Abstract
Promoting rapid healing is a concern in skin wound treatment, as the increased pain and the loss of functional ability when wounds become chronic create a complex problem to manage. This scoping review aimed to explore the literature and synthesize existing knowledge on the therapeutic use of CO2 in treating cutaneous wounds. The literature was selected using previously defined inclusion and exclusion criteria, and 22 articles were selected for data extraction. The most researched type of injury was chronic wounds located on the extremities of the limbs. Carboxytherapy was performed in five different ways: subcutaneous, intradermal, or intralesional injections; in hot water baths with temperatures ranging from 30 to 42 °C; transcutaneous application; intra-abdominal insufflation; and a paste for transcutaneous local application. The main effects of CO2 therapy described were as follows: improved blood flow and local oxygenation, reduction of the inflammatory process, increased collagen production, and improved clinical aspects of wounds, with faster healing. Carboxytherapy can be considered a good alternative for treating skin wounds, although further studies should be pursued to elucidate its molecular mechanisms and enhance its efficacy.
1. Introduction
Chronic or difficult-to-heal wounds significantly impact health systems, and the primary wound-related healthcare resources include hospital outpatient visits, practice nurse visits, hospitalizations, healthcare assistants home visits, laboratory tests, medications, and dressing materials [1,2]. The quality of life of affected individuals is also impacted in terms of function, mobility, daily activities, and mental health [3]. In veterinary medicine, they are also a frequent cause of euthanasia [4].
The wound healing process is complex, involving four overlapping phases: hemostasis, inflammation, proliferation, and maturation. Chronic wounds are those in which the healing process does not occur normally, either in time or in the sequence of phases. They are more common on the extremities but are also associated with other co-morbidities [5].
The therapeutic application of gases has been used in wound treatment, including oxygen (O2), hyperbaric oxygen (HBO), nitric oxide (NO), hydrogen sulfide (H2S), ozone (O3), carbon monoxide (CO), and carbon dioxide (CO2) [6]. The latter has been used in different administration forms, such as bathing in water enriched with CO2, transdermal administration, and subcutaneous injection [7].
Currently, CO2 therapy is attracting attention in various fields, such as health [8], beauty and well-being [9], and sports [10], due to the therapeutic effects attributed to an increase in the partial pressure of oxygen in the local area, known as the Bohr effect, where elevated CO2 levels lower the blood’s affinity for oxygen, facilitating its release into tissues. However, the detailed mechanism of these therapeutic effects is not yet completely understood [11]. Exploring CO2’s effect on critical processes, such as endothelial cell function, fibroblast proliferation, and immune cell activity, could provide mechanistic insights that not only enhance our understanding but also optimize its application in clinical wound healing.
A scoping review is a specific type of review that uses a systematic and well-defined method to identify and synthesize existing literature on a given topic [12]. This study aimed to explore the literature and provide a scoping review of current knowledge resulting from research and clinical application of CO2 in the treatment of skin wounds. We hypothesize that CO2 therapy may help treat cutaneous wounds, especially chronic wounds associated with circulatory disorders. From a scoping review, it is possible to understand what exists in the literature on this topic and identify knowledge gaps to direct future research.
2. Materials and Methods
This scoping review was prepared according to the framework described by Arksey and O’Malley (2005) [13], following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Scoping Reviews (PRISMA-ScR) [14], and the methodological updates for scoping reviews [15].
2.1. Identifying the Research Question
“What do we know about the therapeutic use of carbon dioxide in skin wound management?” was the research question proposed for this scoping review.
2.2. Identifying Relevant Studies
The search strategy used was as follows: skin wound AND (healing OR treatment OR management) AND (CO2 OR carbon dioxide) NOT laser. Search filter was used for languages (English, French, Spanish, and Portuguese). The search was performed on July 2024, and the databases used were Pubmed, Web of Science, and Scopus.
2.3. Study Selection
All articles found in the three databases were imported into Ryyan software (https://rayyan.qcri.org/welcome, accessed on 5 July 2024). Mak and Thomas (2022) [16] recommended this software as a good tool to identify duplicates and help in the first screening level, allowing researchers to analyze articles individually and blindly.
Studies describing the therapeutic use of carbon dioxide in treating skin wounds were included. Articles selected could have addressed protocols for all types of wounds, and any form of carboxytherapy application, in all species, in vivo or in vitro. The types of articles included were as follows: experimental, case reports and case series, and retrospective.
The research addressing aesthetic purposes such as rejuvenation or wrinkle treatment were excluded. CO2 laser treatments were also excluded.
The articles underwent two stages of screening and one of data extraction. The first screening was performed by analyzing the titles and abstracts, and the second by reading the articles in full. Two researchers performed screening and data extraction independently and resolved conflicts by consensus.
2.4. Charting the Data
Two researchers independently reviewed the full articles and extracted the following data:
- -
- Authors and date;
- -
- Manuscript language;
- -
- Study location;
- -
- Study design;
- -
- Participant count;
- -
- Species;
- -
- Type of wound;
- -
- Wound location;
- -
- Treatments (protocol details);
- -
- Outcomes measured;
- -
- Results (intervention effects).
2.5. Collating, Summarizing, and Reporting Data
The extracted data were presented descriptively and in tables. When appropriate, figures were also used to summarize the data.
