Apelinergic System Affects Electrocardiographic Abnormalities Induced by Doxorubicin
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
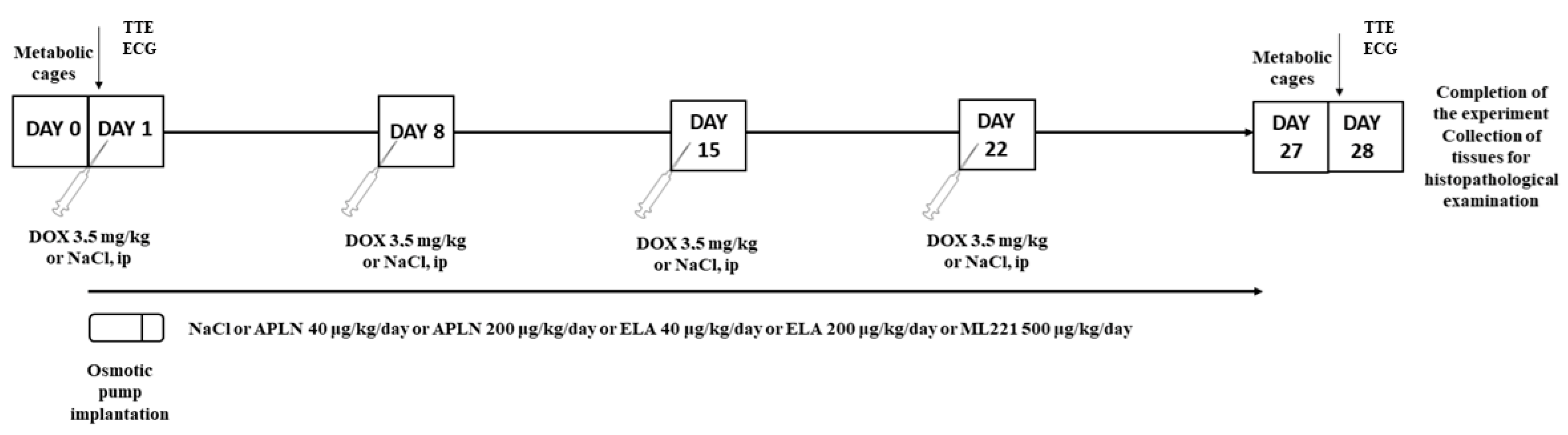
2.2. Experimental Protocol
- Control group (Control)—rats receiving saline i.p. (0.9% NaCl) in adequate volume every 7 days for 4 consecutive weeks (4 doses in total), and saline (0.9% NaCl) 2.5 μL/h in osmotic pump for 28 days starting on day 1.
- Doxorubicin group (DOX)—rats receiving i.p. doxorubicin at the dose of 3.5 mg/kg/b.w. every 7 days for 4 consecutive weeks (4 doses in total), and saline (0.9% NaCl) 2.5 μL/h in osmotic pump for 28 days starting on day 1.
- Apelin 40 group (APLN 40)—rats receiving i.p. doxorubicin at the dose of 3.5 mg/kg/b.w. every 7 days for 4 consecutive weeks (4 doses in total), and apelin-13 at the dose of 40 μg/kg b.w./day (2.5 μL/h) in osmotic pump for 28 days starting on day 1.
- Apelin 200 group (APLN 200)—rats receiving i.p. doxorubicin at the dose of 3.5 mg/kg/b.w. every 7 days for 4 consecutive weeks (4 doses in total), and apelin-13 at the dose of 200 μg/kg b.w./day (2.5 μL/h) in osmotic pump for 28 days starting on day 1.
- Elabela 40 group (ELA 40)—rats receiving i.p. doxorubicin 3.5 mg/kg/b.w. every 7 days for 4 consecutive weeks (4 doses in total) with Elabela 40 μg/kg b.w./day (2.5 μL/h) in osmotic pump for 28 days starting on day 1.
- Elabela 200 group (ELA 200)—rats receiving i.p. doxorubicin 3.5 mg/kg/b.w. every 7 days for 4 consecutive weeks (4 doses in total) with Elabela 200 μg/kg b.w./day (2.5 μL/h) in osmotic pump for 28 days starting on day 1.
- ML221 group (ML221)—rats receiving i.p. doxorubicin 3.5 mg/kg/b.w. every 7 days for 4 consecutive weeks (4 doses in total) with ML 221,500 μg/kg b.w./day (2.5 μL/h) in osmotic pump for 28 days starting on day 1.
