Optimized Method to Generate Well-Characterized Macrophages from Induced Pluripotent Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Detailed Step-by-Step Protocol for Differentiation of iPSMs
2.1.1. Human iPSC Culture
- Matrigel-coated plates:
- Thaw a tube of growth factor-reduced Matrigel (Corning, Corning, NY, USA # 354230) on ice;
- Add 120 µL Matrigel to 12 mL of DMEM/F12 (Thermo Fisher, Waltham, MA, USA # 12500096) at 4 °C (enough for 1–2 plates) and put it in ice;
- Plate 0.5 mL of the mix per well in two 12-well plates (Falcon Corning, Corning, NY, USA # 353043, or equivalent).
- Place plates in a 37 °C incubator for at least 30 min. Plates may be kept for up to 2 weeks in a 37 °C incubator without drying out.
- Thawing and initial plating of iPSCs:
- The following iPS cell lines were obtained from the CIRM hPSC Repository funded by the California Institute of Regenerative Medicine (CIRM, South San Francisco, CA, USA): CW50067BB1, CW50009BB1, CW20105DD1, CW50075AA1, and CW30391CC1. All donors consented to research using their cell lines; the donor consent forms followed guidelines set by CIRM and were approved by Institutional Review Boards (IRBs). All the materials were collected under IRB from the Institutions that collected the material;
- Thaw the frozen iPS vial in a 37 °C water bath until just thawed. Add thawed cells to 5 mL of mTeSR Plus medium (Stemcell Technologies, Vancouver, BC, Canada # 100-0276) in a 15 mL conical tube (Falcon Corning, Corning, NY, USA # 352097). Spin and wash cells with ~1 mL of mTeSR Plus medium. Spin cells and resuspend in 2 mL of mTeSR Plus medium containing 10 uM Y2763 ROCK inhibitor (Tocris, Minneapolis, MN, USA # 1254). Transfer 1 mL of cells each into two wells of the Matrigel-coated 12-well plate. Place into a hypoxic (5% O2) incubator;
- Change media every day with 1 mL of mTeSR+ medium (w/o Rock inhibitor).
- Passage of iPSCs by the clump culture method:
- Ideally, cells should be growing in small clumps and reach 65–80% confluence before passage. Split at 1:3 to 1:5 every 3–4 days to avoid confluence which can lead to spontaneous loss of pluripotency;
- Rinse cells w/PBS, add 300 μL Accutase (Gibco, Waltham, MA, USA # A1110501), and incubate for 5 min at 37 °C. With a P1000 tip, add 1 mL of mTeSR+ w/ROCK inhibitor, and gently pipet up and down to lift cells from the plate in small clumps without dissociating into single cells. Dilute cells as needed in mTeSR+ w/ROCK inhibitor and plate cells into 3–5 wells of a Matrigel-coated 12-well plate;
- Change media every day with 1 mL of mTeSR+ medium (w/o Rock inhibitor).
- Cryopreservation of iPSCs:
- Rinse cells w/PBS, add 300 μL Accutase, and incubate for 5 min at 37 °C. With a P1000 tip, add 1 mL of mTeSR+ w/ROCK inhibitor, and gently pipet up and down to lift cells from the plate in small clumps without dissociating into single cells;
- Spin down the cells, resuspend w/1 mL of mFreSR medium (Stemcell Technologies, Vancouver, BC, Canada # 05855) containing 10 μM Rock inhibitor;
- Transfer 1 mL of cells to cryovial. Transfer the vial to a slow freezing container and store at −80 °C overnight, then transfer the frozen tubes to a liquid nitrogen tank for long-term storage.
