Potential Modulatory Role of Phoenixin-14 in Epithelial–Mesenchymal Transition of Endometriotic 12Z Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line Culture
2.2. Stimulation with PNX-14
2.3. CDH1 and THBS2 Expression Analysis
2.3.1. RNA Quality and Quantity Assessment
2.3.2. Reverse Transcription
2.3.3. Quantitative Polymerase Chain Reaction
2.4. Immunocytochemistry
2.4.1. Cadherin 1 Immunolocalization
2.4.2. Thrombospondin 2 Immunolocalization
2.5. XTT Viability and Cytotoxicity Assays
2.6. Statistical Analysis
3. Results
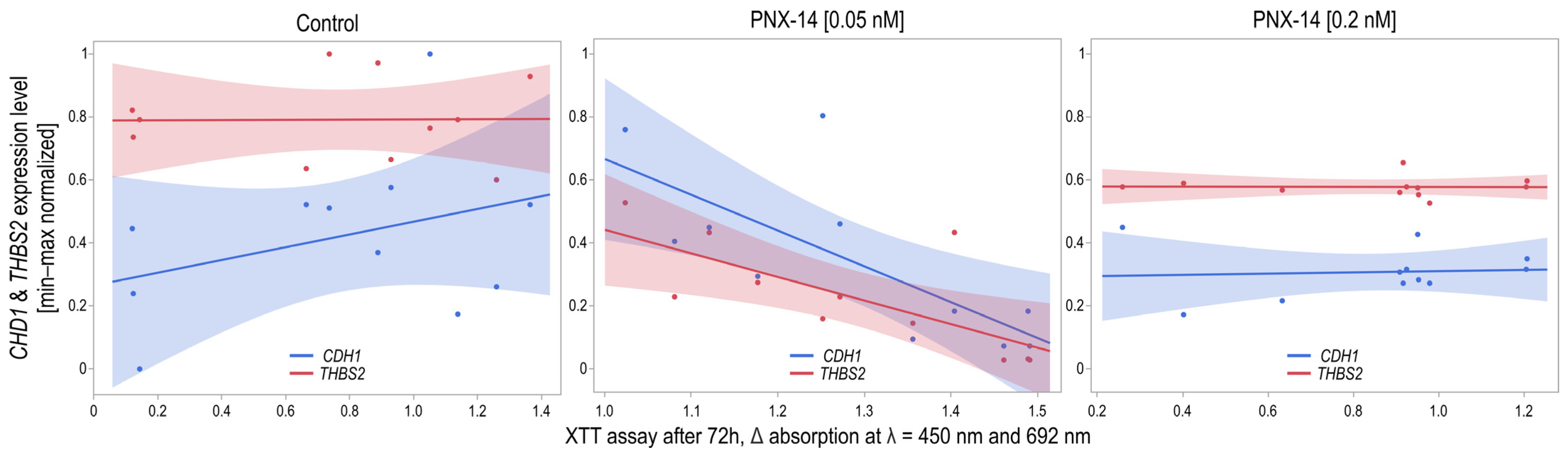
3.1. CDH1 and THBS2 Expression
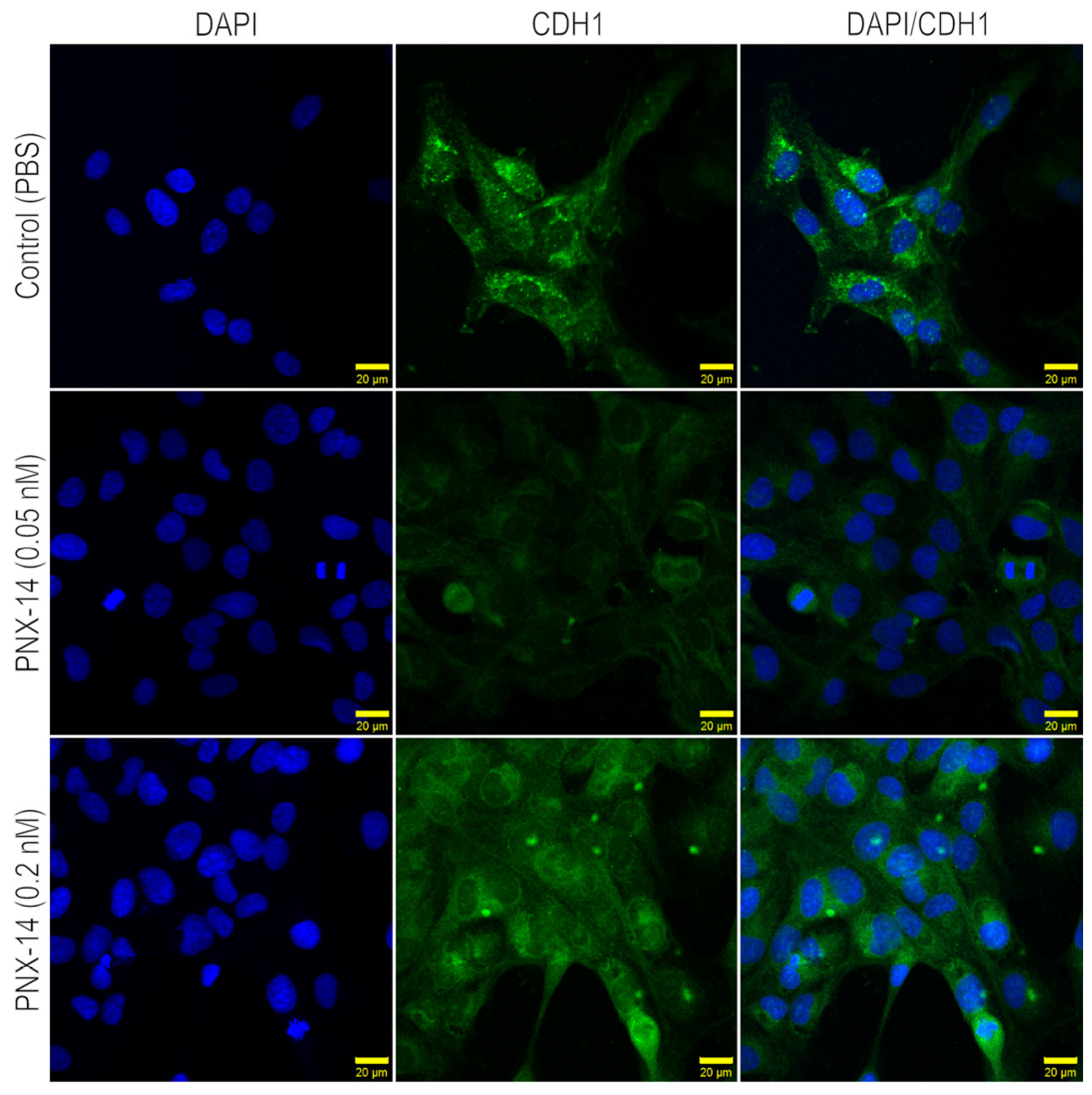
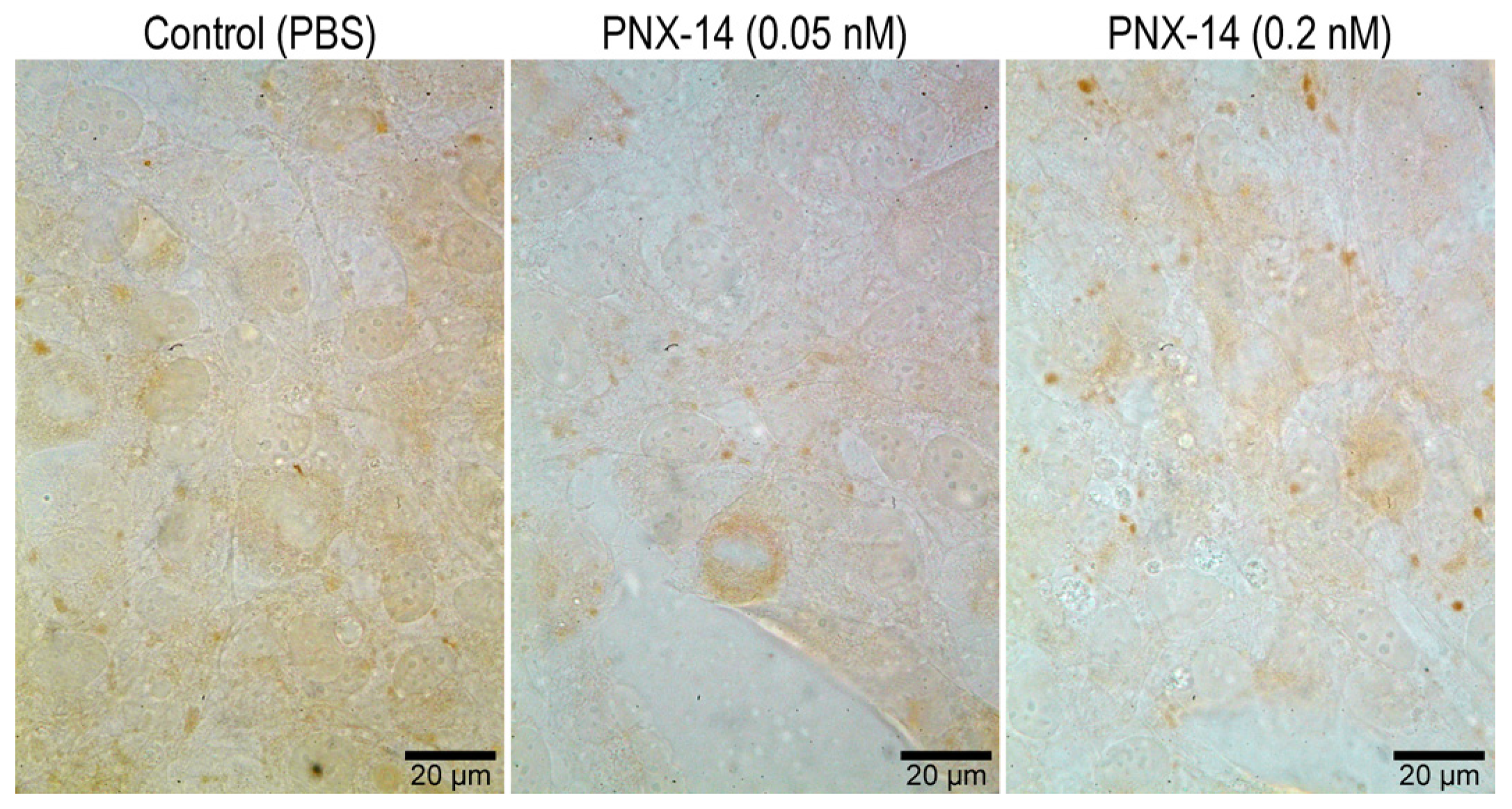
3.2. Immunocytochemical Localization of CDH1 and THBS2 in 12Z Cells
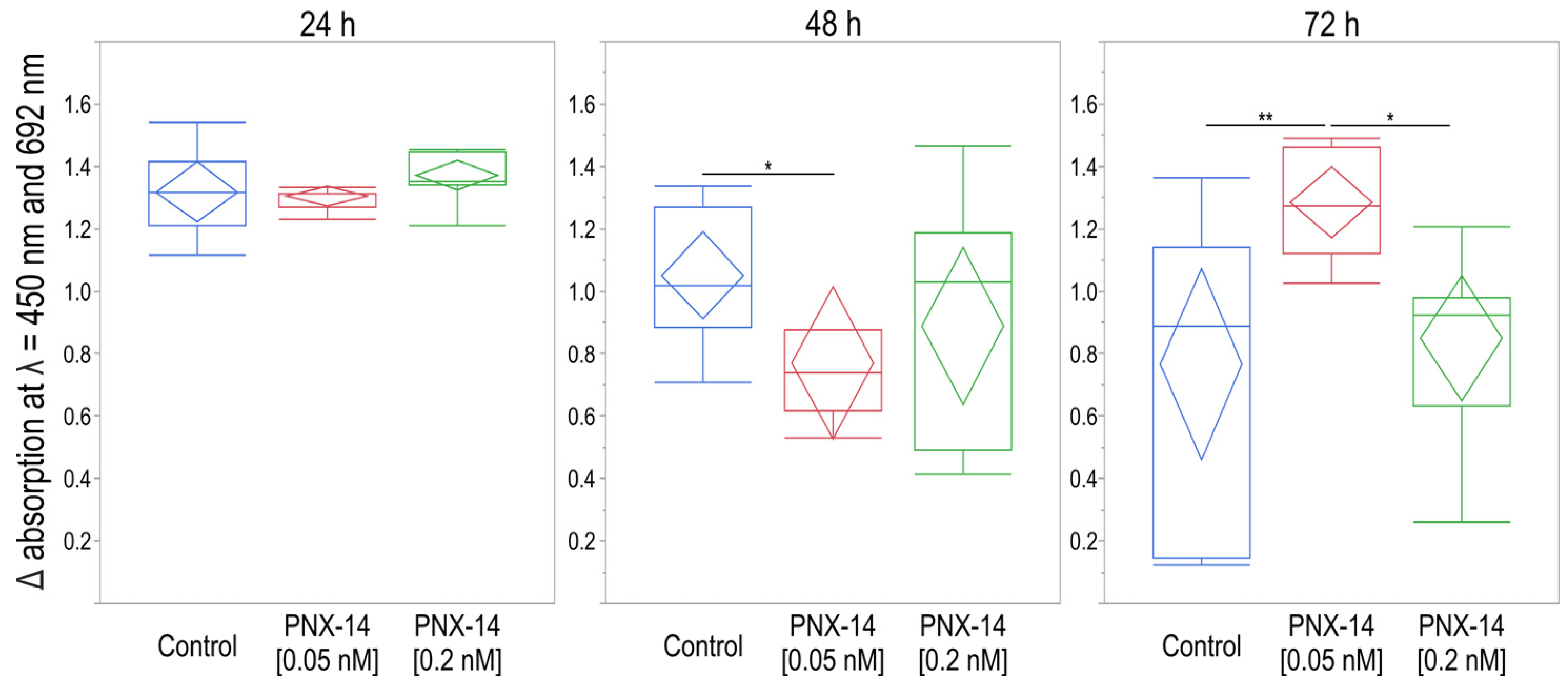
3.3. XTT Viability and Cytotoxicity Assay
4. Discussion
4.1. PNX-14-Stimulated CDH1 and THBS2 Expression
4.2. PNX-14 Effect on 12Z Cell Viability
4.3. PNX-14 and EMT Process of Ectopic Endometrium—Future Perspectives
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obs. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, Pathobiology, and Therapeutic Prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, Y.; Xu, H.; Hill, C.; Ewing, R.M.; He, D.; Zhang, X.; Wang, Y. Bioinformatic Analysis Reveals the Importance of Epithelial-Mesenchymal Transition in the Development of Endometriosis. Sci. Rep. 2020, 10, 8442. [Google Scholar] [CrossRef]
- Konrad, L.; Dietze, R.; Riaz, M.A.; Scheiner-Bobis, G.; Behnke, J.; Horné, F.; Hoerscher, A.; Reising, C.; Meinhold-Heerlein, I. Epithelial–Mesenchymal Transition in Endometriosis—When Does It Happen? J. Clin. Med. 2020, 9, 1915. [Google Scholar] [CrossRef] [PubMed]