2.3. Drugs and Reagents
2.4. Electrocardiographic Examination
- QRS complex
- QT and QTc
- HR
2.5. Transthoracic Echocardiographic (TTE) Examination
2.6. Multimedia
3. Results
3.1. Electrocardiographic Measurements
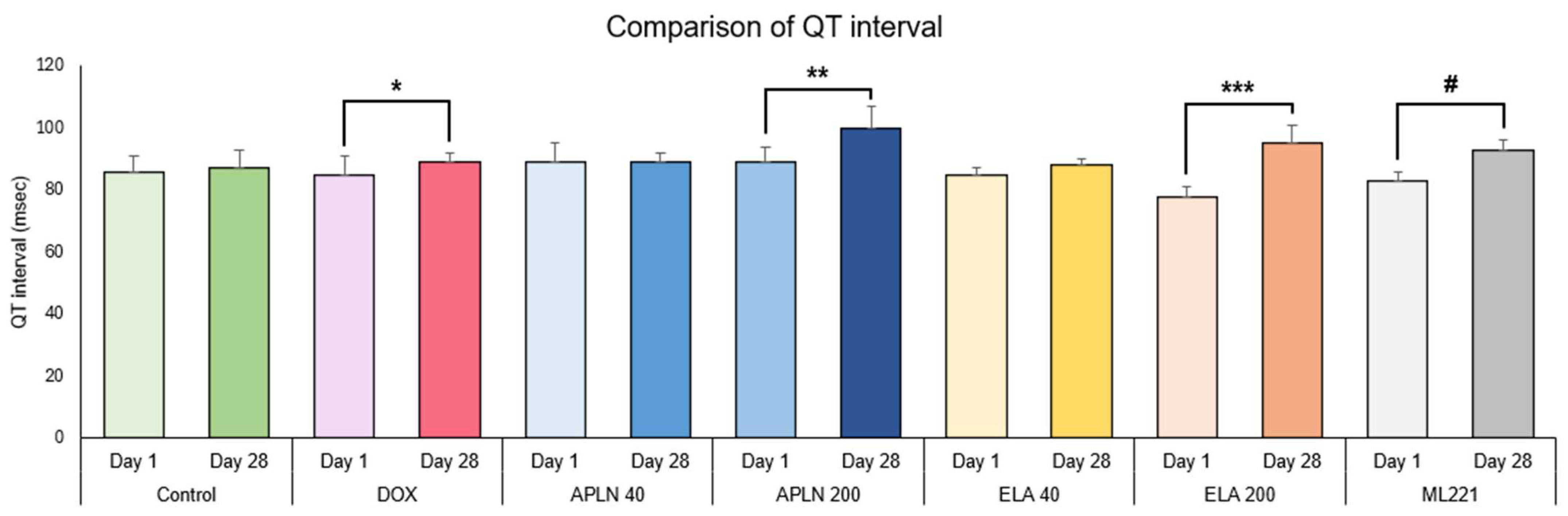
3.1.1. Comparison of QT Interval Between Day 1 and Day 28 in the Groups
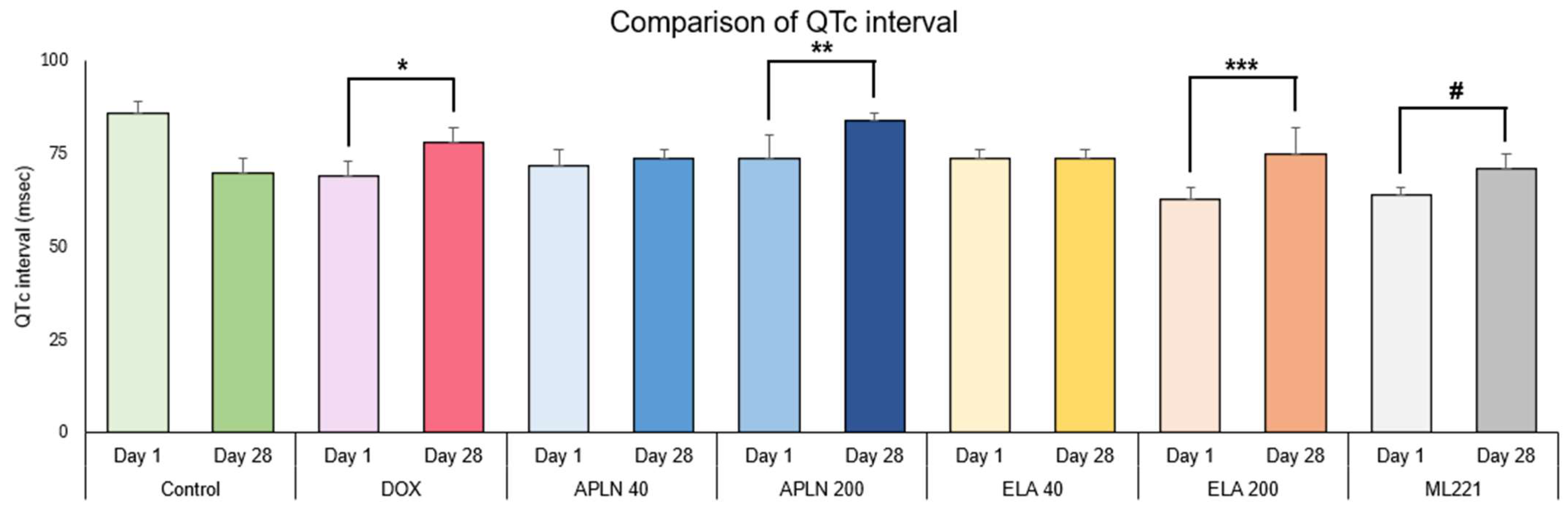
3.1.2. Comparison of QTc Interval Between Day 1 and Day 28 in the Groups
3.1.3. Comparison of QRS Duration Between Day 1 and Day 28 in the Groups
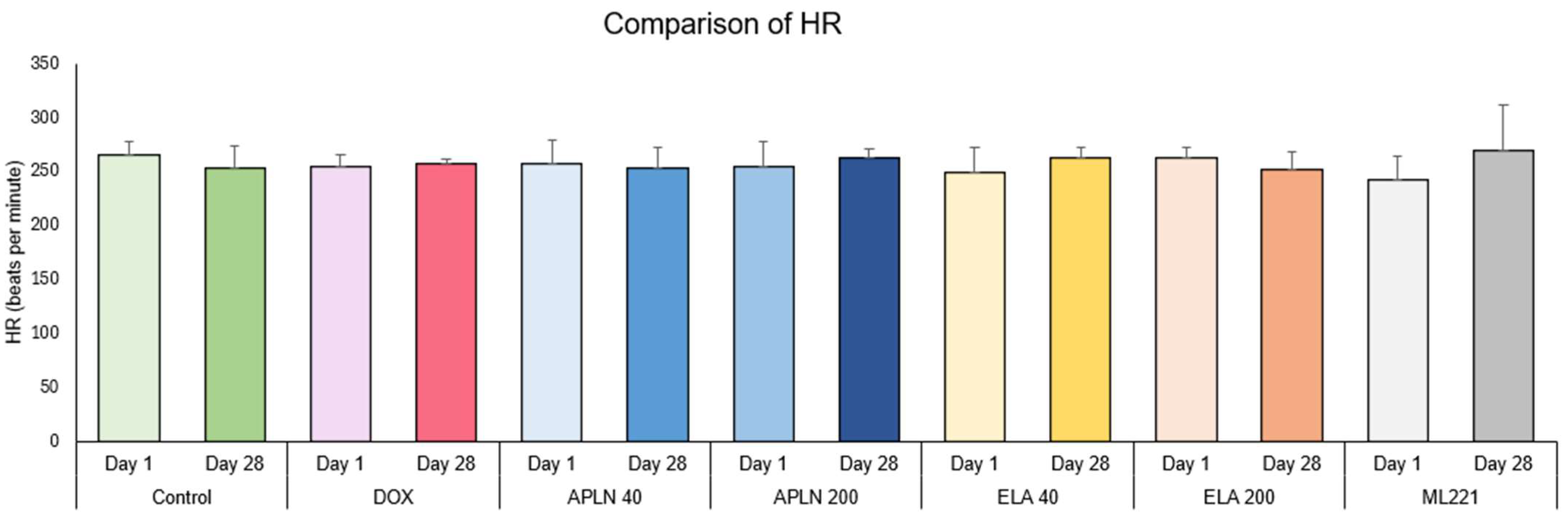
3.1.4. Comparison of HR Between Day 1 and Day 28 in the Groups
3.2. Echocardiographic Measurements
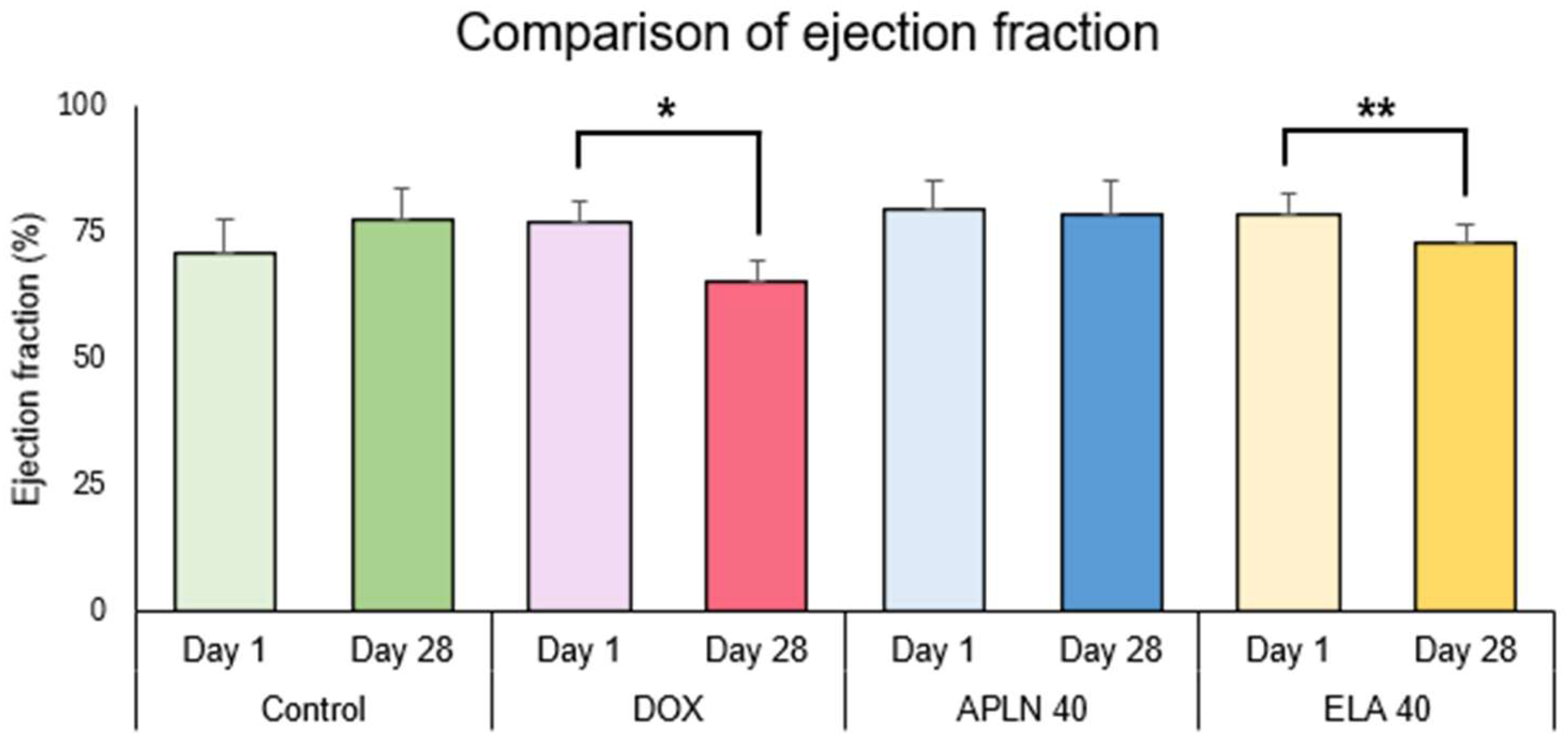
3.2.1. Comparison of Ejection Fraction Between Day 1 and Day 28 in the Groups
3.2.2. Comparison of Fractional Shortening Between Day 1 and Day 28 in the Groups
3.2.3. Comparison of Stroke Volume Between Day 1 and Day 28 in the Groups
3.2.4. Comparison of Cardiac Output Between Day 1 and Day 28 in the Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Trouet, A.; Deprez-De Campeneere, D. Daunorubicin-DNA and doxorubicin-DNA. A review of experimental and clinical data. Cancer Chemother. Pharmacol. 1979, 2, 77–79. [Google Scholar] [CrossRef]
- Damiani, R.M.; Moura, D.J.; Viau, C.M.; Caceres, R.A.; Henriques, J.A.P.; Saffi, J. Pathways of cardiac toxicity: Comparison between chemotherapeutic drugs doxorubicin and mitoxantrone. Arch. Toxicol. 2016, 90, 2063–2076. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.S.; dos Santos Goldenberg, R.C. Doxorubicin-Induced Cardiotoxicity: From Mechanisms to Development of Efficient Therapy; Tan, W., Ed.; InTech: Houston, TX, USA, 2018. [Google Scholar] [CrossRef]
- Lyon, A.R.; Lopez-Fernandez, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.A.; Acton, E.M.; Peters, J.H. Doxorubicin cardiotoxicity in the rat: Comparison of electrocardiogram, transmembrane potential, and structural effects. J. Cardiovasc. Pharmacol. 1984, 6, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Villani, F.; Monti, E.; Piccinini, F.; Favalli, L.; Lanza, E.; Rozza Dionigi, A.; Poggi, P. Relationship between doxorubicin-induced ECG changes and myocardial alterations in rats. Tumori 1986, 72, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Warhol, A.; George, S.A.; Obaid, S.N.; Efimova, T.; Efimov, I.R. Differential cardiotoxic electrocardiographic response to doxorubicin treatment in conscious versus anesthetized mice. Physiol. Rep. 2021, 9, e14987. [Google Scholar] [CrossRef] [PubMed]