3. Results
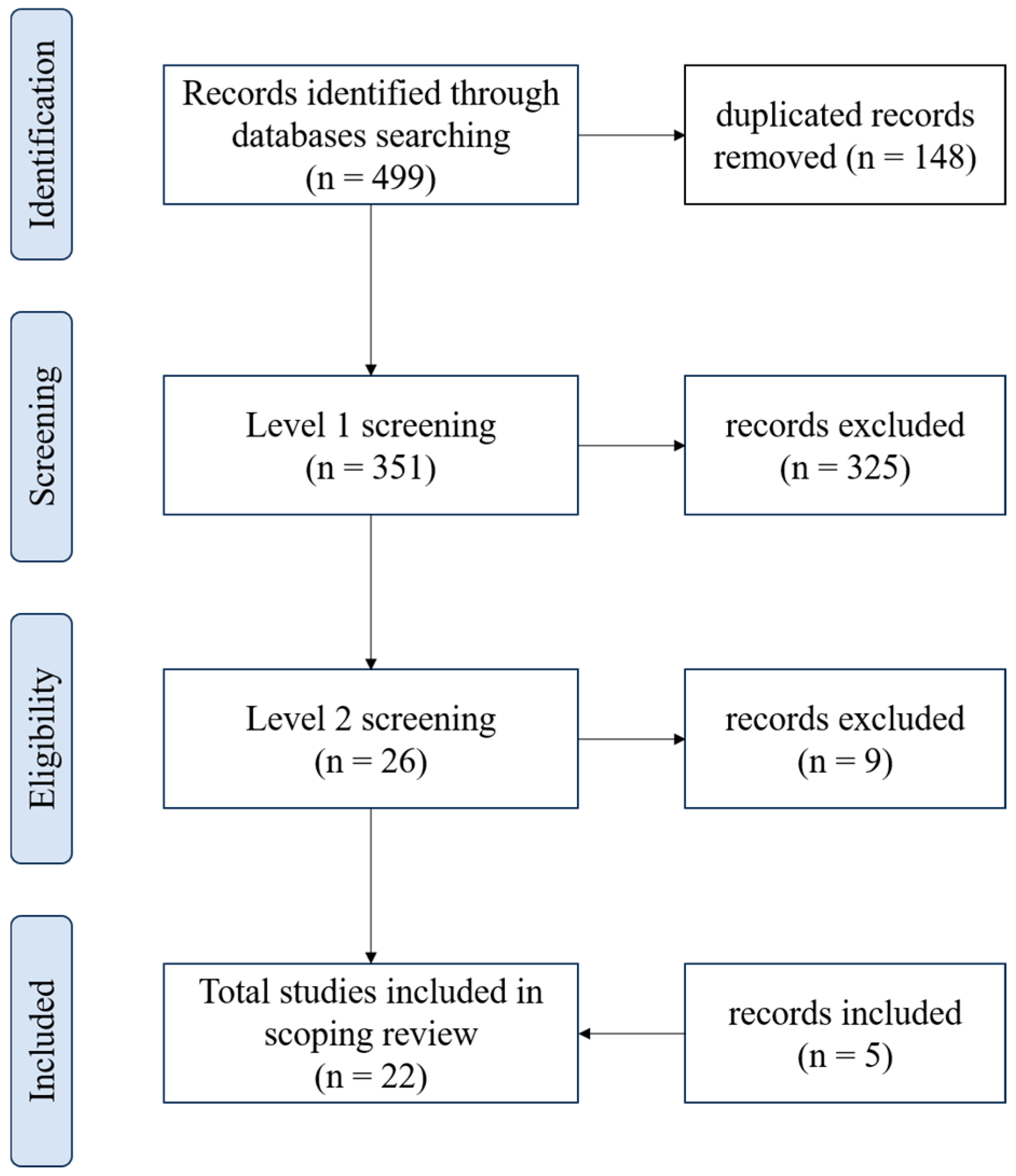
The results of the database search and the flow of articles through the selection process for inclusion in the scoping review are presented in Figure 1. Four hundred ninety-nine papers were found (Pubmed: 201, Scopus: 183, and Web of Science: 115) and 148 were duplicated. After the stage 1 screening, 26 articles were identified as potentially relevant, and 9 of these records were excluded at level 2 because they did not exactly address the use of CO2 for treating skin wounds. Five studies not found in the initial search but which met the eligibility criteria were included.
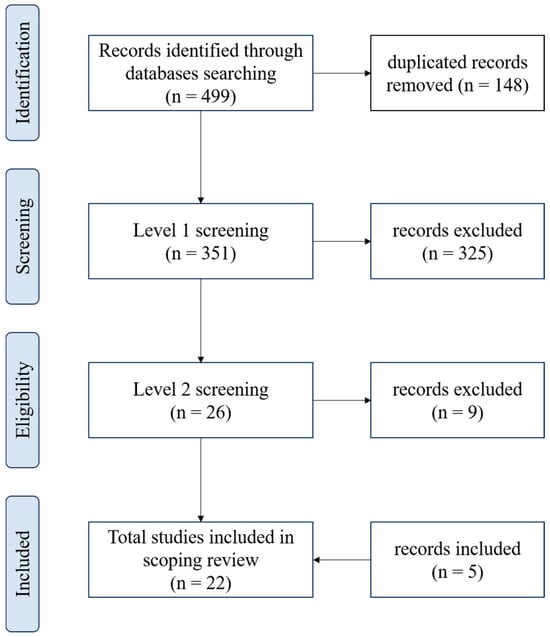
Figure 1.
Flowchart showing the process of selecting articles included in the scoping review, according to PRISMA.
The 22 articles eligible for the scoping review were published between 2000–2024, and the number of articles per year was as follows: 2000 (1), 2003 (1), 2004 (1), 2008 (1), 2010 (1), 2011 (1), 2012 (1), 2013 (2), 2015 (2), 2018 (2), 2020 (4), 2021 (1), 2022 (1), 2023 (2), and 2024 (1).
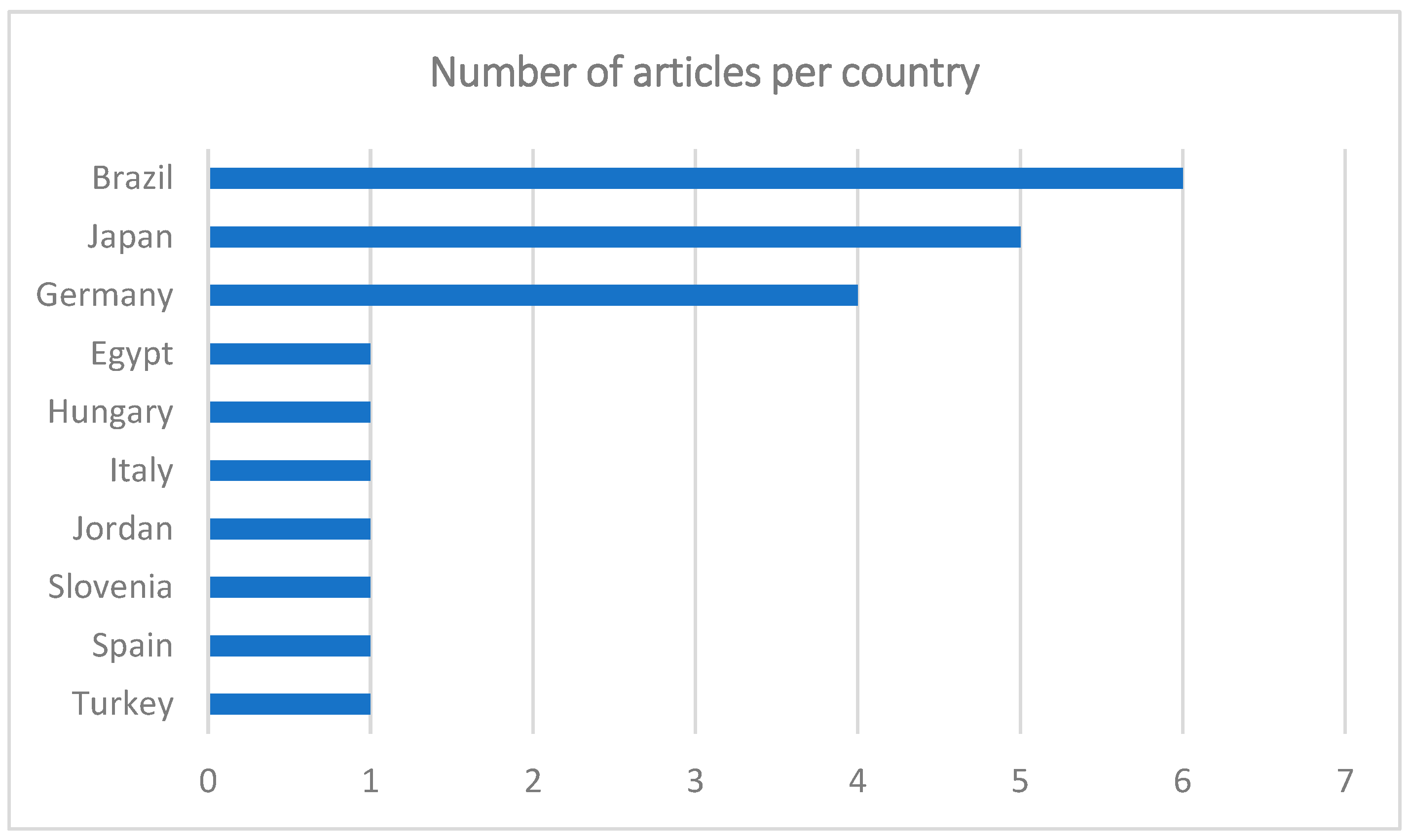