- Proestling, K.; Birner, P.; Gamperl, S.; Nirtl, N.; Marton, E.; Yerlikaya, G.; Wenzl, R.; Streubel, B.; Husslein, H. Enhanced Epithelial to Mesenchymal Transition (EMT) and Upregulated MYC in Ectopic Lesions Contribute Independently to Endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Yang, W.-X. Epithelial-to-Mesenchymal Transition in the Development of Endometriosis. Oncotarget 2017, 8, 41679–41689. [Google Scholar] [CrossRef]
- Rout-Pitt, N.; Farrow, N.; Parsons, D.; Donnelley, M. Epithelial Mesenchymal Transition (EMT): A Universal Process in Lung Diseases with Implications for Cystic Fibrosis Pathophysiology. Respir. Res. 2018, 19, 136. [Google Scholar] [CrossRef] [PubMed]
- Sarrand, J.; Soyfoo, M.S. Involvement of Epithelial-Mesenchymal Transition (EMT) in Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 14481. [Google Scholar] [CrossRef]
- Zvaifler, N.J. Relevance of the Stroma and Epithelial-Mesenchymal Transition (EMT) for the Rheumatic Diseases. Arthritis Res. Ther. 2006, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, U.D.; Joseph, J.V.; Kruyt, F.A.E. EMT- and MET-Related Processes in Nonepithelial Tumors: Importance for Disease Progression, Prognosis, and Therapeutic Opportunities. Mol. Oncol. 2017, 11, 860–877. [Google Scholar] [CrossRef]
- Soni, U.K.; Chadchan, S.B.; Kumar, V.; Ubba, V.; Khan, M.T.A.; Vinod, B.S.V.; Konwar, R.; Bora, H.K.; Rath, S.K.; Sharma, S.; et al. A High Level of TGF-B1 Promotes Endometriosis Development via Cell Migration, Adhesiveness, Colonization, and Invasiveness. Biol. Reprod. 2019, 100, 917–938. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Darcha, C. Epithelial to Mesenchymal Transition-like and Mesenchymal to Epithelial Transition-like Processes Might Be Involved in the Pathogenesis of Pelvic Endometriosis. Hum. Reprod. 2012, 27, 712–721. [Google Scholar] [CrossRef]