- Nousiainen, T.; Vanninen, E.; Rantala, A.; Jantunen, E.; Hartikainen, J. QT dispersion and late potentials during doxorubicin therapy for non-Hodgkin’s lymphoma. J. Intern. Med. 1999, 245, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Yuzawa, H.; Natori, K.; Wada, R.; Yao, S.; Yano, K.; Akitsu, K.; Koike, H.; Shinohara, M.; Fujino, T.; et al. Early electrocardiographic indices for predicting chronic doxorubicin-induced cardiotoxicity. J. Cardiol. 2021, 77, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Bazoukis, G.; Garcia-Zamora, S.; Cinier, G.; Lee, S.; Elvin Gul, E.; Alvarez-Garcia, J.; Miana, G.; Hayiroglu, M.I.; Tse, G.; Liu, T.; et al. Association of electrocardiographic markers with myocardial fibrosis as assessed by cardiac magnetic resonance in different clinical settings. World J. Cardiol. 2022, 14, 483–495. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ruan, Y.; Huang, X.; Dou, L.; Lan, M.; Cui, J.; Chen, B.; Gong, H.; Wang, Q.; Yan, M.; et al. Dexrazoxane ameliorates doxorubicin-induced cardiotoxicity by inhibiting both apoptosis and necroptosis in cardiomyocytes. Biochem. Biophys. Res. Commun. 2020, 523, 140–146. [Google Scholar] [CrossRef]
- Kopp, L.M.; Womer, R.B.; Schwartz, C.L.; Ebb, D.H.; Franco, V.I.; Hall, D.; Barkauskas, D.A.; Krailo, M.D.; Grier, H.E.; Meyers, P.A.; et al. Effects of dexrazoxane on doxorubicin-related cardiotoxicity and second malignant neoplasms in children with osteosarcoma: A report from the Children’s Oncology Group. Cardio-Oncology 2019, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Narayan, H.K.; Putt, M.E.; Kosaraju, N.; Paz, A.; Bhatt, S.; Plappert, T.; Mercer-Rosa, L.; Armenian, S.H.; Desai, A.V.; Womer, R.B.; et al. Dexrazoxane preferentially mitigates doxorubicin cardiotoxicity in female children with sarcoma. Open Heart 2019, 6, e001025. [Google Scholar] [CrossRef] [PubMed]
- de Baat, E.C.; van Dalen, E.C.; Mulder, R.L.; Hudson, M.M.; Ehrhardt, M.J.; Engels, F.K.; Feijen, E.A.M.; Grotenhuis, H.B.; Leerink, J.M.; Kapusta, L.; et al. Primary cardioprotection with dexrazoxane in patients with childhood cancer who are expected to receive anthracyclines: Recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Child. Adolesc. Health 2022, 6, 885–894. [Google Scholar] [CrossRef]
- Titus, A.; Cheema, H.A.; Shafiee, A.; Seighali, N.; Shahid, A.; Bhanushali, K.B.; Kumar, A.; Khan, S.U.; Khadke, S.; Thavendiranathan, P.; et al. Statins for Attenuating Cardiotoxicity in Patients Receiving Anthracyclines: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101885. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C.; et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998, 251, 471–476. [Google Scholar] [CrossRef]
