Connections and Unmet Needs: Severe Asthma Biologics and Osteoporosis
Abstract
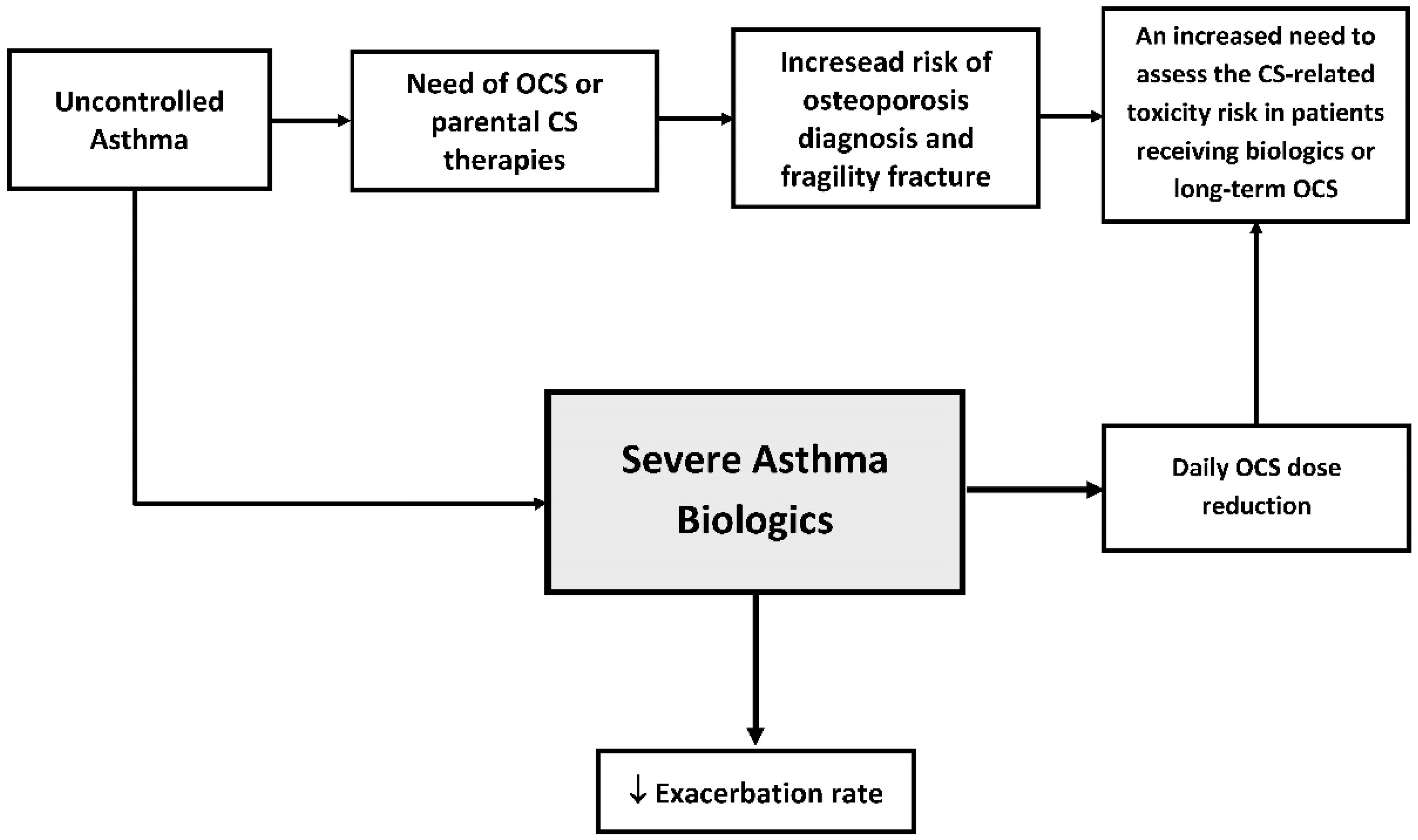
1. Introduction
2. Materials and Methods
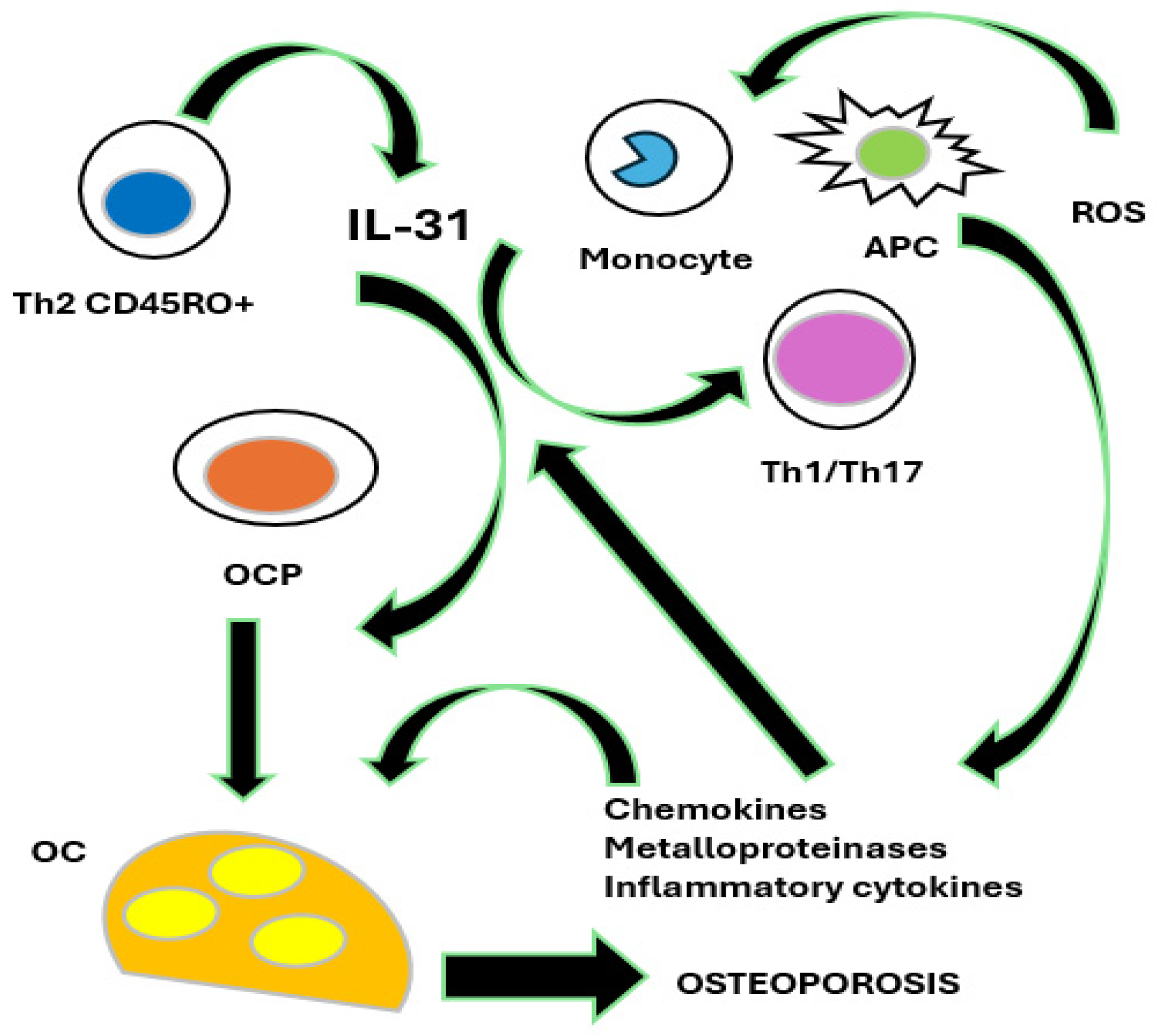