The greatest number of publications was from Brazil (n = 6), followed by Japan (n = 5), Germany (n = 4), Egypt, Hungary, Italy, Jordan, Slovenia, Spain, and Turkey (n = 1) (Figure 2). Most of the articles were in English (n = 21), only one in Portuguese, and none in French or Spanish. Although the articles are written in English, the lack of contributions from areas such as North America or the United Kingdom is curious.
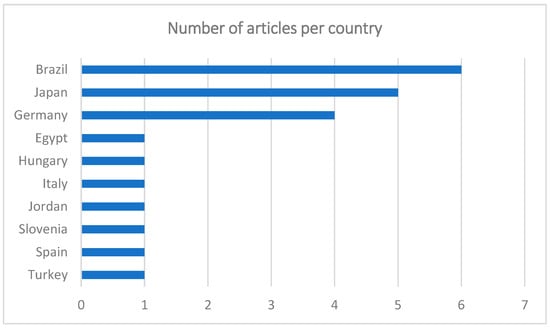
Figure 2.
Graph showing the number of articles per country.
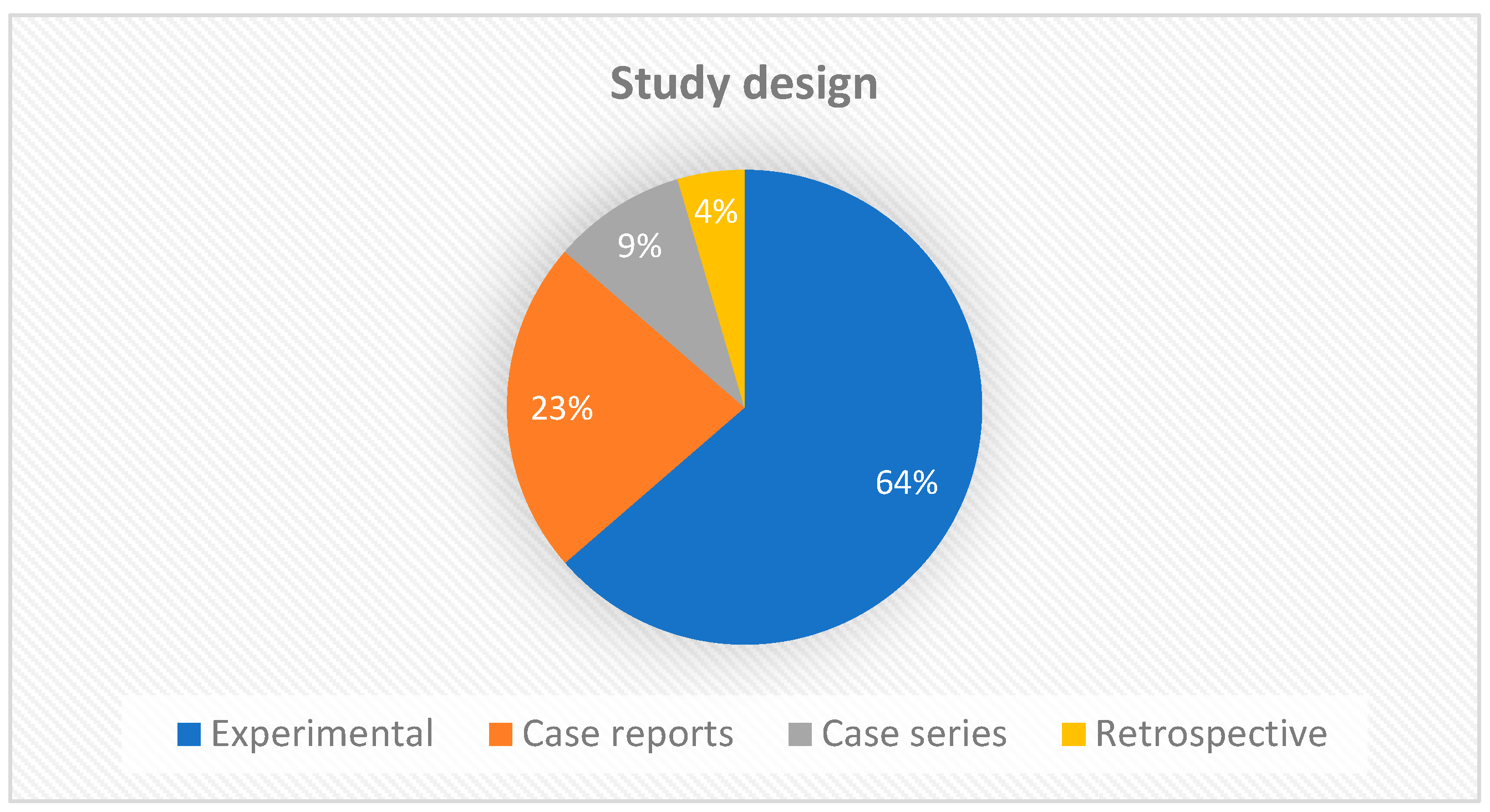
Regarding the study design, the largest number of manuscripts were experimental studies (14/22), followed by case reports (5/22). Two articles were case series and only one was a retrospective study (Figure 3). This result is expected, since new therapies require experimental studies to better understand and standardize protocols before clinical trials can be initiated. Experimental studies add value to the strength of evidence, but clinical reports also provide constraints and clues for further investigations.