- Hugo, H.J.; Kokkinos, M.I.; Blick, T.; Ackland, M.L.; Thompson, E.W.; Newgreen, D.F. Defining the E-Cadherin Repressor Interactome in Epithelial-Mesenchymal Transition: The PMC42 Model as a Case Study. Cells Tissues Organs 2011, 193, 23–40. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Liu, D.; Yang, N.; Liang, Y.; Chen, M.; Yang, F.; Liu, L.; Yao, S. Increased Expression of Epithelial Cell Adhesion Molecule and Its Possible Role in Epithelial-Mesenchymal Transition in Endometriosis. J. Obs. Gynaecol. Res. 2020, 46, 2066–2075. [Google Scholar] [CrossRef]
- Cheon, D.-J.; Tong, Y.; Sim, M.-S.; Dering, J.; Berel, D.; Cui, X.; Lester, J.; Beach, J.A.; Tighiouart, M.; Walts, A.E.; et al. A Collagen-Remodeling Gene Signature Regulated by TGF-β Signaling Is Associated with Metastasis and Poor Survival in Serous Ovarian Cancer. Clin. Cancer Res. 2014, 20, 711–723. [Google Scholar] [CrossRef]
- Tuszynski, G.P.; Rothman, V.; Murphy, A.; Siegler, K.; Smith, L.; Smith, S.; Karczewski, J.; Knudsen, K.A. Thrombospondin Promotes Cell-Substratum Adhesion. Science 1987, 236, 1570–1573. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Li, H.; Sun, W.; Zhang, H.; Lai, M. THBS2 Is a Potential Prognostic Biomarker in Colorectal Cancer. Sci. Rep. 2016, 6, 33366. [Google Scholar] [CrossRef] [PubMed]
- Risher, W.C.; Eroglu, C. Thrombospondins as Key Regulators of Synaptogenesis in the Central Nervous System. Matrix Biol. 2012, 31, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Seki, N.; Kodama, J.; Hashimoto, I.; Hongo, A.; Yoshinouchi, M.; Kudo, T. Thrombospondin-1 and -2 Messenger RNA Expression in Normal and Neoplastic Endometrial Tissues: Correlation with Angiogenesis and Prognosis. Int. J. Oncol. 2001, 19, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Yosten, G.L.C.; Lyu, R.-M.; Hsueh, A.J.W.; Avsian-Kretchmer, O.; Chang, J.-K.; Tullock, C.W.; Le Dun, S.; Dun, N.; Samson, W.K. A Novel Reproductive Peptide, Phoenixin. J. Neuroendocr. 