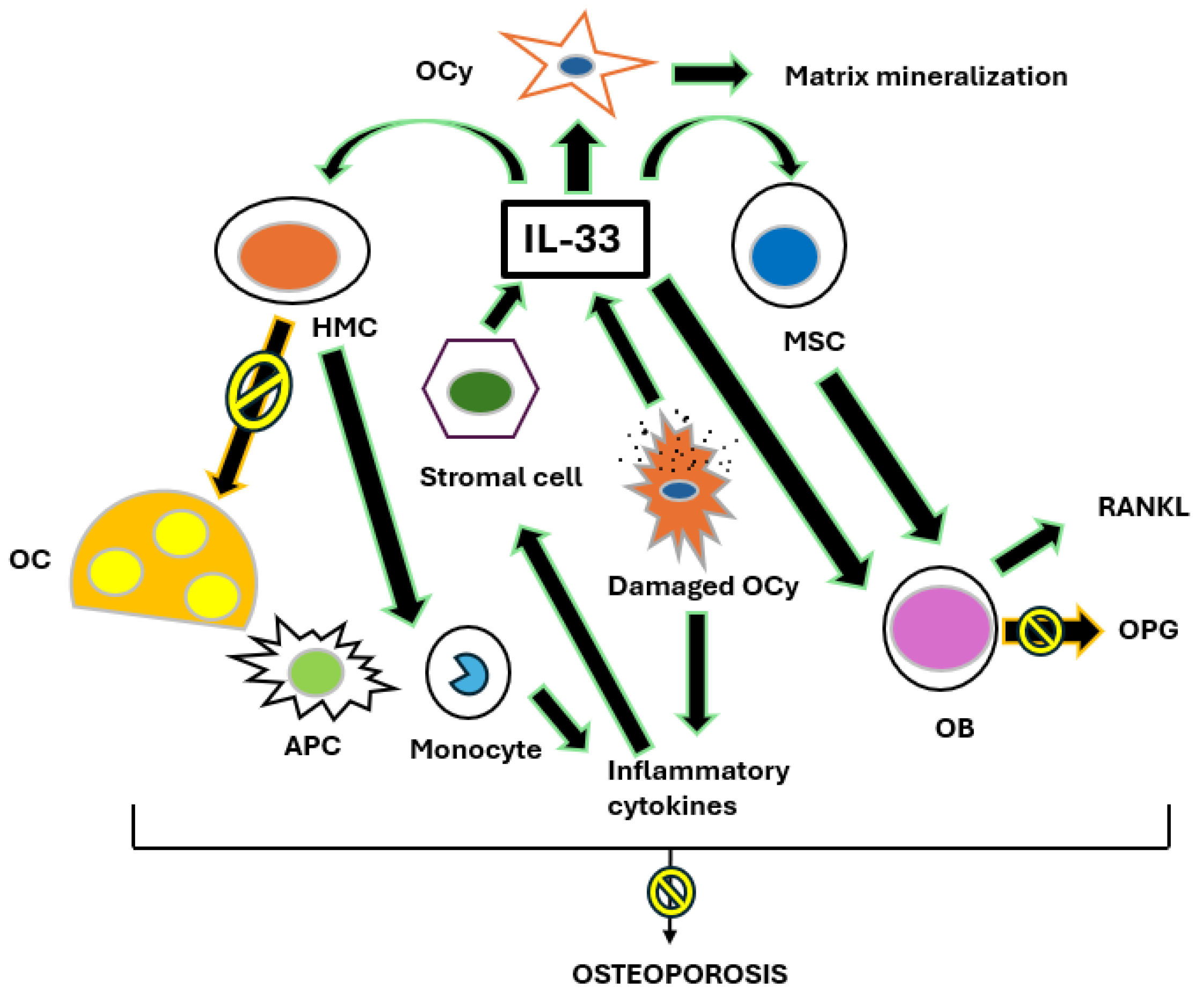
2.1. Osteoporosis and the IL-31/33 Axis
2.2. Overview of Available OCS and Conversion of Doses of OCS
2.3. The Impact of Severe Asthma Biologics in Sparing Steroid Therapy
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2024. Update May 2024. Available online: http://www.ginasthma.org/ (accessed on 31 December 2024).