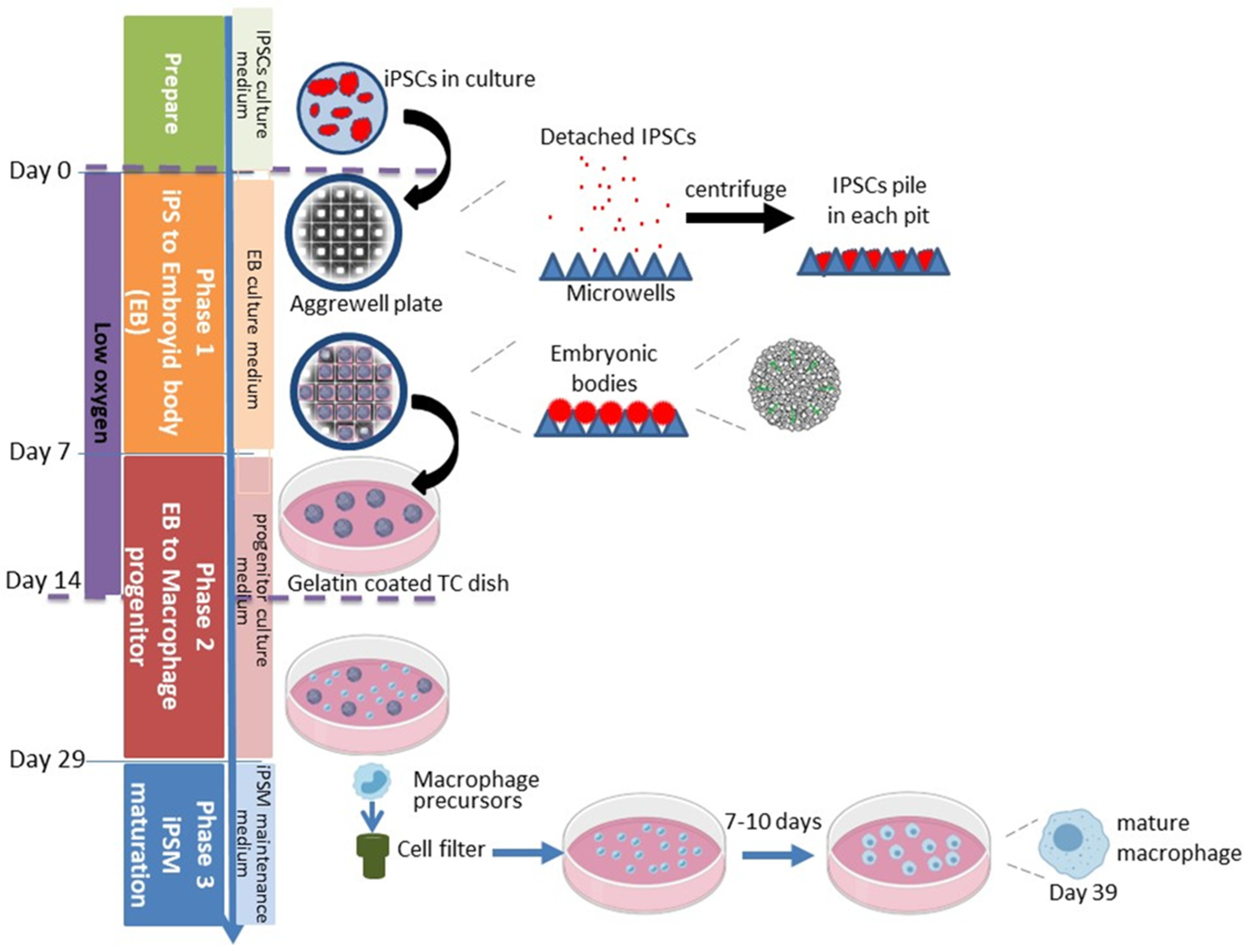
2.1.2. Phase 1: Embryoid Body Formation
- Growth of iPSCs to not more than 50% confluence on a Matrigel-coated P100 TC dish:
- When iPSCs reach 65–80% confluence in a well of a 12-well plate, detach iPSC with Accutase as above, but pipet cells thoroughly to obtain single-cell suspension;
- Count cells and plate 7 × 105 cells onto a Matrigel-coated P100 plate (Corning, Corning, NY, USA # 353003) as described above;
- Change to mTeSR+ medium w/o Rock inhibitor the next day;
- About 2 days later the iPSC should not be higher than 50% confluent. Keeping the cells at this density is a critical step for successful iPSM differentiation.