- Li, Y.; Bai, Y.J.; Jiang, Y.R.; Yu, W.Z.; Shi, X.; Chen, L.; Feng, J.; Sun, G.B. Apelin-13 Is an Early Promoter of Cytoskeleton and Tight Junction in Diabetic Macular Edema via PI-3K/Akt and MAPK/Erk Signaling Pathways. BioMed Res. Int. 2018, 2018, 3242574. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.R.; Mao, D.A.; Yi, L.; Wang, C.; Zhang, X.X.; Xie, H.; Wu, X.P.; Liao, X.B.; Zhou, H.; Meng, J.C.; et al. Apelin suppresses apoptosis of human vascular smooth muscle cells via APJ/PI3-K/Akt signaling pathways. Amino Acids 2010, 39, 1193–1200. [Google Scholar] [CrossRef]
- Xie, F.; Lv, D.; Chen, L. ELABELA: A novel hormone in cardiac development acting as a new endogenous ligand for the APJ receptor. Acta Biochim. Biophys. Sin. 2014, 46, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yan, J.; Pan, W.; Tang, M. Apelin/Elabela-APJ: A novel therapeutic target in the cardiovascular system. Ann. Transl. Med. 2020, 8, 243. [Google Scholar] [CrossRef]
- Czarzasta, K.; Cudnoch-Jedrzejewska, A.; Szczepanska-Sadowska, E.; Fus, L.; Puchalska, L.; Gondek, A.; Dobruch, J.; Gomolka, R.; Wrzesien, R.; Zera, T.; et al. The role of apelin in central cardiovascular regulation in rats with post-infarct heart failure maintained on a normal fat or high fat diet. Clin. Exp. Pharmacol. Physiol. 2016, 43, 983–994. [Google Scholar] [CrossRef]
- Czarzasta, K.; Wojno, O.; Zera, T.; Puchalska, L.; Dobruch, J.; Cudnoch-Jedrzejewska, A. The influence of post-infarct heart failure and high fat diet on the expression of apelin APJ and vasopressin V1a and V1b receptors. Neuropeptides 2019, 78, 101975. [Google Scholar] [CrossRef]
- Charo, D.N.; Ho, M.; Fajardo, G.; Kawana, M.; Kundu, R.K.; Sheikh, A.Y.; Finsterbach, T.P.; Leeper, N.J.; Ernst, K.V.; Chen, M.M.; et al. Endogenous regulation of cardiovascular function by apelin-APJ. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1904–H1913. [Google Scholar] [CrossRef]
- Dolanbay, T.; Makav, M.; Gul, H.F.; Karakurt, E. The effect of diclofenac sodium intoxication on the cardiovascular system in rats. Am. J. Emerg. Med. 2021, 46, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Powers, J.; Chen, M.; Kundu, R.; Finsterbach, T.; Caffarelli, A.; Deng, A.; Eichhorn, J.; Mahajan, R.; Agrawal, R.; et al. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc. Res. 2005, 65, 73–82. [Google Scholar] [CrossRef]