- Kearney, D.M.; Lockey, R.F. Osteoporosis and asthma. Ann. Allergy Asthma Immunol. 2006, 96, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Chalitsios, C.V.; Shaw, D.E.; McKeever, T.M. Corticosteroids and bone health in people with asthma: A systematic review and meta-analysis. Respir. Med. 2021, 181, 106374. [Google Scholar] [CrossRef] [PubMed]
- Chalitsios, C.V.; McKeever, T.M.; Shaw, D.E. Incidence of osteoporosis and fragility fractures in asthma: A UK population-based matched cohort study. Eur. Respir. J. 2021, 57, 2001251. [Google Scholar] [CrossRef] [PubMed]
- Heffler, E.; Madeira, L.N.G.; Ferrando, M.; Puggioni, F.; Racca, F.; Malvezzi, L.; Passalacqua, G.; Canonica, G.W. Inhaled Corticosteroids Safety and Adverse Effects in Patients with Asthma. J. Allergy Clin. Immunol. Pract. 2018, 6, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Phillips, P.J.; Heller, R.F. Asthma and chronic obstructive airway diseases are associated with osteoporosis and fractures: A literature review. Respirology 1999, 4, 101–109. [Google Scholar] [CrossRef]
- Eastell, R. Management of corticosteroid-induced osteoporosis. UK Consensus Group. Meeting on Osteoporosis. J. Intern. Med. 1995, 237, 439–447. [Google Scholar] [CrossRef]
- Woodcock, A. Effects of inhaled corticosteroids on bone density and metabolism. J. Allergy Clin. Immunol. 1998, 101, S456–S459. [Google Scholar] [CrossRef]
- Chalitsios, C.V.; Shaw, D.E.; McKeever, T.M. Risk of osteoporosis and fragility fractures in asthma due to oral and inhaled corticosteroids: Two population-based nested case-control studies. Thorax 2021, 76, 21–28. [Google Scholar] [CrossRef]
- Cardet, J.C.; Bulkhi, A.A.; Lockey, R.F. Nonrespiratory Comorbidities in Asthma. J. Allergy Clin. Immunol. Pract. 2021, 9, 3887–3897. [Google Scholar] [CrossRef]
- Weare-Regales, N.; Hudey, S.N.; Lockey, R.F. Practical Guidance for Prevention and Management of Glucocorticoid-Induced Osteoporosis for the Allergist/Immunologist. J. Allergy Clin. Immunol. Pract. 2021, 9, 1841–1850. [Google Scholar] [CrossRef]
- Carpaij, O.A.; van den Berge, M. The asthma-obesity relationship: Underlying mechanisms and treatment implications. Curr. Opin. Pulm. Med. 2018, 24, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Maslan, J.; Mims, J.W. What is asthma? Pathophysiology, demographics, and health care costs. Otolaryngol. Clin. N. Am. 2014, 47, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Domingo, C.; Sogo, A.; Casado, E.; Martinez-Moragon, E.; Blanco-Aparicio, M.; Carrillo, T. Potential impact of mepolizumab in stepping down anti-osteporotic treatment in corticosteroid-dependent asthma. Front. Pharmacol. 2023, 14, 1183156. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.; Guyatt, G.; Fink, H.A.; Cannon, M.; Grossman, J.; Hansen, K.E.; Humphrey, M.B.; Lane, N.E.; Magrey, M.; Miller, M.; et al. American college of rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Rheumatol. 2017, 69, 1521–1537. [Google Scholar] [CrossRef]
