloidosis |
| Hfe | Hereditary hemochromatosis gene |
| HF | Heart failure |
| HFpEF | Heart failure preserved ejection fraction |
| IBD | Inflammatory bowel diseases |
| IL-1 | Interleukin 1 |
| IL-1B | Interleukin 1B |
| IL-6 | Interleukin 6 |
| IPA | Indole-3-propionic acid |
| Irp2 | Iron regulatory protein 2 |
| LDL | Low-density lipoprotein |
| LPS | Lipopolysaccharides |
| Lyso-Gb3 | Globotriaosylsphingosine |
| NAFLD | Non-alcoholic fatty liver diseases |
| NASH | Non-alcoholic steatohepatitis |
| NF-κB | Nuclear factor kappa B |
| PD | Pompe’s disease |
| ONUS-HF | Optimal Nutraceutical Supplementation in Heart Failure |
| RCM | Restrictive cardiomyopathies |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SGLT2 | Sodium-glucose cotransporter-2 |
| SS | Systemic sclerosis |
| SSc | Cardiac systemic sclerosis |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| TLR4 | Toll-like receptor 4 |
| TTR | Transthyretin |
| WD | Wilson’s disease |
| wt-ATTR | Wild-type TTR amyloidosis |
References
- Mc Namara, K.; Alzubaidi, H.; Jackson, J.K. Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integr. Pharm. Res. Pract. 2019, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.M.; Odell, P.M.; Wilson, P.W.; Kannel, W.B. Cardiovascular disease risk profiles. Am. Heart J. 1991, 121, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human gut microbiome: Function matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Bui, T.V.A.; Hwangbo, H.; Lai, Y.; Hong, S.B.; Choi, Y.-J.; Park, H.-J.; Ban, K. The Gut-Heart Axis: Updated Review for The Roles of Microbiome in Cardiovascular Health. Korean Circ. J. 2023, 53, 499–518. [Google Scholar] [CrossRef]
- Poll, B.G.; Cheema, M.U.; Pluznick, J.L. Gut microbial metabolites and blood pressure regulation: Focus on SCFAs and TMAO. Physiology 2020, 35, 275–284. [Google Scholar] [CrossRef]
- Kallio, K.E.; Hätönen, K.A.; Lehto, M.; Salomaa, V.; Männistö, S.; Pussinen, P.J. Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetol. 2015, 52, 395–404. [Google Scholar] [CrossRef]
- Tang, W.W.; Hazen, S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014, 124, 4204–4211. [Google Scholar] [CrossRef]
- Hernández-Ruiz, P.; Montaño, A.R.E.; Amezcua-Guerra, L.M.; González-Pacheco, H.; Niccolai, E.; Amedei, A.; Aguirre-García, M.M. Potential Association of the Oral Microbiome with Trimethylamine N-Oxide Quantification in Mexican Patients with Myocardial Infarction. Mediat. Inflamm. 2024, 2024, 3985731. [Google Scholar] [CrossRef]
- Niccolai, E.; Baldi, S.; Ricci, F.; Russo, E.; Nannini, G.; Menicatti, M.; Poli, G.; Taddei, A.; Bartolucci, G.; Calabrò, A.S.; et al. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J. Gastroenterol. 2019, 25, 5543–5558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, X.; Bao, X.; Xiao, W.; Chen, G. Toll-like Receptor 4 (TLR4) Inhibitors: Current Research and Prospective. Eur. J. Med. Chem. 2022, 235, 114291. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Ahlehoff, O.; Lindhardsen, J.; Erichsen, R.; Lamberts, M.; Khalid, U.; Nielsen, O.H.; Torp-Pedersen, C.; Gislason, G.H.; Hansen, P.R. Inflammatory bowel disease is associated with an increased risk of hospitalization for heart failure: A Danish Na-tionwide Cohort study. Circ. Heart Fail. 2014, 7, 717–722. [Google Scholar] [CrossRef]
- Yarur, A.J.; Deshpande, A.R.; Pechman, D.M.; Tamariz, L.; Abreu, M.T.; A Sussman, D. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am. J. Gastroenterol. 2011, 106, 741–747. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Ahlehoff, O.; Lindhardsen, J.; Erichsen, R.; Jensen, G.V.; Torp-Pedersen, C.; Nielsen, O.H.; Gislason, G.H.; Hansen, P.R. Disease activity in inflammatory bowel disease is associated with increased risk of myocardial infarction, stroke and cardio-vascular death—A Danish nationwide cohort study. PLoS ONE 2013, 8, e56944. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Principi, M.; Ierardi, E.; Di Leo, A.; Ricci, G.; Carbonara, S.; Gesualdo, M.; Devito, F.; Zito, A.; Cortese, F.; et al. Inflammatory bowel disease, liver diseases and endothelial function: Is there a linkage? J. Cardiovasc. Med. 2015, 16, 11–21. [Google Scholar] [CrossRef]