- Akhondali, Z.; Badavi, M.; Dianat, M.; Faraji, F. Co-administration of Apelin and T4 protects inotropic and chronotropic changes occurring in hypothyroid rats. Arq. Bras. Cardiol. 2015, 105, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Q.; Wu, C.; Chen, L.; Bi, F.; Zhou, Y.; Shan, H. Apelin shorten QT interval by inhibiting Kir2.1/I(K1) via a PI3K way in acute myocardial infarction. Biochem. Biophys. Res. Commun. 2019, 517, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.B.; Jennings, M.; Buckwell, A.; Ewbank, R.; Godfrey, C.; Holgate, B.; Inglis, I.; James, R.; Page, C.; Sharman, I.; et al. Refining procedures for the administration of substances. Report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. British Veterinary Association Animal Welfare Foundation/Fund for the Replacement of Animals in Medical Experiments/Royal Society for the Prevention of Cruelty to Animals/Universities Federation for Animal Welfare. Lab. Anim. 2001, 35, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Coria-Avila, G.A.; Gavrila, A.M.; Menard, S.; Ismail, N.; Pfaus, J.G. Cecum location in rats and the implications for intraperitoneal injections. Lab. Anim. 2007, 36, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Konopelski, P.; Ufnal, M. Electrocardiography in rats: A comparison to human. Physiol. Res. 2016, 65, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, H.; Hanada, E.; Yamamoto, K.; Sawada, Y.; Iga, T. Pharmacokinetic-pharmacodynamic analysis of the electrocardiographic effects of terfenadine and quinidine in rats. Biol. Pharm. Bull. 1996, 19, 1189–1196. [Google Scholar] [CrossRef]
- Hanada, E.; Ohtani, H.; Kotaki, H.; Sawada, Y.; Sato, H.; Iga, T. Pharmacodynamic analysis of the electrocardiographic interaction between disopyramide and erythromycin in rats. J. Pharm. Sci. 1999, 88, 234–240. [Google Scholar] [CrossRef]
- Roden, D.M. Drug-induced prolongation of the QT interval. N. Engl. J. Med. 2004, 350, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Krol, M.; Ufnal, M.; Szulczyk, B.; Podsadni, P.; Drapala, A.; Turlo, J.; Dawidowski, M. Characterization of Disopyramide derivative ADD424042 as a non-cardiotoxic neuronal sodium channel blocker with broad-spectrum anticonvulsant activity in rodent seizure models. Eur. J. Pharm. Sci. 2016, 81, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Kmecova, J.; Klimas, J. Heart rate correction of the QT duration in rats. Eur. J. Pharmacol. 2010, 641, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Sztechman, D.; Zera, T.; Czarzasta, K.; Wojciechowska, M.; Szczepanska-Sadowska, E.; Cudnoch-Jedrzejewska, A. Transthoracic echocardiography: From guidelines for humans to cardiac ultrasound of the heart in rats. Physiol. Meas. 2020, 41, 10TR02. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, S.; Paldino, A.; Falcao-Pires, I.; Daskalopoulos, E.P.; Dal Ferro, M.; Vodret, S.; Lesizza, P.; Cannata, A.; Miranda-Silva, D.; Lourenco, A.P.; et al. Towards standardization of echocardiography for the evaluation of left ventricular function in adult rodents: A position paper of the ESC Working Group on Myocardial Function. Cardiovasc. Res. 2021, 117, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Rozwadowski, J.; Borodzicz-Jazdzyk, S.; Czarzasta, K.; Cudnoch-Jedrzejewska, A. A Review of the Roles of Apelin and ELABELA Peptide Ligands in Cardiovascular Disease, Including Heart Failure and Hypertension. Med. Sci. Monit. 2022, 28, e938112. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, H. Therapeutic potential of apelin and Elabela in cardiovascular disease. Biomed. Pharmacother. 2023, 166, 115268. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Shi, M.; Xu, F.; Zhang, X.; Gong, D.W. Comparative Study of Elabela and Apelin on Apelin Receptor Activation Through beta-Arrestin Recruitment. Mol. Biotechnol. 2023, 65, 394–400. [Google Scholar] [CrossRef]
- He, L.; Chen, L.; Li, L. The mechanosensitive APJ internalization via clathrin-mediated endocytosis: A new molecular mechanism of cardiac hypertrophy. Med. Hypotheses 2016, 90, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Iturrioz, X.; Gerbier, R.; Leroux, V.; Alvear-Perez, R.; Maigret, B.; Llorens-Cortes, C. By interacting with the C-terminal Phe of apelin, Phe255 and Trp259 in helix VI of the apelin receptor are critical for internalization. J. Biol. Chem. 2010, 285, 32627–32637. [Google Scholar] [CrossRef]
- Zhou, N.; Fan, X.; Mukhtar, M.; Fang, J.; Patel, C.A.; DuBois, G.C.; Pomerantz, R.J. Cell-cell fusion and internalization of the CNS-based, HIV-1 co-receptor, APJ. Virology 2003, 307, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Yassin, N.G.; Aly Seif, A.; Abd el Hamid, M.S.; Nassef, N.A.-A. Effect of Apelin on Hepatic Dysfunction in a Rat Model of Heart Failure. QJM An. Int. J. Med. 2023, 116, hcad069.687. [Google Scholar] [CrossRef]
- Cheng, C.C.; Weerateerangkul, P.; Lu, Y.Y.; Chen, Y.C.; Lin, Y.K.; Chen, S.A.; Chen, Y.J. Apelin regulates the electrophysiological characteristics of atrial myocytes. Eur. J. Clin. Investig. 2013, 43, 34–40. [Google Scholar] [CrossRef]
- Cai, X.; Liu, L.; Xia, F.; Papadimos, T.J.; Wang, Q. Apelin-13 reverses bupivacaine-induced cardiotoxicity: An experimental study. Braz. J. Anesthesiol. 2024, 74, 844501. [Google Scholar] [CrossRef] [PubMed]
- Wyderka, R.; Diakowska, D.; Loboz-Rudnicka, M.; Mercik, J.; Borger, M.; Osuch, L.; Brzezinska, B.; Leskow, A.; Krzystek-Korpacka, M.; Jaroch, J. Influence of the Apelinergic System on Conduction Disorders in Patients after Myocardial Infarction. J. Clin. Med. 2023, 12, 7603. [Google Scholar] [CrossRef] [PubMed]