- Chen, W.; Tran, T.N.; Sadatsafavi, M.; Murray, R.; Wong, N.C.B.; Ali, N.; Ariti, C.; Bulathsinhala, L.; Gil, E.G.; Fitzgerald, J.M.; et al. Impact of Initiating Biologics in Patients with Severe Asthma on Long-Term Oral Corticosteroids or Frequent Rescue Steroids (GLITTER): Data From the International Severe Asthma Registry. J. Allergy Clin. Immunol. Pract. 2023, 11, 2732–2747. [Google Scholar] [CrossRef]
- González-Barcala, F.J.; Muñoz-Gall, X.; Mariscal, E.; García, A.; Yang, S.; van de Wetering, G.; Izquierdo-Alonso, J.L. Cost-effectiveness analysis of anti-IL-5 therapies of severe eosinophilic asthma in Spain. J. Med. Econ. 2021, 24, 874–882. [Google Scholar] [CrossRef]
- Virchow, J.C.; Barnes, P.J. Asthma. Semin. Respir. Crit. Care Med. 2012, 33, 577. [Google Scholar]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef]
- Barnes, P.J.; Adcock, I.M. How do corticosteroids work in asthma? Ann. Intern. Med. 2003, 139, 359–370. [Google Scholar] [CrossRef]
- Ginaldi, L.; De Martinis, M.; Ciccarelli, F.; Saitta, S.; Imbesi, S.; Mannucci, C.; Gangemi, S. Increasedlevels of interleukin 31 (IL-31) in osteoporosis. BMC Immunol. 2015, 16, 60. [Google Scholar] [CrossRef]
- De Martinis, M.; Sirufo, M.M.; Suppa, M.; Ginaldi, L. IL-33/IL-31 Axis in Osteoporosis. Int. J. Mol. Sci. 2020, 21, 1239. [Google Scholar] [CrossRef] [PubMed]
- Sirufo, M.M.; Suppa, M.; Ginaldi, L.; De Martinis, M. Does Allergy Break Bones? Osteoporosis and Its Connection to Allergy. Int. J. Mol. Sci. 2020, 21, 712. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Romanelli, G.; Grassi, V. Glucocorticoid-induced osteoporosis in asthma and respiratory diseases. Front. Horm. Res. 2002, 30, 86–93. [Google Scholar] [PubMed]
- Barnes, P.J. Pathophysiology of asthma. Br. J. Clin. Pharmacol. 1996, 42, 3–10. [Google Scholar] [CrossRef]
- Chung, K.F.; Barnes, P.J. Role of inflammatory mediators in asthma. Br. Med. Bull. 1992, 48, 135–148. [Google Scholar] [CrossRef]
- Parente, L. Deflazacort: Therapeutic index, relative potency and equivalent doses versus other corticosteroids. BMC Pharmacol. Toxicol. 2017, 18, 1. [Google Scholar] [CrossRef]
- Cortisone. Summary of Product Characteristics. Available online: https://it.wikipedia.org/wiki/Cortisone (accessed on 31 December 2024).
- Hydrocortisone. Summary of Product Characteristics. Available online: https://it.wikipedia.org/wiki/Idrocortisone (accessed on 31 December 2024).
- Deflazacort. Summary of Product Characteristics. Available online: https://it.wikipedia.org/wiki/Deflazacort (accessed on 31 December 2024).
- Prednisolone. Summary of Product Characteristics. Available online: https://it.wikipedia.org/wiki/Prednisolone (accessed on 31 December 2024).
- Prednisone. Summary of Product Characteristics. Available online: https://it.wikipedia.org/wiki/Prednisone (accessed on 31 December 2024).
- Methylprednisolone. Summary of Product Characteristics. Available online: https://it.wikipedia.org/wiki/Metilprednisolone (accessed on 31 December 2024).
- Triamcinolone. Summary of Product Characteristics. Available online: https://it.wikipedia.org/wiki/Triamcinolone_acetonide (accessed on 31 December 2024).
- Betamethasone. Summary of Product Characteristics. Available online: https://it.wikipedia.org/wiki/Betametasone (accessed on 31 December 2024).