- Pieniazek, P.; Karczewska, E.; Duda, A.; Tracz, W.; Pasowicz, M.; Konturek, S.J. Association of Helicobacter pylori infection with coronary heart disease. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 1999, 50, 743–751. [Google Scholar]
- Kim, H.-L.; Jeon, H.H.; Park, I.Y.; Choi, J.M.; Kang, J.S.; Min, K.-W. Helicobacter pylori infection is associated with elevated low density lipoprotein cholesterol levels in elderly Koreans. J. Korean Med. Sci. 2011, 26, 654–658. [Google Scholar] [CrossRef]
- Gen, R.; Demir, M.; Ataseven, H. Effect of helicobacter pylori eradication on insulin resistance, serum lipids and low-grade inflammation. Singap. Med. J. 2010, 103, 190–196. [Google Scholar] [CrossRef]
- Tamura, A.; Fujioka, T.; Nasu, M. Relation of Helicobacter pylori infection to plasma vitamin B12, folic acid, and homocysteine levels in patients who underwent diagnostic coronary arteriography. Am. J. Gastroenterol. 2002, 97, 861–866. [Google Scholar] [CrossRef]
- Kunz, J.S.; Hemann, B.; Edwin Atwood, J.; Jackson, J.; Wu, T.; Hamm, C. Is there a link between gastroesophageal reflux disease and atrial fibrillation? Clin. Cardiol. 2009, 32, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Chan, W.-L.; Luo, J.-C.; Chen, Y.-C.; Chen, T.-J.; Chung, C.-M.; Huang, P.-H.; Lin, S.-J.; Chen, J.-W.; Leu, H.-B. Gastroesophageal reflux disease and atrial fibrillation: A nationwide population-based study. PLoS ONE 2012, 7, e47575. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.; Dumitraşcu, D.L. Cardiovascular risk in fatty liver disease: The liver-heart axis—Literature review. Front. Med. 2019, 6, 202. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Hear. J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Pereira, N.L.; Grogan, M.; Dec, G.W. Spectrum of restrictive and infiltrative cardiomyopathies: Part 1 of a 2-part series. J. Am. Coll. Cardiol. 2018, 71, 1130–1148. [Google Scholar] [CrossRef]
- Muchtar, E.; Blauwet, L.A.; Gertz, M.A. Restrictive cardiomyopathy: Genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 2017, 121, 819–837. [Google Scholar] [CrossRef]
- Madan, N.; Kalra, D. Clinical evaluation of infiltrative cardiomyopathies resulting in heart failure with preserved ejection fraction. Rev. Cardiovasc. Med. 2020, 21, 181–190. [Google Scholar] [CrossRef]
- Kubo, T.; Gimeno, J.R.; Bahl, A.; Steffensen, U.; Steffensen, M.; Osman, E.; Thaman, R.; Mogensen, J.; Elliott, P.M.; Doi, Y.; et al. Prevalence, clinical significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J. Am. College Cardiol. 2007, 49, 2419–2426. [Google Scholar] [CrossRef]
- Hsu, C.L.; Schnabl, B. The Gut-Liver Axis and Gut Microbiota in Health and Liver Disease. Nat. Rev. Microbiol. 2023, 21, 719–733. [Google Scholar] [CrossRef]
- Kirundi, J.; Moghadamrad, S.; Urbaniak, C. Microbiome-Liver Crosstalk: A Multihit Therapeutic Target for Liver Disease. World J. Gastroenterol. 2023, 29, 1651–1668. [Google Scholar] [CrossRef]
- Xu, M.; Luo, K.; Li, J.; Li, Y.; Zhang, Y.; Yuan, Z.; Xu, Q.; Wu, X. Role of Intestinal Microbes in Chronic Liver Diseases. Int. J. Mol. Sci. 2022, 23, 12661. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.M.; Kummen, M.; Trivedi, P.J.; Hov, J.R. Role of Microbiome in Autoimmune Liver Diseases. Hepatology 2024, 80, 965–987. [Google Scholar] [CrossRef] [PubMed]
- Przybyszewski, E.M.; Targher, G.; Roden, M.; Corey, K.E. Nonalcoholic fatty liver disease and cardiovascular disease. Clin. Liver. Dis. 2021, 17, 19–22. [Google Scholar] [CrossRef]
- Gananandan, K.; Wiese, S.; Møller, S.; Mookerjee, R.P. Cardiac Dysfunction in Patients with Cirrhosis and Acute Decompensation. Liver Int. Off. J. Int. Assoc. Study Liver 2024, 44, 1832–1841. [Google Scholar] [CrossRef]