- Formation of embryoid bodies in AggreWell microwell plate:
- Pre-treat one well for each iPS line in a 24-well AggreWell 800 plate (each well has 300 micropits) with a 500 μL Anti-Adherence Rinsing Solution (both contained in Stemcell Technologies, Vancouver, BC, Canada # 34850);
- Centrifuge plate at 1300× g for 5 min in a swinging bucket rotor fitted with plate holders. Observe the plate under a microscope to ensure bubbles have been removed from microwells. If bubbles remain trapped in any microwells, centrifuge at 1300× g for an additional 5 min;
- Aspirate Anti-Adherence Rinsing Solution from the wells. Rinse each well with 2 mL of warm mTeSR Plus medium;
- Aspirate medium from the well. Add 1 mL of warm EB medium (Table 1) supplemented w/Rock inhibitor to each well to be used;
- Rinse 50% confluent iPSC w/PBS and detach iPSCs with Accutase, as described above, but pipet thoroughly into single cells. Then add mTeSR Plus to quench Accutase, as shown above;
- Count cells, spin down 4.5 × 106 IPSCs at 250× g for 5 min. Resuspend pellet in 1 mL of EB medium supplemented w/Rock inhibitor (10 μM);
- Add cells to well to yield a final volume of 2 mL;
- Incubate the plate at 37 °C in a 5% O2 5% CO2 incubator. Feed with 1 mL of EB medium (w/o rock inhibitor) every day for 7 days. Compact EB should be observed by day 2 (Figure 2).
2.1.3. Phase 2: Generation of Macrophage Progenitors
- Add 10 mL of macrophage progenitor culture medium (Table 2) to gelatin-coated (4 mL of 0.1% gelatin incubated at 37 °C for 1 h, MP, catalog number: 901771) P100 tissue culture dish;
- Remove and discard 0.5 mL of media from each well of the EB culture, yielding 1.5 mL remaining. Cut a P1000 pipet tip with a sterile blade to widen the opening. Gently suspend EBs with a P1000 pipet tip to dislocate each EB from the microwells;
- Transfer free EBs to a 1.5 mL sterile microfuge tube. EBs will spontaneously sediment in 1 min. Discard the supernatant and use wide-open P1000 pipet tip to resuspend the EBs with progenitor culture medium;
- Transfer resuspended EBs into the previously prepared P100 tissue culture dish mentioned above;
- Move the P100 dish back-and-forth and side-to-side, without rotating, to evenly distribute EBs and incubate in a hypoxic incubator for the first 7 days of phase 2, then move to a regular incubator. Replenish with progenitor culture medium every 4 days. EBs will attach to the P100 dish and begin to grow out.
2.1.4. Harvesting Macrophage Progenitors
2.1.5. Phase 3: iPSMs Maturation and Maintenance
2.1.6. Cryopreservation of iPSMs
2.2. Immunological Profiling of iPSCs, Macrophage Progenitors, and iPSMs by Flow Cytometry
2.3. Acetylated LDL Uptake Assay
2.4. Bacteria Phagocytosis Assay
2.5. Cholesterol Efflux Assay
2.6. Polarization of iPSMs
2.7. Cytokine Release Assay
2.8. Inflammasome Activation and IL-1β Secretion Assay
2.9. RNAseq Analysis
2.10. General Statistical Analysis
3. Results
3.1. Generation of iPSMs from iPSCs
3.2. Immunological Characterization of iPSMs and Their Precursors
3.3. Optimal iPSM Differentiation Time Course
3.4. Functional Characterization of iPSMs
3.5. Polarization of iPSMs
3.6. RNAseq Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| iPSC | induced pluripotent stem cell |
| EB | embryoid body |
| iPSMs | iPSC-derived macrophages |