2013, 25, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.M.; Tullock, C.W.; Mathews, S.K.; Garcia-Galiano, D.; Elias, C.F.; Samson, W.K.; Yosten, G.L.C. Hypothalamic Action of Phoenixin to Control Reproductive Hormone Secretion in Females: Importance of the Orphan G Protein-Coupled Receptor Gpr173. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R489–R496. [Google Scholar] [CrossRef] [PubMed]
- Treen, A.K.; Luo, V.; Belsham, D.D. Phoenixin Activates Immortalized GnRH and Kisspeptin Neurons Through the Novel Receptor GPR173. Mol. Endocrinol. 2016, 30, 872–888. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.P.; Nakamura, T.; Osuka, S.; Bayasula, B.; Nakanishi, N.; Kasahara, Y.; Muraoka, A.; Hayashi, S.; Nagai, T.; Murase, T.; et al. Effect of the Neuropeptide Phoenixin and Its Receptor GPR173 during Folliculogenesis. Reproduction 2019, 158, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Gershon, E.; Dekel, N. Newly Identified Regulators of Ovarian Folliculogenesis and Ovulation. Int. J. Mol. Sci. 2020, 21, 4565. [Google Scholar] [CrossRef]
- Kulinska, K.I.; Andrusiewicz, M.; Dera-Szymanowska, A.; Billert, M.; Skrzypski, M.; Szymanowski, K.; Nowak-Markwitz, E.; Kotwicka, M.; Wołuń-Cholewa, M. Phoenixin as a New Target in the Development of Strategies for Endometriosis Diagnosis and Treatment. Biomedicines 2021, 9, 1427. [Google Scholar] [CrossRef]
- Kulinska, K.I.; Białas, P.; Dera-Szymanowska, A.; Billert, M.; Kotwicka, M.; Szymanowski, K.; Wołun-Cholewa, M. The Role of Phoenixin in the Proliferation and Migration of Ectopic Epithelial Cells in Vitro. Biochem. Biophys. Res. Commun. 2023, 646, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Gołąbek-Grenda, A.; Olejnik, A. In Vitro Modeling of Endometriosis and Endometriotic Microenvironment—Challenges and Recent Advances. Cell. Signal. 2022, 97, 110375. [Google Scholar] [CrossRef] [PubMed]