- Wiese, S.; Voiosu, A.; Hove, J.D.; Danielsen, K.V.; Voiosu, T.; Grønbæk, H.; Møller, H.J.; Genovese, F.; Reese-Petersen, A.L.; Mookerjee, R.P.; et al. Fibrogenesis and Inflammation Contribute to the Pathogenesis of Cirrhotic Cardiomyopathy. Aliment. Pharmacol. Ther. 2020, 52, 340–350. [Google Scholar] [CrossRef]
- Desai, M.S. Mechanistic insights into the pathophysiology of cirrhotic cardiomyopathy. Anal Biochem. 2022, 636, 114388. [Google Scholar] [CrossRef]
- Isaak, A.; Praktiknjo, M.; Jansen, C.; Faron, A.; Sprinkart, A.M.; Pieper, C.C.; Chang, J.; Fimmers, R.; Meyer, C.; Dabir, D.; et al. Myocardial Fibrosis and Inflammation in Liver Cirrhosis: MRI Study of the Liver-Heart Axis. Radiology 2020, 297, 51–61. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Mantovani, A.; Tilg, H.; Targher, G. Risk of Cardiomyopathy and Cardiac Arrhythmias in Patients with Nonalcoholic Fatty Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 425–439. [Google Scholar] [CrossRef]
- Njoku, D.B.; Schilling, J.D.; Finck, B.N. Mechanisms of Nonalcoholic Steatohepatitis-Associated Cardiomyopathy: Key Roles for Liver–Heart Crosstalk. Curr. Opin. Infect. Dis. 2022, 33, 295–299. [Google Scholar] [CrossRef]
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Hear. J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef]
- Rubin, J.; Maurer, M.S. Cardiac amyloidosis: Overlooked, underappreciated, and treatable. Annu. Rev. Med. 2020, 71, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, A.B.; Kataria, R.; Zannad, F.; Sauer, A.J.; Damman, K.; Sharma, K.; Shah, S.J.; Van Spall, H.G.C. Heart failure with preserved ejection fraction: Recent concepts in diagnosis, mechanisms and management. Heart 2022, 108, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, A.M.C.; Janssen, A.; Boorsma, P.C.; Mannens, M.M.A.M.; Wilde, A.A.M.; Christiaans, I. Transthyretin amyloidosis: A phenocopy of hypertrophic cardiomyopathy. Amyloid 2017, 24, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Law, S.; Petrie, A.; Chacko, L.; Cohen, O.C.; Ravichandran, S.; Gilbertson, J.A.; Rowczenio, D.; Wechalekar, A.; Martinez-Naharro, A.; Lachmann, H.J.; et al. Disease progression in cardiac transthyretin amyloidosis is indicated by serial calculation of National Amyloidosis Centre transthyretin amyloidosis stage. ESC Hear. Fail. 2020, 7, 3942–3949. [Google Scholar] [CrossRef] [PubMed]
- Marizzoni, M.; Cattaneo, A.; Mirabelli, P.; Festari, C.; Lopizzo, N.; Nicolosi, V.; Mombelli, E.; Mazzelli, M.; Luongo, D.; Naviglio, D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Medi-ators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 78, 683–697. [Google Scholar] [CrossRef]
- Peterle, D.; Pontarollo, G.; Spada, S.; Brun, P.; Palazzi, L.; Sokolov, A.V.; Spolaore, B.; de Laureto, P.P.; Vasilyev, V.B.; Castagliuolo, I.; et al. A serine protease secreted from Bacillus subtilis cleaves human plasma transthyretin to generate an amyloidogenic fragment. Commun. Biol. 2020, 3, 764. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Li, D.Y.; Hazen, S.L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 2019, 16, 137–154. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, J.; Ning, X.; Qin, Y.; Xing, Y.; Wang, Y.; Jia, Q.; Huang, B.; Ma, R.; Lei, C.; et al. Alterations of the gut microbiota in patients with immunoglobulin light chain amyloidosis. Front. Immunol. 2022, 13, 973760. [Google Scholar] [CrossRef]
- Hu, X.; Li, H.; Wang, Z.; He, S.; Zhao, X.; Wu, Q.; Sun, Y.; Fan, Y.; Tian, Z.; Zhang, S. Gut Dysbiosis and Metabolism Alteration in Variant Transthyretin Amyloidosis. SSRN 2023, 441650. [Google Scholar] [CrossRef]
- Teng, C.; Li, P.; Bae, J.Y.; Pan, S.; Dixon, R.A.F.; Liu, Q. Diagnosis and treatment of transthyretin-related amyloidosis cardiomyopathy. Clin. Cardiol. 2020, 43, 1223–1231. [Google Scholar] [CrossRef]
- Kandolin, R.; Lehtonen, J.; Kupari, M. Cardiac sarcoidosis. J. Intern. Med. 2016, 280, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Fussner, L.A.; Karlstedt, E.; Hodge, D.O.; Fine, N.M.; Kalra, S.; Carmona, E.M.; Utz, J.P.; Isaac, D.L.; Cooper, L.T. Management and outcomes of cardiac sarcoidosis: A 20-year experience in two tertiary care centres. Eur. J. Hear. Fail. 2018, 20, 1713–1720. [Google Scholar] [CrossRef]