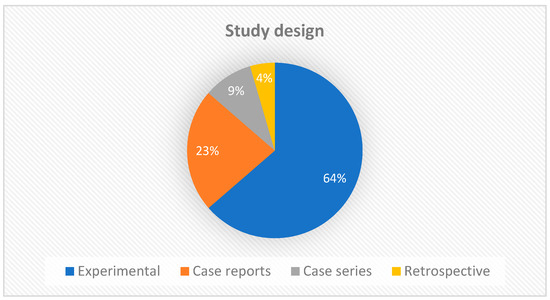
Figure 3.
Graph showing the types of studies included in the scoping review.
The species studied in the included manuscripts were humans (14/22), rats (6/22), mice (1/22), and rabbits (1/22). The case reports, case series, and retrospective study analyzed injuries in humans. Of the 14 experimental studies, 7 analyzed injuries in rats, 6 in humans, 1 in mice, and 1 in rabbits. The average number of individuals participating in experimental studies was 45, with a minimum number of 9 and a maximum of 96 participants.
The anatomical location and type of injury varied between manuscripts. In experimental studies conducted on humans, the most researched type of injury was chronic wounds located at the extremities of the limbs. In experimental research with rats, the type of skin injury was incisional or made with a Punch and the most common region was the back. Among case reports and case series, ulcers on limbs also prevailed, followed by heat press injury. The retrospective study compared chronic wounds and acute surgical wounds, in different areas.
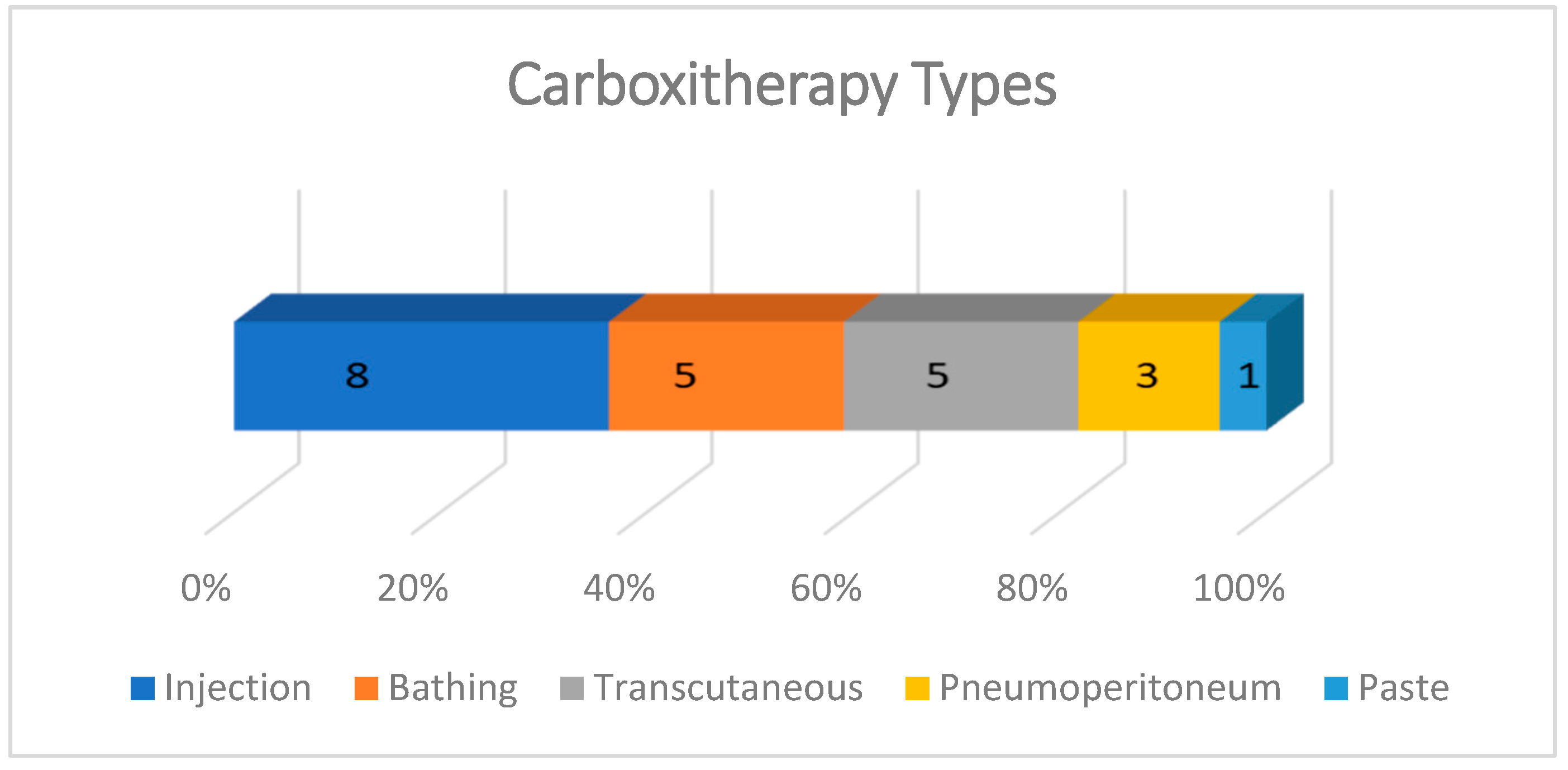
Carboxytherapy was performed in five different ways: subcutaneous, intradermal, or intralesional injections; in hot water baths with temperatures ranging from 30 to 42 °C; transcutaneous application; intra-abdominal insufflation; and a paste for transcutaneous local application. The most frequent form of administration was injectable, described in 8/22 studies, almost all experimental. In case reports, the most common carboxytherapy was through bathing (5/22) and transcutaneous administration (5/22). The effect of CO2 pneumoperitoneum on skin wound healing was evaluated in three experimental studies and the use of CO2 paste was described in one study (Figure 4.)