- Dexamethasone. Summary of Product Characteristics. Available online: https://it.wikipedia.org/wiki/Desametasone (accessed on 31 December 2024).
- Chung, K.F.; Wenzel, S.E.; Brozek, J.L.; Bush, A.; Castro, M.; Sterk, P.J.; Adcock, I.M.; Bateman, E.D.; Bel, E.H.; Bleecker, E.R.; et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur. Respir. J. 2014, 43, 343–373. [Google Scholar] [CrossRef]
- Price, D.B.; Trudo, F.; Voorham, J.; Xu, X.; Kerkhof, M.; Ling Zhi Jie, J.; Tran, T.N. Adverse outcomes from initiation of systemic corticosteroids for asthma: Long-term observational study. J. Asthma Allergy 2018, 11, 193–204. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The cytokines of asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Teach, S.J.; Gill, M.A.; Togias, A.; Sorkness, C.A.; Arbes, S.J., Jr.; Calatroni, A.; Wildfire, J.J.; Gergen, P.J.; Cohen, R.T.; Pongracic, J.A.; et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J. Allergy Clin. Immunol. 2015, 136, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Kozawa, M.; Yoshisue, H.; Milligan, K.L.; Nagasaki, M.; Sasajima, T.; Miyamoto, T.; Ohta, K. Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: A long-term post-marketing study in Japan. Respir. Med. 2018, 141, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.; Platts-Mills, T.A.; Finegold, I.; Schwartz, L.B.; Simons, F.E.R.; Wallace, D.V. American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma and Immunology joint task force report on omalizumab-associated anaphylaxis. J. Allergy Clin. Immunol. 2007, 120, 1373–1377. [Google Scholar] [PubMed]
- Smith, D.A.; Minthorn, E.A.; Beerahee, M. Pharmacokinetics and pharmacodynamics of mepolizumab, an anti-interleukin-5 monoclonal antibody. Clin. Pharmacokinet. 2011, 50, 215–227. [Google Scholar] [CrossRef]
- Flood-Page, P.T.; Menzies-Gow, A.N.; Kay, A.B.; Robinson, D.S. Eosinophil’s role remains uncertain as anti–interleukin-5 only partially depletes numbers in asthmatic airway. Am. J. Respir. Crit. Care Med. 2003, 167, 199–204. [Google Scholar] [CrossRef]
- Ortega, H.G.; Liu, M.C.; Pavord, I.D.; Brusselle, G.G.; FitzGerald, J.M.; Chetta, A.; Humbert, M.; Katz, L.E.; Keene, O.N.; MENSA Investigators; et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1198–1207. [Google Scholar] [CrossRef]
- Bel, E.H.; Wenzel, S.E.; Thompson, P.J.; Prazma, C.M.; Keene, O.N.; Yancey, S.W.; Ortega, H.G.; Pavord, I.D. SIRIUS Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N. Engl. J. Med. 2014, 371, 1189–1197. [Google Scholar] [CrossRef]
- Kavanagh, J.E.; d’Ancona, G.; Elstad, M.; Green, L.; Fernandes, M.; Thomson, L.; Roxas, C.; Dhariwal, J.; Nanzer, A.M.; Kent, B.D.; et al. Real-world effectiveness and the characteristics of a ‘super-responder’ to mepolizumab in severe eosinophilic asthma. Chest 2020, 158, 491–500. [Google Scholar] [CrossRef]
- Ghazi, A.; Trikha, A.; Calhoun, W.J. Benralizumab—A humanized mAb to IL-5Rα with enhanced antibody-dependent cell-mediated cytotoxicity—A novel approach for the treatment of asthma. Expert. Opin. Biol. Ther. 2012, 12, 113–118. [Google Scholar] [CrossRef]