- Banu, S.K.; Lee, J.; Starzinski-Powitz, A.; Arosh, J.A. Gene Expression Profiles and Functional Characterization of Human Immortalized Endometriotic Epithelial and Stromal Cells. Fertil. Steril. 2008, 90, 972–987. [Google Scholar] [CrossRef] [PubMed]
- Zeitvogel, A.; Baumann, R.; Starzinski-Powitz, A. Identification of an Invasive, N-Cadherin-Expressing Epithelial Cell Type in Endometriosis Using a New Cell Culture Model. Am. J. Pathol. 2001, 159, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-Phenol-Chloroform Extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Andrusiewicz, M.; Słowikowski, B.; Skibińska, I.; Wołuń-Cholewa, M.; Dera-Szymanowska, A. Selection of Reliable Reference Genes in Eutopic and Ectopic Endometrium for Quantitative Expression Studies. Biomed. Pharmacother. 2016, 78, 66–73. [Google Scholar] [CrossRef]
- Zubrzycka, A.; Migdalska-Sęk, M.; Jędrzejczyk, S.; Brzeziańska-Lasota, E. Assessment of BMP7, SMAD4, and CDH1 Expression Profile and Regulatory miRNA-542-3p in Eutopic and Ectopic Endometrium of Women with Endometriosis. Int. J. Mol. Sci. 2023, 24, 6637. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, M.; Kouvaras, E.; Papamichali, R.; Samara, M.; Chiotoglou, I.; Koukoulis, G. Smad4 and Epithelial-Mesenchymal Transition Proteins in Colorectal Carcinoma: An Immunohistochemical Study. J. Mol. Histol. 2018, 49, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhang, L.; Liu, H.; Li, N.; Du, Y.; He, H.; Zhang, Z.; Liu, Y. E2-mediated EMT by Activation of Β-catenin/Snail Signalling during the Development of Ovarian Endometriosis. J. Cell Mol. Med. 2019, 23, 8035–8045. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ma, Y.; He, H.-W.; Zhao, W.-L.; Shao, R.-G. SPHK1 (Sphingosine Kinase 1) Induces Epithelial-Mesenchymal Transition by Promoting the Autophagy-Linked Lysosomal Degradation of CDH1/E-Cadherin in Hepatoma Cells. Autophagy 2017, 13, 900–913. [Google Scholar] [CrossRef]