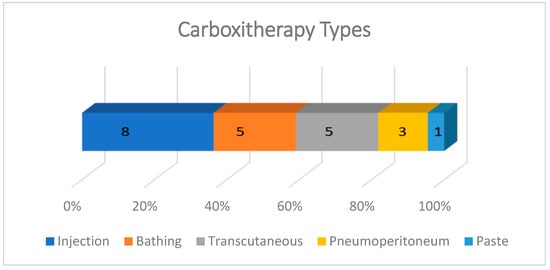
Figure 4.
Graph showing the types of carboxytherapy described in studies included in the scoping review. The most frequent forms of application were injection, bathing, and transcutaneous. The intraperitoneal route was used only in experiments with rats involving laparotomy, and the CO2-rich paste was tested in only one experimental study with rats.
The “outcomes measured” indicate which tools were used in the studies to assess the effectiveness of the treatment performed. Among the manuscripts included in this review, clinical evaluation was the main tool used. As a clinical evaluation, we included all macroscopic evaluations of the wounds, such as the size, color, presence of abscess and foreign body, evaluation of the appearance of the wound bed, sensation, and patient satisfaction. Of the 22 articles analyzed, 9 used only clinical evaluation to validate the effectiveness of the treatment described. These articles included all case reports, case series, and retrospective study, and only one experimental study. In addition to clinical evaluation, all other experimental studies used additionally tools such as histological and molecular exams, blood flow by a Doppler flowmeter, and the tensile strength of the healing wound.
Regarding the results obtained in the studies included in this review, Table 1 summarizes the data extracted. In general, the manuscripts presented good results with the use of CO2 for the treatment of skin wounds, regardless of the route of administration. Studies that evaluated the effects of the pneumoperitoneum reported that the use of CO2 does not interfere with the healing process of the abdominal wall or distant injuries (such as the back). One of these studies compared abdominal insufflation with CO2, helium, and air, finding beneficial effects on healing with helium pneumoperitoneum.

Table 1.
Summary of data extracted from the 22 articles included in the scoping review about the effects of carboxytherapy on skin wound healing.
4. Discussion
Carbon dioxide therapy refers to CO2 administration for therapeutic purposes, and, to produce the desired effects, an adequate amount of CO2 needs to be delivered to local tissues without difficulty and invasion. Three methods of CO2 administration that were able to accomplish this are as follows: bathing in CO2-enriched water, direct subcutaneous CO2 injection, and the transcutaneous administration of CO2 [7].
According to Frangež et al. (2021) [39], the role of natural water rich in CO2 is known for its positive effects on wound healing and it is used more frequently than gaseous CO2. However, the form of medical application has been modified in recent decades, as the transcutaneous application of gaseous CO2 avoids hydration of the wound and the inhalation of CO2 evaporated from water. All case reports included in this review showed positive effects with the use of carboxytherapy, both in the form of bathing [24,25,26] and transcutaneous application [27,37,38]. The evaluation of the treatment was carried out clinically and the main effects found were as follows: the removal of necrotic tissue, an improvement in granulation, a reduction in secretion, and bad odor.
The majority of experimental studies included in this review used injection as a form of CO2 administration. Subcutaneous CO2 injection, although it can provide pure CO2 in local tissues, is an invasive method, that involves the risk of infection, and it is difficult to use over a large area of the body [7]. Additionally, some patients found the treatments quite painful [36]. In addition to the improvement in clinical aspects described in case reports, experimental studies observed significantly faster healing in wounds treated with CO2 compared to control groups. Faster healing was observed with hot spring water rich in carbonate ion [28], with transcutaneous application of CO2 gas [29], and also with a CO2 paste covering the wound [19].
The improvement in clinical aspects and faster healing may be related to the better blood perfusion in wounds treated with CO2. According to Finzgar et al. (2015) [40], the inefficient healing of chronic wounds is the result of poor blood perfusion in the wound and surrounding tissues and artificially applied carbon dioxide has the potential to improve tissue perfusion and oxygenation, therefore being useful for the healing of chronic wounds. In fact, Shalan et al. (2015) [35] reported improved blood flow in the feet of patients after CO2 therapy, and Liang et al. (2015) [28] showed histologically increased vessel density in wounds treated with thermal water rich in carbonate ion compared to control groups. The significant increase in tissue oxygenation values was confirmed by the better evolution of the lesions, both in the improvement in healing and in the reduction in the injured area in the group treated with CO2 [20].
The Bohr effect is characterized by the stimulation of dissociation between oxygen and hemoglobin (Hb), causing the release of oxygen into the blood when there is an increase in the concentration of carbon dioxide; that is, when CO2 levels become high, the blood’s affinity for oxygen decreases, facilitating its release into the tissues. Near-infrared spectroscopy was used to confirm that the transcutaneous application of CO2 actually causes the dissociation of O2 from oxy-Hb, which is a characteristic phenomenon of the Bohr effect. The experimental results showed scientific evidence that the transcutaneous application of CO2 can cause an “artificial Bohr effect”. This artificial Bohr effect could be a potential new therapy for disorders in which a high amount of O2 in local tissues is required for treatment, as well as in peripheral vascular disorder [7].
In 2020, Oda et al. [41] investigated whether CO2 treatment would promote fracture repair in cases with type I Diabetes mellitus. This study showed that the gene expression levels of vascular endothelial growth factor (VEGF) in newly generated callus tissue were significantly higher in the CO2 group at all time points. Through fluorescent immunostaining with isolectin B4, the researchers also observed that angiogenesis around the fracture was stimulated in the CO2 group. Therefore, the results showed that CO2 therapy reverses the reduced levels of VEGF gene expression in the fracture region and improves angiogenesis, contributing to fracture repair. Kuroiwa et al. (2019) [11] also reported that the expression of the VEGF gene in the CO2 group was significantly higher than in the control group.
VEGF plays a critical role in wound healing by promoting angiogenesis and enhancing oxygen and nutrient delivery to hypoxic tissues. Various therapeutic approaches, such as the administration of recombinant growth factors (e.g., VEGF-A or PDGF-BB) [42,43], the use of biomaterials [44], and mesenchymal stem cell (MSC)-based therapies [45], have been developed to upregulate VEGF expression and improve tissue regeneration. Compared to these strategies, CO2 treatment offers a singular method to stimulate VEGF’s expression and enhances angiogenesis without the need for exogenous growth factors or complex interventions.
Collagen gene expression levels have also been reported to be significantly higher in the CO2 group than in the control group [41]. Oliveira et al. (2020) [32] observed an increase in collagen and elastic fibers in the group treated with a single application of carboxytherapy with an infusion rate of 100 mL/min and 0.6 mL/kg of weight in an area of 25 cm2 in the abdominal region in humans. The use of CO2 injection around grafts also increased the amount of collagen in 2 cm grafts in rabbits [23].