- Gentile, P.; Bollano, E.; Vergaro, G.; Bobbio, E. Editorial: Myocarditis and inflammatory cardiomyopathies: Diagnosis, treatment and future directions. Front. Cardiovasc. Med. 2023, 10, 1321494. [Google Scholar] [CrossRef]
- Ishibashi, K.; Eishi, Y.; Tahara, N.; Asakura, M.; Sakamoto, N.; Nakamura, K.; Ta-kaya, Y.; Nakamura, T.; Yazaki, Y.; Yamaguchi, T.; et al. Japanese Antibacterial Drug Management for Cardiac Sarcoidosis (J-ACNES): A Multicenter, Open-label, Randomized Controlled Study. J. Arrhythmia 2018, 34, 520–526. [Google Scholar] [CrossRef]
- Delprete, C.; Rimondini Giorgini, R.; Lucarini, E.; Bastiaanssen, T.F.S.; Scicchitano, D.; Interino, N.; Formaggio, F.; Uhlig, F.; Ghelardini, C.; Caprini, M. Disruption of the Microbiota-Gut-Brain Axis Is a Defining Characteristic of the A-Gal a (-/0) Mouse Model of Fabry Disease. Gut Microbes 2023, 15, 2256045. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.-J.; Madrazo-Clemente, P.; Martínez-Cuesta, M.d.C.; Peláez, C.; Ortiz, A.; Sánchez-Niño, M.D.; Esteban, J.; Requena, T. Lyso-Gb3 Modulates the Gut Microbiota and Decreases Butyrate Production. Sci. Rep. 2019, 9, 12010. [Google Scholar] [CrossRef]
- Teschke, R. Hemochromatosis: Ferroptosis, ROS, Gut Microbiome, and Clinical Challenges with Alcohol as Con-founding Variable. Int. J. Mol. Sci. 2024, 25, 2668. [Google Scholar] [CrossRef]
- Buhnik-Rosenblau, K.; Moshe-Belizowski, S.; Danin-Poleg, Y.; Meyron-Holtz, E.G. Genetic Modifi-cation of Iron Metabolism in Mice Affects the Gut Microbiota. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2012, 25, 883–892. [Google Scholar]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef]
- Kim, S.-J.; Bale, S.; Verma, P.; Wan, Q.; Ma, F.; Gudjonsson, J.E.; Hazen, S.L.; Harms, P.W.; Tsou, P.-S.; Khanna, D.; et al. Gut microbe-derived metabolite trimethylamine N-oxide activates PERK to drive fibrogenic mesenchymal differentiation. iScience 2022, 25, 104669. [Google Scholar] [CrossRef]
- Stec, A.; Maciejewska, M.; Paralusz-Stec, K.; Michalska, M.; Giebułtowicz, J.; Rudnicka, L.; Sikora, M. The Gut Microbial Metabolite Trimethylamine N-Oxide is Linked to Specific Complications of Systemic Sclerosis. J. Inflamm. Res. 2023, 16, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.J.; Muhammad, L.N.; Khanh, L.N.; Li, X.S.; Carns, M.; Aren, K.; Kim, S.-J.; Verma, P.; Hazen, S.L.; Varga, J. Elevated Circulating Levels of Gut Microbe-Derived Trimethylamine N-Oxide Are Associated with Systemic Sclerosis. J. Clin. Med. 2024, 13, 5984. [Google Scholar] [CrossRef] [PubMed]
- Amodio, F.; Caiazza, M.; Monda, E.; Rubino, M.; Capodicasa, L.; Chiosi, F.; Simonelli, V.; Dongiglio, F.; Fimiani, F.; Pepe, N.; et al. An Overview of Molecular Mechanisms in Fabry Disease. Biomolecules 2022, 12, 1460. [Google Scholar] [CrossRef]
- Lenders, M.; Brand, E. Fabry Disease: The Current Treatment Landscape. Drugs 2021, 81, 635–645. [Google Scholar] [CrossRef]
- Lenders, M.; Brand, E. Precision Medicine in Fabry Disease. Nephrol. Dialysis Tran. Off. Publ. Eur. Dial. Trans. Assoc.-Eur. Renal Assoc. 2021, 36 (Suppl. S2), 14–23. [Google Scholar] [CrossRef]
- Azevedo, O.; Cordeiro, F.; Gago, M.F.; Miltenberger-Miltenyi, G.; Ferreira, C.; Sousa, N.; Cunha, D. Fabry Disease and the Heart: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 4434. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Givertz, M.M.; Ho, C.Y.; Judge, D.P.; Kantor, P.F.; McBride, K.L.; Morales, A.; Taylor, M.R.; Vatta, M.; Ware, S.M. Genetic Evaluation of Cardiomyopathy—A Heart Failure Society of America Practice Guideline. J. Card. Fail. 2018, 24, 281–302. [Google Scholar] [CrossRef]
- Izhar, R.; Borriello, M.; La Russa, A.; Di Paola, R.; De, A.; Capasso, G.; Ingrosso, D.; Perna, A.F.; Simeoni, M. Fabry Disease in Women: Genetic Basis, Available Biomarkers, and Clinical Manifestations. Genes 2023, 15, 37. [Google Scholar] [CrossRef]