- Kolbeck, R.; Kozhich, A.; Koike, M.; Peng, L.; Andersson, C.K.; Damschroder, M.M.; Reed, J.L.; Woods, R.; Dall’acqua, W.W.; Stephens, G.L. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J. Allergy Clin. Immunol. 2010, 125, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldamn, M.; et al. Oral glucocorticoid–sparing effect of benralizumab in severe asthma. N. Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Gurnell, M.; Heaney, L.G.; Corren, J.; Bel, E.H.; Maspero, J.; Harrison, T.; Jackson, D.J.; Price, D.; Lugogo, N.; et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): A multicentre, open-label, single-arm study. Lancet Respir. Med. 2022, 10, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Nair, P.; Brusselle, G.; Maspero, J.F.; Castro, M.; Sher, L.; Zhu, H.; Hamilton, J.D.; Swanson, B.N.; Khan, A.; et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N. Engl. J. Med. 2018, 378, 2475–2485. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, J.E.; Hearn, A.P.; Jackson, D.J. A pragmatic guide to choosing biologic therapies in severe asthma. Breathe 2021, 17, 210144. [Google Scholar] [CrossRef]
- Ziegler, S.F.; Roan, F.; Bell, B.D.; Stoklasek, T.A.; Kitajima, M.; Han, H. The biology of thymic stromal lymphopoietin (TSLP). Adv. Pharmacol. 2013, 66, 129–155. [Google Scholar]
- Ying, S.; O’Connor, B.; Ratoff, J.; Meng, Q.; Mallett, K.; Cousins, D.; Robinson, D.; Zhang, G.; Lee, T.H.; Corrigan, C. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005, 174, 8183–8190. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Colice, G.; Griffiths, J.M.; Almqvist, G.; Skärby, T.; Piechowiak, T.; Kaur, P.; Bowen, K.; Hellqvist, A.; Mo, M.; et al. SOURCE: A phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir. Res. 2020, 21, 264. [Google Scholar] [CrossRef]
- AstraZeneca. Update on SOURCE Phase III Trial for Tezepelumab in Patients with Severe, Oral Corticosteroid-Dependent Asthma. Updated: 22 December 2020. Available online: https://www.astrazeneca.com/media-centre/press-releases/2020/update-on-source-phase-iii-trial-for-tezepelumab-in-patients-with-severe-oral-corticosteroid-dependent-asthma.html (accessed on 14 September 2021).
- MA, Y.; Qiu, S.; Zhou, R. Osteoporosis in Patients With Respiratory Diseases. Front. Physiol. 2022, 13, 939253. [Google Scholar] [CrossRef]
- Naranjo Hernández, A.; Díaz Del Campo Fontecha, P.; Aguado Acín, M.P.; Arboleya Rodríguez, L.; Casado Burgos, E.; Castañeda, S.; Arestè, J.F.; Gifre, L.; Gòmez Vaquero, C.; Rodrìguez, G.C.; et al. Recommendations by the Spanish society of rheumatology on osteoporosis. Reumatol. Clin. Engl. 2019, 15, 188–210. [Google Scholar] [CrossRef]
- McDowell, P.J.; Stone, J.H.; Zhang, Y.; Honeyford, K.; Dunn, L.; Logan, R.J.; Butler, C.A.; McGarvey, L.P.A.; Heaney, L.G. Quantification of glucocorticoid-associated morbidity in severe asthma using the Glucocorticoid Toxicity Index. J. Allergy Clin. Immunol. Pract. 2021, 9, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Portacci, A.; Dragonieri, S.; Carpagnano, G.E. Super-Responders to Biologic Treatment in Type 2-High Severe Asthma: Passing Fad or a Meaningful Phenotype? J. Allergy Clin. Immunol. Pract. 2023, 11, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Caminati, M.; Vaia, R.; Furci, F.; Guarnieri, G.; Senna, G. Uncontrolled Asthma: Unmet Needs in the Management of Patients. J Asthma. Allergy 2021, 14, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Cutroneo, P.M.; Arzeton, E.; Furci, F.; Scapini, F.; Bulzomì, M.; Luxi, N.; Caminati, M.; Senna, G.; Moretti, U.; Trifirò, G. Safety of Biological Therapies for Severe Asthma: An Analysis of Suspected Adverse Reactions Reported in the WHO Pharmacovigilance Database. BioDrugs 2024, 38, 425–448. [Google Scholar] [CrossRef]