Some studies included in this review reported a decreased inflammatory process in wounds treated with CO2 in rats [21], and human [31]. In 2021, Sayama et al. [46] clarified the anti-inflammatory mechanism of CO2 using a UVB-induced inflammation model. The researchers observed that CO2 can decrease the production of IL-6 and TNFα in human keratinocytes and the 3D epidermis, thereby decreasing the formation of UVB-induced erythema in human skin. TNFα and IL-6 are cytokines that play essential roles in skin inflammation. Therefore, CO2 can reduce UV-induced inflammation by decreasing the production of these inflammatory cytokines.
Although further clinical studies are needed to quantify the effects of TNF-α and IL-6 suppression on specific clinical metrics, such as pain scores, wound size reduction, and edema resolution, the overall literature suggests that CO2 therapy can benefit clinical outcomes such as wound pain and edema. Firstly, studies show that CO2 therapy suppresses TNF-α and IL-6 production, thereby reducing the inflammatory burden in both animal models and human subjects [46]. Usually, elevated levels of these cytokines are associated with pain, tissue damage, and edema, as they enhance vascular permeability, recruit inflammatory cells, and amplify nociceptive signaling pathways [47,48]. The suppression of TNF-α and IL-6 in CO2 treatments correlates with clinical observations of decreased erythema, reduced swelling, and improved granulation tissue formation. Moreover, the attenuation of inflammation likely contributes to lower nociceptive signaling, as suggested by the documented improvement in patient-reported discomfort and overall wound condition in case reports [47]. Edema may also be alleviated through CO2 therapy due to its effects on tissue oxygenation, blood flow, and vascular leakage reduction, as observed in studies such as Shalan et al. (2015) [35], all of which may directly counteract edema formation and facilitate its resolution.
CO2 can permeate through the stratum corneum and promote mild extracellular acidification by reacting with H2O in the interstitial fluid and producing H+. Considering this, it was investigated whether the anti-inflammatory effects of CO2 could be associated with this change in extracellular pH. In vitro studies using human keratinocytes revealed that the CO2-induced suppression of pro-inflammatory cytokines TNF-a and IL-6 was pH-dependent. These findings suggest that extracellular pH is crucial in modulating skin inflammation [46].
Changes in the intracellular and extracellular pH can exert different physiological effects. In the skin, it is known that the pH of the stratum corneum plays an important role in several pathological conditions. It is generally maintained in an acidic range of 4.1–5.8; however, there is an increase in inflammatory skin diseases [49]. Previous research has demonstrated that changes in pH lead to the dysregulation of several skin functions, including antimicrobial defense mechanisms, barrier action, and inflammatory responses. Thus, the topical application of CO2 may enhance the stratum corneum’s barrier and antimicrobial properties while attenuating the epidermis’s excessive inflammatory reactions through CO2-induced acidification [46].
Another study [16] demonstrates that CO₂ paste promotes wound healing by modulating the hypoxic environment, reducing inflammation, and accelerating angiogenesis. Specifically, it enhanced the upregulation of growth factors such as VEGF, TGF-β, and platelet-derived growth factor (PDGF), which, in turn, stimulate angiogenesis and fibroblast proliferation. CO2 also decreased the levels of interleukins (IL-1β and IL-6), which are critical for regulating the inflammatory phase and promoting cellular recruitment. The signaling cascade involved the activation of nitric oxide synthase (NOS) and the production of nitric oxide (NO), along with downregulation of Hypoxia-Inducible Factor 1-alpha (HIF-1α), indicating an improved oxygenation status in the wound area. These pathways converge to accelerate granulation tissue formation, epithelialization, and wound closure.
Concerning TGF-β and VEGF, the literature shows they play complementary roles in distinct but interdependent phases of the process. On the one hand, TGF-β regulates the inflammatory and proliferative phases of wound healing, promoting fibroblast migration, extracellular matrix deposition, and myofibroblast differentiation necessary for wound contraction and closure [50]. On the other hand, VEGF mainly facilitates angiogenesis, increasing vascular density and oxygen supply to the wound site, which are critical for the reparative processes and epithelialization [51]. Although its contributions are well-established at the molecular level, linking them to clinical outcomes such as wound closure can be challenging as clinical outcomes are influenced by multiple factors (such as patient comorbidities, and wound type and location), but, overall, CO2 therapy has been shown to have the potential to modulate TGF-β and VEGF in a manner that aligns with physiological healing processes.
Overall, while the results of existing studies on CO2 in wound healing are promising, the current body of research remains limited. Future work should focus on elucidating the precise molecular mechanisms, particularly the interactions between CO2, inflammatory cytokines, growth factors, and hypoxia-inducible factors. Important clinical information such as the dosage, duration of exposure, and administration methods should also be further addressed to enhance efficacy and safety.
5. Conclusions
Although carboxytherapy is widely used for different purposes, the existing literature on the use of CO2 as a specific treatment for skin wounds is not that extensive. The vast majority of articles report the beneficial effects of the use of CO2 on skin healing, in all forms of use. The main effects of carboxytherapy described were the following: an improvement in blood flow and local oxygenation, a reduction in the inflammatory process, increased collagen production, and an improvement in the clinical aspects of wounds, with faster healing. Given this, the use of carboxytherapy in treating skin wounds seems very promising, and it should be studied in more depth to better understand the mechanisms of action and the effects obtained with the treatment, as well as to define the best protocol for clinical use.
Author Contributions
Conceptualization, G.R. and J.P.; methodology, G.R., A.L. and J.P.; data curation, G.R., A.L. and J.P.; writing—original draft preparation, G.R.; writing—review and editing, G.R., A.L. and J.P.; funding acquisition, G.R., A.L. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Faculty of Veterinary Medicine, Lusófona University, through the project named WOUNDHEALFAST (2023–2024).