- Gugelmo, G.; Vitturi, N.; Francini-Pesenti, F.; Fasan, I.; Lenzini, L.; Valentini, R.; Carraro, G.; Avogaro, A.; Spinella, P. Gastrointestinal Manifestations and Low-FODMAP Protocol in a Cohort of Fabry Disease Adult Patients. Nutrients 2023, 15, 658. [Google Scholar] [CrossRef]
- Lenders, M.; Brand, E. Fabry Disease—A Multisystemic Disease with Gastrointestinal Manifestations. Gut Microbes 2022, 14, 2027852. [Google Scholar] [CrossRef]
- Lucena-Valera, A.; Perez-Palacios, D.; Muñoz-Hernandez, R.; Romero-Gómez, M.; Ampuero, J. Wilson’s Disease: Revisiting an Old Friend. World J. Hepatol. 2021, 13, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Eickhoff, A. Wilson Disease: Copper-Mediated Cuproptosis, Iron-Related Ferroptosis, and Clinical Highlights, With Comprehensive and Critical Analysis Update. Int. J. Mol. Sci. 2024, 25, 4753. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Wang, W.; Zhabyeyev, P.; Basu, R.; McLean, B.; Fan, D.; Parajuli, N.; DesAulniers, J.; Patel, V.B.; Hajjar, R.J.; et al. Iron-overload injury and cardiomyopathy in acquired and genetic models is attenuated by resveratrol therapy. Sci. Rep. 2015, 5, 18132. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, K.; Benyounes, N.; Obadia, M.A.; Van Der Vynckt, C.; Morvan, E.; Tibi, T.; Poujois, A. Cardiac involvement in Wilson disease: Review of the literature and description of three cases of sudden death. J. Inherit. Metab. Dis. 2021, 44, 1099–1112. [Google Scholar] [CrossRef]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G.; et al. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 2010, 31, 1872–1880. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic Sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef]
- Jerjen, R.; Nikpour, M.; Krieg, T.; Denton, C.P.; Saracino, A.M. Systemic Sclerosis in Adults. Part I: Clinical Features and Pathogenesis. J. Am. Acad. Dermatol. 2022, 87, 937–954. [Google Scholar] [CrossRef]
- Truchetet, M.E.; Brembilla, N.C.; Chizzolini, C. Current Concepts on the Pathogenesis of Systemic Sclerosis. Clin. Rev. Allergy Immunol. 2023, 64, 262–283. [Google Scholar] [CrossRef]
- Chizzolini, C. Update on pathophysiology of scleroderma with special reference to immunoinflammatory events. Ann. Med. 2007, 39, 42–53. [Google Scholar] [CrossRef]
- Asano, Y. Systemic Sclerosis. J. Dermatol. 2018, 45, 128–138. [Google Scholar] [CrossRef]
- Asano, Y. The Pathogenesis of Systemic Sclerosis: An Understanding Based on a Common Pathologic Cascade across Multiple Organs and Additional Organ-Specific Pathologies. J. Clin. Med. 2020, 9, 2687. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, D.; Brown, M.; Postlethwaite, B.C.; Postlethwaite, A.E. Pathogenesis of Systemic Sclerosis. Front. Immunol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Cavalli, G.; Campochiaro, C.; Bruni, C.; Tomelleri, A.; Dagna, L.; Matucci-Cerinic, M. Interleukin-1 and Systemic Sclerosis: Getting to the Heart of Cardiac Involvement. Front. Immunol. 2021, 12, 653950. [Google Scholar] [CrossRef]
- Nadel, A.; Nadel, M.; Taborska, N.; Stępień, B.; Gajdecki, J.; Brzezińska, O.; Opinc-Rosiak, A.; Makowska, J.; Lewandowska-Polak, A. Heart involvement in patients with systemic sclerosis—What have we learned about it in the last 5 years. Rheumatol. Int. 2024, 44, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D.; Andréasson, K.; McMahan, Z.H.; Bukiri, H.; Howlett, N.; Lagishetty, V.; Lee, S.M.; Jacobs, J.P.; Volkmann, E.R. Gastrointestinal tract involvement in systemic sclerosis: The roles of diet and the microbiome. Semin. Arthritis Rheum. 2023, 60, 152185. [Google Scholar] [CrossRef]
- Bellando-Randone, S.; Russo, E.; Di Gloria, L.; Lepri, G.; Baldi, S.; Fioretto, B.S.; Romano, E.; Ghezzi, G.; Bertorello, S.; El Aoufy, K.; et al. Gut microbiota in very early systemic sclerosis: The first case-control taxonomic and functional characterisation highlighting an altered butyric acid profile. RMD Open 2024, 10, e004647. [Google Scholar] [CrossRef]