- El Amrousy, D.; El-Mahdy, H. Prognostic Value of Serum Apelin Level in Children with Heart Failure Secondary to Congenital Heart Disease. Pediatr. Cardiol. 2018, 39, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Plana, J.C.; Galderisi, M.; Barac, A.; Ewer, M.S.; Ky, B.; Scherrer-Crosbie, M.; Ganame, J.; Sebag, I.A.; Agler, D.A.; Badano, L.P.; et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2014, 27, 911–939. [Google Scholar] [CrossRef]
- Podyacheva, E.Y.; Kushnareva, E.A.; Karpov, A.A.; Toropova, Y.G. Analysis of Models of Doxorubicin-Induced Cardiomyopathy in Rats and Mice. A Modern View From the Perspective of the Pathophysiologist and the Clinician. Front. Pharmacol. 2021, 12, 670479. [Google Scholar] [CrossRef] [PubMed]
- Belger, C.; Abrahams, C.; Imamdin, A.; Lecour, S. Doxorubicin-induced cardiotoxicity and risk factors. Int. J. Cardiol. Heart Vasc. 2024, 50, 101332. [Google Scholar] [CrossRef] [PubMed]
- Babaei, H.; Razmaraii, N.; Assadnassab, G.; Mohajjel Nayebi, A.; Azarmi, Y.; Mohammadnejad, D.; Azami, A. Ultrastructural and Echocardiographic Assessment of Chronic Doxorubicin-Induced Cardiotoxicity in Rats. Arch. Razi Inst. 2020, 75, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, J.A.M.; Linschoten, M.; Cramer, M.J.; Doevendans, P.A.; Asselbergs, F.W.; Teske, A.J. Early- and late anthracycline-induced cardiac dysfunction: Echocardiographic characterization and response to heart failure therapy. Cardio-Oncology 2020, 6, 23. [Google Scholar] [CrossRef]
- Berry, M.F.; Pirolli, T.J.; Jayasankar, V.; Burdick, J.; Morine, K.J.; Gardner, T.J.; Woo, Y.J. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation 2004, 110, II187–II193. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.X.; Pan, C.S.; Zhang, J.; Geng, B.; Zhao, J.; Gerns, H.; Yang, J.; Chang, J.K.; Tang, C.S.; Qi, Y.F. Apelin protects myocardial injury induced by isoproterenol in rats. Regul. Pept. 2006, 133, 147–154. [Google Scholar] [CrossRef]
- Japp, A.G.; Cruden, N.L.; Barnes, G.; van Gemeren, N.; Mathews, J.; Adamson, J.; Johnston, N.R.; Denvir, M.A.; Megson, I.L.; Flapan, A.D.; et al. Acute cardiovascular effects of apelin in humans: Potential role in patients with chronic heart failure. Circulation 2010, 121, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Szponar, J.; Ciechanski, E.; Ostrowska-Lesko, M.; Gorska, A.; Tchorz, M.; Dabrowska, A.; Dudka, J.; Murias, M.; Kowalczyk, M.; Korga-Plewko, A.; et al. The Lack of Synergy between Carvedilol and the Preventive Effect of Dexrazoxane in the Model of Chronic Anthracycline-Induced Cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 10202. [Google Scholar] [CrossRef]