Institutional Review Board Statement
Not applicable for review of literature.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Queen, D.; Harding, K. What’s the True Costs of Wounds Faced by Different Healthcare Systems around the World? Int. Wound J. 2023, 20, 3935–3938. [Google Scholar] [CrossRef]
- Guest, J.F.; Fuller, G.W.; Vowden, P. Cohort Study Evaluating the Burden of Wounds to the UK’s National Health Service in 2017/2018: Update from 2012/2013. BMJ Open 2020, 10, e045253. [Google Scholar] [CrossRef] [PubMed]
- Lentsck, M.H.; Baratieri, T.; Trincaus, M.R.; Mattei, A.P.; Miyahara, C.T.S. Quality of Life Related to Clinical Aspects in People with Chronic Wound. Rev. Esc. Enferm. USP 2018, 52, e03384. [Google Scholar] [CrossRef] [PubMed]
- Sivula, C.P.; Suckow, M.A. Euthanasia. In Management of Animal Care and Use Programs in Research, Education, and Testing, 2nd ed.; Weichbrod, R.H., Thompson, G.A., Norton, J.N., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2017; pp. 827–840. ISBN 978-1-315-15218-9. [Google Scholar]
- Wolny, D.; Štěpánek, L.; Horáková, D.; Thomas, J.; Zapletalová, J.; Patel, M.S. Risk Factors for Non-Healing Wounds—A Single-Centre Study. J. Clin. Med. 2024, 13, 1003. [Google Scholar] [CrossRef]
- Ding, J.; Xu, K.; Xu, H.; Ji, J.; Qian, Y.; Shen, J. Advances in Gas Therapeutics for Wound Healing: Mechanisms, Delivery Materials, and Prospects. Small Struct. 2024, 5, 2300151. [Google Scholar] [CrossRef]
- Sakai, Y.; Miwa, M.; Oe, K.; Ueha, T.; Koh, A.; Niikura, T.; Iwakura, T.; Lee, S.Y.; Tanaka, M.; Kurosaka, M. A Novel System for Transcutaneous Application of Carbon Dioxide Causing an “Artificial Bohr Effect” in the Human Body. PLoS ONE 2011, 6, e24137. [Google Scholar] [CrossRef] [PubMed]
- Pagourelias, E.D.; Zorou, P.G.; Tsaligopoulos, M.; Athyros, V.G.; Karagiannis, A.; Efthimiadis, G.K. Carbon Dioxide Balneotherapy and Cardiovascular Disease. Int. J. Biometeorol. 2011, 55, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Lippi, L.; Ferrillo, M.; Losco, L.; Folli, A.; Marcasciano, M.; Curci, C.; Moalli, S.; Ammendolia, A.; De Sire, A.; Invernizzi, M. Aesthetic Rehabilitation Medicine: Enhancing Wellbeing beyond Functional Recovery. Medicina 2024, 60, 603. [Google Scholar] [CrossRef] [PubMed]
- Ueha, T.; Oe, K.; Miwa, M.; Hasegawa, T.; Koh, A.; Nishimoto, H.; Lee, S.Y.; Niikura, T.; Kurosaka, M.; Kuroda, R.; et al. Increase in Carbon Dioxide Accelerates the Performance of Endurance Exercise in Rats. J. Physiol. Sci. 2018, 68, 463–470. [Google Scholar] [CrossRef]
- Kuroiwa, Y.; Fukui, T.; Takahara, S.; Lee, S.Y.; Oe, K.; Arakura, M.; Kumabe, Y.; Oda, T.; Matsumoto, T.; Matsushita, T.; et al. Topical Cutaneous Application of CO2 Accelerates Bone Healing in a Rat Femoral Defect Model. BMC Musculoskelet. Disord. 2019, 20, 237. [Google Scholar] [CrossRef]
- Thomas, A.; Lubarsky, S.; Durning, S.J.; Young, M.E. Knowledge Syntheses in Medical Education: Demystifying Scoping Reviews. Acad. Med. 2017, 92, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated Methodological Guidance for the Conduct of Scoping Reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Mak, S.; Thomas, A. Steps for Conducting a Scoping Review. J. Grad. Med. Educ. 2022, 14, 565–567. [Google Scholar] [CrossRef]
- Abramo, A.C.; Teixeira, T.T. Carboinsuflação em úlceras crônicas dos membros inferiores. Rev. Bras. Cir. Plástica Impresso 2011, 26, 205–210. [Google Scholar] [CrossRef]
- Agalar, F.; Hamaloglu, E.; Daphan, C.; Tarim, A.; Onur, R.; Renda, N.; Sayek, I. Effects of CO2 Insufflation and Laparotomy on Wound Healing in Mice. Aust. N. Z. J. Surg. 2000, 70, 739–742. [Google Scholar] [CrossRef]
- Amano-Iga, R.; Hasegawa, T.; Takeda, D.; Murakami, A.; Yatagai, N.; Saito, I.; Arimoto, S.; Kakei, Y.; Sakakibara, A.; Akashi, M. Local Application of Transcutaneous Carbon Dioxide Paste Decreases Inflammation and Accelerates Wound Healing. Cureus 2021, 13, e19518. [Google Scholar] [CrossRef] [PubMed]
- Brandi, C.; Grimaldi, L.; Nisi, G.; Brafa, A.; Campa, A.; Calabrò, M.; Campana, M.; D’Aniello, C. The Role of Carbon Dioxide Therapy in the Treatment of Chronic Wounds. Vivo Athens Greece 2010, 24, 223–226. [Google Scholar]
- Brochado, T.M.M.; De Carvalho Schweich, L.; Di Pietro Simões, N.; Oliveira, R.J.; Antoniolli-Silva, A.C.M.B. Carboxytherapy: Controls the Inflammation and Enhances the Production of Fibronectin on Wound Healing under Venous Insufficiency. Int. Wound J. 2019, 16, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Csonka, Á.; Gárgyán, I.; Varga, E. Multidisciplinary Treatment of a Complicated Crural Degloving Injury in a Diabetic Patient. Injury 2021, 52, S74–S77. [Google Scholar] [CrossRef] [PubMed]
- Durães, E.F.R.; Durães, L.D.C.; Carneiro, F.P.; Lino Júnior, R.D.S.; Sousa, J.B.D. The Effect of Carbon Dioxide Therapy on Composite Graft Survival. Acta Cir. Bras. 2013, 28, 589–593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hihara, M.; Fukui, M.; Mitsui, T.; Kakudo, N.; Kuro, A. Osteolytic Metatarsal Osteomyelitis Regenerated by Combined Treatment of Artificial Carbon Dioxide Foot Bathing and Povidone–Iodine Sugar Ointment: A Case Report. J. Med. Case Reports 2022, 16, 434. [Google Scholar] [CrossRef] [PubMed]
- Hihara, M.; Takeji, K.; Mitsui, T.; Kuro, A.; Kakudo, N. Functional and Cosmetic Reconstruction of Palmar Heat Press Injury Following Wound Bed Preparation Combined with Artificial Highly Concentrated Carbon Dioxide Bathing: A Case Report. Scars Burns Health 2023, 9, 20595131231213705. [Google Scholar] [CrossRef] [PubMed]