- Russo, E.; Bellando-Randone, S.; Carboni, D.; Fioretto, B.S.; Romano, E.; Baldi, S.; El Aoufy, K.; Ramazzotti, M.; Rosa, I.; Lepri, G.; et al. The differential crosstalk of the skin–gut microbiome axis as a new emerging actor in systemic sclerosis. Rheumatology 2024, 63, 226–234. [Google Scholar] [CrossRef]
- Sève, P.; Pacheco, Y.; Durupt, F.; Jamilloux, Y.; Gerfaud-Valentin, M.; Isaac, S.; Boussel, L.; Calender, A.; Androdias, G.; Valeyre, D.; et al. Sarcoidosis: A Clinical Overview from Symptoms to Diagnosis. Cells 2021, 10, 766. [Google Scholar] [CrossRef]
- Drent, M.; Crouser, E.D.; Grunewald, J. Challenges of Sarcoidosis and Its Management. N. Engl. J. Med. 2021, 385, 1018–1032. [Google Scholar] [CrossRef]
- Markatis, E.; Afthinos, A.; Antonakis, E.; Papanikolaou, I.C. Cardiac sarcoidosis: Diagnosis and management. Rev. Cardiovasc. Med. 2020, 21, 321–338. [Google Scholar] [CrossRef]
- Hattori, T.; Konno, S.; Shijubo, N.; Yamaguchi, T.; Sugiyama, Y.; Honma, S.; Inase, N.; Ito, Y.M.; Nishimura, M. Nationwide survey on the organ-specific prevalence and its interaction with sarcoidosis in Japan. Sci. Rep. 2018, 8, 9440. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Cen, J.; Dai, Q.; Tao, H.; Peng, L. Gut microbiota does not play a mediating role in the causal association between inflam-matory bowel disease and several its associated extraintestinal manifestations: A Mendelian randomization study. Front Im-Munol. 2024, 14, 1296889. [Google Scholar] [CrossRef] [PubMed]
- Brownell, I.; Ramírez-Valle, F.; Sanchez, M.; Prystowsky, S. Evidence for mycobacteria in sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2011, 45, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.S.; Lehmann, S.; Nielsen, R.; Tangedal, S.; Paytuvi-Gallart, A.; Sanseverino, W.; Martinsen, E.M.H.; Hiemstra, P.S.; Eagan, T.M. The lower airways microbiota and antimicrobial peptides indicate dysbiosis in sarcoidosis. Microbiome 2022, 10, 175. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, T.; Geng, R.; Sun, L.; Fan, H. The associations between gut microbiota and chronic respiratory diseases: A Mendelian randomization study. Front. Microbiol. 2023, 14, 1200937. [Google Scholar] [CrossRef]
- Asakawa, N.; Uchida, K.; Sakakibara, M.; Omote, K.; Noguchi, K.; Tokuda, Y.; Kamiya, K.; Hatanaka, K.C.; Matsuno, Y.; Yamada, S.; et al. Immunohistochemical Identi-fication of Propionibacterium Acnes in Granuloma and Inflammatory Cells of Myocardial Tissues Obtained from Cardiac Sarcoidosis Patients. PLoS ONE 2017, 12, e0179980. [Google Scholar] [CrossRef]
- Taverna, S.; Cammarata, G.; Colomba, P.; Sciarrino, S.; Zizzo, C.; Francofonte, D.; Zora, M.; Scalia, S.; Brando, C.; Lo Curto, A.; et al. Pompe disease: Pathogenesis, molecular genetics and diagnosis. Aging 2020, 12, 15856–15874. [Google Scholar] [CrossRef]
- Dow, D.E.; Seed, P.C. Clostridium Difficile Cure with Fecal Microbiota Transplantation in a Child with Pompe Disease: A Case Report. J. Med. Case Rep. 2018, 12, 112. [Google Scholar] [CrossRef]
- Van Nood, E.; Vrieze, A.; Nieuwdorp, M.; Fuentes, S.; Zoetendal, E.G.; De Vos, W.M.; Visser, C.E.; Kuijper, E.J.; Bartelsman, J.F.W.M.; Tijssen, J.G.P.; et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N. Engl. J. Med. 2013, 368, 407–415. [Google Scholar] [CrossRef]
- Khanna, S.; Pardi, D.S.; Kelly, C.R.; Kraft, C.S.; Dhere, T.; Henn, M.R.; Lombardo, M.-J.; Vulic, M.; Ohsumi, T.; Winkler, J.; et al. A Novel Microbiome Therapeutic Increases Gut Microbial Diversity and Prevents Recurrent Clostridium difficile Infection. J. Infect. Dis. 2016, 214, 173–181. [Google Scholar] [CrossRef]