| FC | flow cytometry |
| AcLDL | Aceteylated LDL |
| ATP | adenosine triphosphate |
| BCA | bicinchoninic acid |
| CPM | counts per million |
| PCA | principal component analysis |
| GEO | Gene Expression Omnibus |
| apoA1 | apolipoprotein A1 |
| LPS | lipopolysaccharide |
| IFNγ | Interferon gamma |
| IL-4 | interleukin-4 |
References
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Mass, E.; Nimmerjahn, F.; Kierdorf, K.; Schlitzer, A. Tissue-specific macrophages: How they develop and choreograph tissue biology. Nat. Rev. Immunol. 2023, 23, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, H.; Feinweber, C.; Luckhardt, S.; Linke, B.; Resch, E.; Geisslinger, G.; Weigert, A.; Parnham, M.J. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol. Immunol. 2017, 88, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Borchers, A.; Pieler, T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes 2010, 1, 413–426. [Google Scholar] [CrossRef]
- van Wilgenburg, B.; Browne, C.; Vowles, J.; Cowley, S.A. Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS ONE 2013, 8, e71098. [Google Scholar] [CrossRef]
- Lachmann, N.; Ackermann, M.; Frenzel, E.; Liebhaber, S.; Brennig, S.; Happle, C.; Hoffmann, D.; Klimenkova, O.; Luttge, D.; Buchegger, T.; et al. Large-scale hematopoietic differentiation of human induced pluripotent stem cells provides granulocytes or macrophages for cell replacement therapies. Stem Cell Rep. 2015, 4, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Nenasheva, T.; Gerasimova, T.; Serdyuk, Y.; Grigor’eva, E.; Kosmiadi, G.; Nikolaev, A.; Dashinimaev, E.; Lyadova, I. Macrophages Derived From Human Induced Pluripotent Stem Cells Are Low-Activated "Naive-Like" Cells Capable of Restricting Mycobacteria Growth. Front. Immunol. 2020, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Klepikova, A.; Nenasheva, T.; Sheveleva, O.; Protasova, E.; Antonov, D.; Gainullina, A.; Chikina, E.; Sakovnich, O.; Gerasimova, T.; Nikitina, I.; et al. iPSC-Derived Macrophages: The Differentiation Protocol Affects Cell Immune Characteristics and Differentiation Trajectories. Int. J. Mol. Sci. 2022, 23, 16087. [Google Scholar] [CrossRef] [PubMed]
- Robinet, P.; Ritchey, B.; Smith, J.D. Physiological difference in autophagic flux in macrophages from 2 mouse strains regulates cholesterol ester metabolism. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, G.; Lorkowski, S.W.; Gulshan, K.; Hazen, S.L.; Gogonea, V.; Smith, J.D. First eight residues of apolipoprotein A-I mediate the C-terminus control of helical bundle unfolding and its lipidation. PLoS ONE 2020, 15, e0221915. [Google Scholar] [CrossRef] [PubMed]
- Ewels, P.A.; Peltzer, A.; Fillinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 2020, 38, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Poplin, R.; Chang, P.C.; Alexander, D.; Schwartz, S.; Colthurst, T.; Ku, A.; Newburger, D.; Dijamco, J.; Nguyen, N.; Afshar, P.T.; et al. A universal SNP and small-indel variant caller using deep neural networks. Nat. Biotechnol. 2018, 36, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.; Li, H.; Chang, P.C.; Lin, M.F.; Carroll, A.; McLean, C.Y. Accurate, scalable cohort variant calls using DeepVariant and GLnexus. Bioinformatics 2021, 36, 5582–5589. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 30 December 2024).