- Galetta, F.; Franzoni, F.; Cervetti, G.; Cecconi, N.; Carpi, A.; Petrini, M.; Santoro, G. Effect of epirubicin-based chemotherapy and dexrazoxane supplementation on QT dispersion in non-Hodgkin lymphoma patients. Biomed. Pharmacother. 2005, 59, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Aggarwal, S.; Doody, D.R.; Aplenc, R.; Armenian, S.H.; Baker, K.S.; Bhatia, S.; Blythe, N.; Colan, S.D.; Constine, L.S.; et al. Dexrazoxane and Long-Term Heart Function in Survivors of Childhood Cancer. J. Clin. Oncol. 2023, 41, 2248–2257. [Google Scholar] [CrossRef] [PubMed]
- Henninger, C.; Huelsenbeck, S.; Wenzel, P.; Brand, M.; Huelsenbeck, J.; Schad, A.; Fritz, G. Chronic heart damage following doxorubicin treatment is alleviated by lovastatin. Pharmacol. Res. 2015, 91, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ohlig, J.; Henninger, C.; Zander, S.; Merx, M.; Kelm, M.; Fritz, G. Rac1-mediated cardiac damage causes diastolic dysfunction in a mouse model of subacute doxorubicin-induced cardiotoxicity. Arch. Toxicol. 2018, 92, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Hundley, W.G.; D’Agostino, R., Jr.; Crotts, T.; Craver, K.; Hackney, M.H.; Jordan, J.H.; Ky, B.; Wagner, L.I.; Herrington, D.M.; Yeboah, J.; et al. Statins and Left Ventricular Ejection Fraction Following Doxorubicin Treatment. NEJM Evid. 2022, 1, EVIDoa2200097. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buczma, K.; Borzuta, H.; Kamińska, K.; Sztechman, D.; Matusik, K.; Pawlonka, J.; Kowara, M.; Buchalska, B.; Cudnoch-Jędrzejewska, A. Apelinergic System Affects Electrocardiographic Abnormalities Induced by Doxorubicin. Biomedicines 2025, 13, 94. https://doi.org/10.3390/biomedicines13010094
Buczma K, Borzuta H, Kamińska K, Sztechman D, Matusik K, Pawlonka J, Kowara M, Buchalska B, Cudnoch-Jędrzejewska A. Apelinergic System Affects Electrocardiographic Abnormalities Induced by Doxorubicin. Biomedicines. 2025; 13(1):94. https://doi.org/10.3390/biomedicines13010094
Chicago/Turabian StyleBuczma, Kasper, Hubert Borzuta, Katarzyna Kamińska, Dorota Sztechman, Katarzyna Matusik, Jan Pawlonka, Michał Kowara, Barbara Buchalska, and Agnieszka Cudnoch-Jędrzejewska. 2025. "Apelinergic System Affects Electrocardiographic Abnormalities Induced by Doxorubicin" Biomedicines 13, no. 1: 94. https://doi.org/10.3390/biomedicines13010094
APA StyleBuczma, K., Borzuta, H., Kamińska, K., Sztechman, D., Matusik, K., Pawlonka, J., Kowara, M., Buchalska, B., & Cudnoch-Jędrzejewska, A. (2025). Apelinergic System Affects Electrocardiographic Abnormalities Induced by Doxorubicin. Biomedicines, 13(1), 94. https://doi.org/10.3390/biomedicines13010094