- Sandek, A.; Bjarnason, I.; Volk, H.-D.; Crane, R.; Meddings, J.B.; Niebauer, J.; Kalra, P.R.; Buhner, S.; Herrmann, R.; Springer, J.; et al. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int. J. Cardiol. 2012, 157, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Swidsinski, A.; Schroedl, W.; Watson, A.; Valentova, M.; Herrmann, R.; Scherbakov, N.; Cramer, L.; Rauchhaus, M.; Grosse-Herrenthey, A.; et al. Intestinal blood flow in patients with chronic heart failure: A link with bacterial growth, gastrointestinal symptoms, and cachexia. J. Am. Coll. Cardiol. 2014, 64, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Doehner, W.; Anker, S.D.; von Haehling, S. Nutrition in heart failure: An update. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Aquilani, R.; Testa, C.; Baiardi, P.; Angioletti, S.; Boschi, F.; Verri, M.; Dioguardi, F. Pathogenic Gut Flora in Patients With Chronic Heart Failure. JACC Heart Fail. 2016, 4, 220–227. [Google Scholar] [CrossRef]
- Beale, A.L.; O’donnell, J.A.; Nakai, M.E.; Nanayakkara, S.; Vizi, D.; Carter, K.; Dean, E.; Ribeiro, R.V.; Yiallourou, S.; Carrington, M.J.; et al. The Gut Microbiome of Heart Failure With Preserved Ejection Fraction. J. Am. Heart Assoc. 2021, 10, e020654. [Google Scholar] [CrossRef]
- Cui, X.; Ye, L.; Li, J.; Jin, L.; Wang, W.; Li, S.; Bao, M.; Wu, S.; Li, L.; Geng, B.; et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 2018, 8, 635. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef]
- Madan, S.; Mehra, M.R. Gut dysbiosis and heart failure: Navigating the universe within. Eur. J. Heart Fail. 2020, 22, 629–637. [Google Scholar] [CrossRef]
- Gan, X.T.; Ettinger, G.; Huang, C.X.; Burton, J.P.; Haist, J.V.; Rajapurohitam, V.; Sidaway, J.E.; Martin, G.; Gloor, G.B.; Swann, J.R.; et al. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ. Heart Fail. 2014, 7, 491–499. [Google Scholar] [CrossRef]
- Costanza, A.C.; Moscavitch, S.D.; Neto, H.C.F.; Mesquita, E.T. Probiotic therapy with Saccharomyces boulardii for heart failure patients: A randomized, double-blind, placebo-controlled pilot trial. Int. J. Cardiol. 2015, 179, 348–350. [Google Scholar] [CrossRef]
- Mayerhofer, C.C.K.; Awoyemi, A.O.; Moscavitch, S.D.; Lappegård, K.T.; Hov, J.R.; Aukrust, P.; Hovland, A.; Lorenzo, A.; Halvorsen, S.; Seljeflot, I.; et al. Design of the GutHeart-targeting gut microbiota to treat heart failure-trial: A Phase II, randomized clinical trial. ESC Heart Fail. 2018, 5, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Vatanen, T.; Cutfield, W.S.; O’Sullivan, J.M. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front. Cell. Infect. Microbiol. 2019, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Gesualdo, M.; Scicchitano, P.; Carbonara, S.; Ricci, G.; Principi, M.; Ierardi, E.; Di Leo, A.; Cortese, F.; Ciccone, M.M. The Association between Cardiac and Gastrointestinal Disorders: Causal or Casual Link? J. Cardiovasc. Med. 2016, 17, 330. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic Bacterial Metabolites Regulate Gastrointestinal Barrier Function via the Xenobiotic Sensor PXR and Toll-like Receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Koay, Y.C.; Pan, C.; Zhou, Z.; Tang, W.; Wilcox, J.; Li, X.S.; Zagouras, A.; Marques, F.; Allayee, H.; et al. Indole-3-Propionic Acid Protects Against Heart Failure With Preserved Ejection Fraction. Circ. Res. 2024, 134, 371–389. [Google Scholar] [CrossRef]
- Bianchi, F.; Cappella, A.; Gagliano, N.; Sfondrini, L.; Stacchiotti, A. Polyphe-nols-Gut-Heart: An Impactful Relationship to Improve Cardiovascular Diseases. Antioxidants 2022, 11, 1700. [Google Scholar] [CrossRef]
- Mollace, V.; Rosano, G.M.C.; Anker, S.D.; Coats, A.J.S.; Seferovic, P.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; et al. Pathophysiological Basis for Nutraceutical Supplementation in Heart Failure: A Comprehensive Review. Nutrients 2021, 13, 257. [Google Scholar] [CrossRef]