- Janda, E.; Nevolo, M.; Lehmann, K.; Downward, J.; Beug, H.; Grieco, M. Raf plus TGFbeta-Dependent EMT Is Initiated by Endocytosis and Lysosomal Degradation of E-Cadherin. Oncogene 2006, 25, 7117–7130. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, I.; Öllinger, K. Lysosomes in Cancer—At the Crossroad of Good and Evil. Cells 2024, 13, 459. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Song, S. Circ-OPHN1 Suppresses the Proliferation, Migration, and Invasion of Trophoblast Cells through Mediating miR-558/THBS2 Axis. Drug Dev. Res. 2022, 83, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Y.; Liu, W.; Lan, R. Circ_0085296 Inhibits the Biological Functions of Trophoblast Cells to Promote the Progression of Preeclampsia via the miR-942-5p/THBS2 Network. Open Med. 2022, 17, 577–588. [Google Scholar] [CrossRef]
- Deng, B.; Liu, X.-P.; Wang, X. Prognostic and Immunological Role of THBS2 in Colorectal Cancer. Biomed. Res. Int. 2021, 2021, 1124985. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.-C.; Wu, M.-H.; Shoji, Y.; Tsai, S.-J. Downregulation of CD36 Results in Reduced Phagocytic Ability of Peritoneal Macrophages of Women with Endometriosis. J. Pathol. 2009, 219, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Moar, K.; Arora, T.K.; Maurya, P.K. Implication of Biosignatures in the Progression of Endometriosis. Pathol.-Res. Pract. 2024, 254, 155103. [Google Scholar] [CrossRef] [PubMed]
- Billert, M.; Wojciechowicz, T.; Jasaszwili, M.; Szczepankiewicz, D.; Waśko, J.; Kaźmierczak, S.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Phoenixin-14 Stimulates Differentiation of 3T3-L1 Preadipocytes via cAMP/Epac-Dependent Mechanism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1449–1457. [Google Scholar] [CrossRef]