- Hihara, M.; Himejima, T.; Takeji, K.; Fujita, M.; Fukui, M.; Matsuoka, Y.; Mitsui, T.; Kuro, A.; Kakudo, N. A Novel Intervention for Wound Bed Preparation in Severe Extremity Trauma: Highly Concentrated Carbon Dioxide Bathing. JPRAS Open 2024, 41, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Hohaus, K.; Bley, B.; Köstler, E.; Schönlebe, J.; Wollina, U. Mineral Oil Granuloma of the Penis. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Kang, D.; Wang, Y.; Yu, Y.; Fan, J.; Takashi, E. Carbonate Ion-Enriched Hot Spring Water Promotes Skin Wound Healing in Nude Rats. PLoS ONE 2015, 10, e0117106. [Google Scholar] [CrossRef] [PubMed]
- Macura, M.; Ban Frangez, H.; Cankar, K.; Finžgar, M.; Frangez, I. The Effect of Transcutaneous Application of Gaseous CO2 on Diabetic Chronic Wound Healing—A Double-blind Randomized Clinical Trial. Int. Wound J. 2020, 17, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Morais, P.H.A.D.; Farias, I.E.C.D.; Durães, L.D.C.; Carneiro, F.P.; Oliveira, P.G.D.; Sousa, J.B.D. Evaluation of the Effects of Carbon Dioxide Pneumoperitoneum on Abdominal Wall Wound Healing in Rats Undergoing Segmental Resection and Anastomosis of the Left Colon. Acta Cir. Bras. 2012, 27, 63–70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nassar, S.O.; Eltatawy, R.A.R.; Hassan, G.F.R. Safety and Efficacy of Platelet-rich Plasma vs. Carboxytherapy in the Treatment of Atrophic Scars: A Comparative Clinical and Histopathological Study. Dermatol. Ther. 2020, 33, e13942. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.M.D.; Rocha, L.B.; Da Cunha, M.T.R.; Cintra, M.M.M.; Pinheiro, N.M.; Mendonça, A.C. Effects of Carboxytherapy on Skin Laxity. J. Cosmet. Dermatol. 2020, 19, 3007–3013. [Google Scholar] [CrossRef] [PubMed]
- Penhavel, M.V.C.; Nascimento, V.H.T.; Durães, E.F.R.; Carneiro, F.P.; Sousa, J.B.D. Effects of Carbon Dioxide Therapy on the Healing of Acute Skin Wounds Induced on the Back of Rats. Acta Cir. Bras. 2013, 28, 334–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosch, R.; Junge, K.; Binnebösel, M.; Mirgartz, N.; Klinge, U.; Schumpelick, V. Gas-Related Impact of Pneumoperitoneum on Systemic Wound Healing. Langenbecks Arch. Surg. 2007, 393, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Shalan, N.; Al-Bazzaz, A.; Al-Ani, I.; Najem, F.; Al-Masri, M. Effect of Carbon Dioxide Therapy on Diabetic Foot Ulcer. J. Diabetes Mellit. 2015, 5, 284–289. [Google Scholar] [CrossRef]
- Waked, K.; Kierdaj, M.; Aslani, A. The Use of Carboxytherapy for the Treatment of Deep Partial-Thickness Skin Burns After Circumferential and High-Definition Liposuction: Promising Clinical Results in 5 Consecutive Cases. Aesthetic Surg. J. Open Forum 2023, 5, ojad096. [Google Scholar] [CrossRef]
- Wollina, U.; Heinig, B.; Uhlemann, C. Transdermal CO2 Application in Chronic Wounds. Int. J. Low. Extrem. Wounds 2004, 3, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Heinig, B.; Stelzner, C.; Hansel, G.; Schönlebe, J.; Tchernev, G.; Lotti, T. The Role of Complex Treatment in Mixed Leg Ulcers—A Case Report of Vascular, Surgical and Physical Therapy. Open Access Maced. J. Med. Sci. 2018, 6, 67–70. [Google Scholar] [CrossRef]
- Ban Frangež, H.; Rodi, Z.; Miklavčič, J.; Frangež, I. The Effect of Transcutaneous Application of Gaseous CO2 on Diabetic Symmetrical Peripheral Neuropathy—A Double-Blind Randomized Clinical Trial. Appl. Sci. 2021, 11, 4911. [Google Scholar] [CrossRef]
- Finzgar, M.; Melik, Z.; Cankar, K. Effect of Transcutaneous Application of Gaseous Carbon Dioxide on Cutaneous Microcirculation. Clin. Hemorheol. Microcirc. 2015, 60, 423–435. [Google Scholar] [CrossRef]
- Oda, T.; Niikura, T.; Fukui, T.; Oe, K.; Kuroiwa, Y.; Kumabe, Y.; Sawauchi, K.; Yoshikawa, R.; Mifune, Y.; Hayashi, S.; et al. Transcutaneous CO2 Application Accelerates Fracture Repair in Streptozotocin-Induced Type I Diabetic Rats. BMJ Open Diabetes Res. Care 2020, 8, e001129. [Google Scholar] [CrossRef]
- Ferrara, N. The Role of Vascular Endothelial Growth Factor in Pathological Angiogenesis. Breast Cancer Res. Treat. 1995, 36, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Mustoe, T.A.; Hunt, T.K. The Future of Recombinant Growth Factors in Wound Healing. Am. J. Surg. 1998, 176, 80S–82S. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Das, P.; Mukherjee, P.; Roy, S.; Chaudhuri, S.; Kesh, S.S.; Ghosh, D.; Nandi, S.K. Biomaterials-Based Strategies to Enhance Angiogenesis in Diabetic Wound Healing. ACS Biomater. Sci. Eng. 2024, 10, 2725–2741. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Gou, M.; Da, L.-C.; Zhang, W.-Q.; Xie, H.-Q. Mesenchymal Stem Cells for Chronic Wound Healing: Current Status of Preclinical and Clinical Studies. Tissue Eng. Part B Rev. 2020, 26, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Sayama, K.; Yuki, K.; Sugata, K.; Fukagawa, S.; Yamamoto, T.; Ikeda, S.; Murase, T. Carbon Dioxide Inhibits UVB-Induced Inflammatory Response by Activating the Proton-Sensing Receptor, GPR65, in Human Keratinocytes. Sci. Rep. 2021, 11, 379. [Google Scholar] [CrossRef]
- Verri, W.A.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive Role of Cytokines and Chemokines: Targets for Analgesic Drug Development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Kawano, S.; Mori, S.; Murase, T. Facial Application of High-Concentration Carbon Dioxide Prevents Epidermal Impairment Associated with Environmental Changes. Clin. Cosmet. Investig. Dermatol. 2019, 12, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. pH in Nature, Humans and Skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Ferrara, N. VEGF as a Therapeutic Target in Cancer. Oncology 2005, 69, 11–16. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).