- Dinu, L.D.; Gatea, F.; Roaming Israel, F.; Lakicevic, M.; Dedović, N.; Vamanu, E. The Modulation Effect of a Fermented Bee Pollen Postbiotic on Cardiovascular Microbiota and Therapeutic Perspectives. Biomedicines 2023, 11, 2712. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Haiser, H.J.; Gootenberg, D.B.; Chatman, K.; Sirasani, G.; Balskus, E.P.; Turnbaugh, P.J. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science 2013, 341, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Steiner, H.E.; Gee, K.; Giles, J.; Knight, H.; Hurwitz, B.L.; Karnes, J.H. Role of the Gut Microbiome in Cardiovascular Drug Response: The Potential for Clinical Application. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2021, 42, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.; Pruteanu, M.; Kuhn, M.; Zeller, G.; Telzerow, A.; Anderson, E.E.; Brochado, A.R.; Fernandez, K.C.; Dose, H.; Mori, H.; et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018, 555, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Tuteja, S.; Ferguson, J.F. Gut Microbiome and Response to Cardiovascular Drugs. Circ. Genom. Precis. Med. 2019, 12, 421–429. [Google Scholar] [CrossRef]
- Singh, P.; Meenatchi, R.; Ahmed, Z.T.; Thacharodi, A.; Rohinth, M.; Kumar, R.R.; Harsha Varthan, M.K.; Hassan, S. Implications of the gut microbiome in cardiovascular diseases: Association of gut microbiome with cardiovascular diseases, therapeutic interventions and multi-omics approach for precision medicine. Med. Microecol. 2023, 19, 100096. [Google Scholar] [CrossRef]
- Kim, I.S.; Yoo, D.-H.; Jung, I.-H.; Lim, S.; Jeong, J.-J.; Kim, K.-A.; Bae, O.-N.; Yoo, H.H.; Kim, D.-H. Reduced metabolic activity of gut microbiota by antibiotics can potentiate the antithrombotic effect of aspirin. Biochem. Pharmacol. 2016, 122, 72–79. [Google Scholar] [CrossRef]
- Rogers, M.a.M.; Aronoff, D.M. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin. Microbiol. Infect Off. Publ. Eur. Soc. Clin. Microbiol. Infect Dis. 2016, 22, 178.e1–178.e9. [Google Scholar] [CrossRef]
- Peters, U.; Falk, L.C.; Kalman, S.M. Digoxin metabolism in patients. Arch. Intern. Med. 1978, 138, 1074–1076. [Google Scholar] [CrossRef]
- Lindenbaum, J.; Rund, D.G.; Butler, V.P.; Tse-Eng, D.; Saha, J.R. Inactivation of digoxin by the gut flora: Reversal by antibiotic therapy. N. Engl. J. Med. 1981, 305, 789–794. [Google Scholar] [CrossRef]
- Konop, M.; Radkowski, M.; Grochowska, M.; Perlejewski, K.; Samborowska, E.; Ufnal, M. Enalapril decreases rat plasma concen-tration of TMAO, a gut bacteria-derived cardiovascular marker. Biomark. Biochem. Indic. Expo Response Susceptibility Chem. 2018, 23, 380–385. [Google Scholar]
- Yoo, H.H.; Kim, I.S.; Yoo, D.-H.; Kim, D.-H. Effects of orally administered antibiotics on the bioavailability of amlodipine: Gut mi-crobiota-mediated drug interaction. J. Hypertens. 2016, 34, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.A.; Ganguly, K.; Mazumdar, K.; Dutta, N.K.; Dastidar, S.G.; Chakrabarty, A.N. Amlodipine: A cardiovascular drug with powerful antimicrobial property. Acta Microbiol. Pol. 2003, 52, 285–292. [Google Scholar] [PubMed]
- Dhillon, S. Correction to: Dapagliflozin: A Review in Type 2 Diabetes. Drugs 2019, 79, 2013. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Bao, N.; Liu, X.; Zhong, X.; Jia, S.; Hua, N.; Zhang, L.; Mo, G. Dapagli-flozin-Affected Endothelial Dysfunction and Altered Gut Microbiota in Mice with Heart Failure. PeerJ 2023, 11, e15589. [Google Scholar] [CrossRef]
- Vrieze, A.; Out, C.; Fuentes, S.; Jonker, L.; Reuling, I.; Kootte, R.S.; van Nood, E.; Holleman, F.; Knaapen, M.; Romijn, J.A.; et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 2014, 60, 824–831. [Google Scholar] [CrossRef]
- Lam, V.; Su, J.; Koprowski, S.; Hsu, A.; Tweddell, J.S.; Rafiee, P.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J. 2012, 26, 1727–1735. [Google Scholar] [CrossRef]
- Lam, V.; Su, J.; Hsu, A.; Gross, G.J.; Salzman, N.H.; Baker, J.E. Intestinal microbial metabolites are linked to severity of myocardial infarction in rats. PLoS ONE 2016, 11, e0160840–e160850. [Google Scholar] [CrossRef]
- Conraads, V.M.; Jorens, P.G.; De Clerck, L.S.; Van Saene, H.K.; Ieven, M.M.; Bosmans, J.M.; Schuerwegh, A.; Bridts, C.H.; Wuyts, F.; Stevens, W.J.; et al. Selective intestinal decontamination in advanced chronic heart failure: A pilot trial. Eur. J. Heart Fail. 2004, 6, 483–491. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).