- Wu, R.-F.; Chen, Z.-X.; Zhou, W.-D.; Li, Y.-Z.; Huang, Z.-X.; Lin, D.-C.; Ren, L.-L.; Chen, Q.-X.; Chen, Q.-H. High Expression of ZEB1 in Endometriosis and Its Role in 17β-Estradiol-Induced Epithelial-Mesenchymal Transition. Int. J. Clin. Exp. Pathol. 2018, 11, 4744–4758. [Google Scholar] [PubMed]
- Chen, Y.-J.; Li, H.-Y.; Huang, C.-H.; Twu, N.-F.; Yen, M.-S.; Wang, P.-H.; Chou, T.-Y.; Liu, Y.-N.; Chao, K.-C.; Yang, M.-H. Oestrogen-Induced Epithelial–Mesenchymal Transition of Endometrial Epithelial Cells Contributes to the Development of Adenomyosis. J. Pathol. 2010, 222, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, Z.; Chen, X.; Lu, J.; Song, Y.; Xia, W. A Review of the Effects of Estrogen and Epithelial-Mesenchymal Transformation on Intrauterine Adhesion and Endometriosis. Transpl. Immunol. 2023, 79, 101679. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Ur Rahman, T.; Wu, D.-D.; Lin, X.-H.; Liu, Y.; Guo, X.-Y.; Leung, P.C.K.; Zhang, R.-J.; Huang, H.-F.; Sheng, J.-Z. Phoenixin-14 Concentrations Are Increased in Association with Luteinizing Hormone and Nesfatin-1 Concentrations in Women with Polycystic Ovary Syndrome. Clin. Chim. Acta 2017, 471, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Suszka-Świtek, A.; Pałasz, A.; Filipczyk, Ł.; Menezes, I.C.; Mordecka-Chamera, K.; Angelone, T.; Bogus, K.; Bacopoulou, F.; Worthington, J.J.; Wiaderkiewicz, R. The GnRH Analogues Affect Novel Neuropeptide SMIM20/Phoenixin and GPR173 Receptor Expressions in the Female Rat Hypothalamic–Pituitary–Gonadal (HPG) Axis. Clin. Exp. Pharmacol. Physiol. 2019, 46, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, I.; Alharbi, K.S.; Al-Abbasi, F.A.; Almalki, W.H.; Kumar, S.; Yasmeen, A.; Khan, A.; Gupta, G. Role of Epithelial-to-Mesenchymal Transition Markers in Different Stages of Endometriosis: Expression of the Snail, Slug, ZEB1, and Twist Genes. Crit. Rev. Eukaryot. Gene Expr. 2021, 31, 89–95. [Google Scholar] [CrossRef] [PubMed]