- Canonica, G.W.; Blasi, F.; Paggiaro, P.; Senna, G.; Passalacqua, G.; Spanevello, A.; Aliberti, S.; Bagnasco, D.; Bonavia, M.; Bonini, M.; et al. Oral CorticoSteroid sparing with biologics in severe asthma: A remark of the Severe Asthma Network in Italy (SANI). World Allergy Organ. J. 2020, 13, 100464. [Google Scholar] [CrossRef]
- Perlato, M.; Mecheri, V.; Vivarelli, E.; Accinno, M.; Vultaggio, A.; Matucci, A. “Patient remodeling” as a consequence of uncontrolled and prolonged OCS use in severe asthma: How biologic therapy can reverse a dangerous trend. J. Asthma. 2024, 61, 72–75. [Google Scholar] [CrossRef]
- McDonald, V.M.; Harrington, J.; Clark, V.L.; Gibson, P.G. Multidisciplinary care in chronic airway diseases: The Newcastle model. ERJ Open Res. 2022, 8, 00215–02022. [Google Scholar] [CrossRef]
- Caminati, M.; Cegolon, L.; Bacchini, M.; Segala, N.; Dama, A.; Bovo, C.; Olivieri, B.; Furci, F.; Senna, G. The potential role of local pharmacies to assess asthma control: An Italian cross-sectional study. BMC Public Health 2021, 21, 19. [Google Scholar] [CrossRef]
- Denton, E.; Lee, J.; Tay, T.; Radhakrishna, N.; Hore-Lacy, F.; Mackay, A.; Hoy, R.; Dabscheck, E.; O’Hehir, R.E.; Hew, M. Systematic assessment for difficult and severe asthma improves outcomes and halves oral corticosteroid burden independent of monoclonal biologic use. J. Allergy Clin. Immunol. Pract. 2020, 8, 1616–1624. [Google Scholar] [CrossRef]
- Clark, V.L.; Gibson, P.G.; Genn, G.; Hiles, S.A.; Pavord, I.D.; McDonald, V.M. Multidimensional assessment of severe asthma: A systematic review and meta-analysis. Respirology 2017, 22, 1262–1275. [Google Scholar] [CrossRef]
| Oral Corticosteroids (OCS) | Approximate Equivalent Dose (mg) |
|---|---|
| Cortisone | 25 mg |
| Hydrocortisone | 20 mg |
| Deflazacort | 7.5 mg |
| Prednisolone | 5 mg |
| Prednisone | 5 mg |
| Methylprednisolone | 4 mg |
| Triamcinolone | 4 mg |
| Betamethasone | 0.75 mg |
| Dexamethasone | 0.75 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furci, F.; Scaramozzino, M.U.; Talesa, G.R.; Pelaia, C. Connections and Unmet Needs: Severe Asthma Biologics and Osteoporosis. Biomedicines 2025, 13, 197. https://doi.org/10.3390/biomedicines13010197
Furci F, Scaramozzino MU, Talesa GR, Pelaia C. Connections and Unmet Needs: Severe Asthma Biologics and Osteoporosis. Biomedicines. 2025; 13(1):197. https://doi.org/10.3390/biomedicines13010197
Chicago/Turabian StyleFurci, Fabiana, Marco Umberto Scaramozzino, Giuseppe Rocco Talesa, and Corrado Pelaia. 2025. "Connections and Unmet Needs: Severe Asthma Biologics and Osteoporosis" Biomedicines 13, no. 1: 197. https://doi.org/10.3390/biomedicines13010197
APA StyleFurci, F., Scaramozzino, M. U., Talesa, G. R., & Pelaia, C. (2025). Connections and Unmet Needs: Severe Asthma Biologics and Osteoporosis. Biomedicines, 13(1), 197. https://doi.org/10.3390/biomedicines13010197