| Gene | NCBI Accession No | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Probe No |
|---|---|---|---|---|
| B2M | NM_004048.4 | GATGAGTATGCCTGCCGTGT | CTGCTTACATGTCTCGATCCCA | 1 |
| CDH1 | NM_001270 | CCACCAAAGTCACGCTGAA | TGCTTGGATTCCAGAAACG | #77 |
| THBS2 | NM_003247.2 | CTTTGCGGAAAATGAAACG | TGATTTGGTGGCAAATGGT | #77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulinska, K.I.; Wierzbicka, M.; Dera-Szymanowska, A.; Szymanowski, K.; Andrusiewicz, M.; Wołuń-Cholewa, M. Potential Modulatory Role of Phoenixin-14 in Epithelial–Mesenchymal Transition of Endometriotic 12Z Cells. Biomedicines 2025, 13, 158. https://doi.org/10.3390/biomedicines13010158
Kulinska KI, Wierzbicka M, Dera-Szymanowska A, Szymanowski K, Andrusiewicz M, Wołuń-Cholewa M. Potential Modulatory Role of Phoenixin-14 in Epithelial–Mesenchymal Transition of Endometriotic 12Z Cells. Biomedicines. 2025; 13(1):158. https://doi.org/10.3390/biomedicines13010158
Chicago/Turabian StyleKulinska, Karolina Iwona, Magdalena Wierzbicka, Anna Dera-Szymanowska, Krzysztof Szymanowski, Mirosław Andrusiewicz, and Maria Wołuń-Cholewa. 2025. "Potential Modulatory Role of Phoenixin-14 in Epithelial–Mesenchymal Transition of Endometriotic 12Z Cells" Biomedicines 13, no. 1: 158. https://doi.org/10.3390/biomedicines13010158
APA StyleKulinska, K. I., Wierzbicka, M., Dera-Szymanowska, A., Szymanowski, K., Andrusiewicz, M., & Wołuń-Cholewa, M. (2025). Potential Modulatory Role of Phoenixin-14 in Epithelial–Mesenchymal Transition of Endometriotic 12Z Cells. Biomedicines, 13(1), 158. https://doi.org/10.3390/biomedicines13010158