- Soneson, C.; Love, M.I.; Robinson, M.D. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Res 2015, 4, 1521. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Chinetti, G.; Lestavel, S.; Bocher, V.; Remaley, A.T.; Neve, B.; Torra, I.P.; Teissier, E.; Minnich, A.; Jaye, M.; Duverger, N.; et al. PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat. Med. 2001, 7, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xue, C.; Liu, W.; Zhang, H. Differentiation of Human-Induced Pluripotent Stem Cells to Macrophages for Disease Modeling and Functional Genomics. Curr. Protoc. Stem Cell 2019, 48, e74. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yakala, G.K.; van den Hil, F.E.; Cochrane, A.; Mummery, C.L.; Orlova, V.V. Differentiation and Functional Comparison of Monocytes and Macrophages from hiPSCs with Peripheral Blood Derivatives. Stem Cell Rep. 2019, 12, 1282–1297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, C.; Shah, R.; Bermingham, K.; Hinkle, C.C.; Li, W.; Rodrigues, A.; Tabita-Martinez, J.; Millar, J.S.; Cuchel, M.; et al. Functional analysis and transcriptomic profiling of iPSC-derived macrophages and their application in modeling Mendelian disease. Circ. Res. 2015, 117, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Acosta, D.; Hoq, M.R.; Khurana, S.; Golding, H.; Zaitseva, M. Pyrogenic and inflammatory mediators are produced by polarized M1 and M2 macrophages activated with D-dimer and SARS-CoV-2 spike immune complexes. Cytokine 2024, 173, 156447. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Smith, J.D. Genetic-genomic replication to identify candidate mouse atherosclerosis modifier genes. J. Am. Heart Assoc. 2013, 2, e005421. [Google Scholar] [CrossRef]
- The GTEx Consortium; Ardlie, K.G.; Deluca, D.S.; Segrè, A.V.; Sullivan, T.J.; Young, T.R.; Gelfand, E.T.; Trowbridge, C.A.; Maller, J.B.; Tukiainen, T.; et al. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef]
- Warren, C.R.; Cowan, C.A. Humanity in a Dish: Population Genetics with iPSCs. Trends Cell Biol. 2018, 28, 46–57. [Google Scholar] [CrossRef]
- Lee, M.N.; Ye, C.; Villani, A.C.; Raj, T.; Li, W.; Eisenhaure, T.M.; Imboywa, S.H.; Chipendo, P.I.; Ran, F.A.; Slowikowski, K.; et al. Common genetic variants modulate pathogen-sensing responses in human dendritic cells. Science 2014, 343, 1246980. [Google Scholar] [CrossRef]







| Composition | Volume (for 50 mL) | Final Concentration |
|---|---|---|
| mTeSR medium | 50 mL | |
| BMP-4 (25 μg/mL stock) 1 | 100 μL | 50 ng/mL |
| SCF (100 μg/mL stock) 2 | 10 μL | 20 ng/mL |
| VEGF (50 μg/mL stock) 3 | 50 μL | 50 ng/mL |
| Composition | Volume (for 50 mL) | Final Concentration |
|---|---|---|
| X-VIVO-15 | 50 mL | |
| M-CSF (100 μg/mL stock) 1 | 50 μL | 100 ng/mL |
| IL-3 (100 μg/mL stock) 2 | 25 μL | 50 ng/mL |
| Glutamax (200 mM stock) | 500 μL | 2 mM |
| 2-Mercaptoethanol (55 mM) | 90 μL | 0.1 mM |
| Penicillin/Streptomycin (10,000 units/mL) | 500 μL | 100 units/mL |
| Composition | Volume (for 10 mL) | Final Concentration |
|---|---|---|
| RPMI1640 | 8 mL | |
| Penicillin/Streptomycin (10,000 units/mL) | 100 μL | 100 units/mL |
| FBS | 2 mL | 20% |
| M-CSF (100 μg/mL stock) | 10 μL | 100 ng/mL |
| Antigen | Fluorochrome | Clone | BD 1 Catalog# |
|---|---|---|---|
| CD45 | BB515 | HI30 | 564585 |
| CD86 | BB700 | 2331(FUN-1) | 566473 |
| CD14 | APC H7 | MoP-9 | 560180 |
| CD163 | BV650 | GHI/61 | 563888 |
| CD11c | PE | BU15 | 566730 |
| CD90 | PECy7 | 5E10 | 561558 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hai, Q.; Bazeley, P.; Han, J.; Brubaker, G.; Powers, J.; Diaz-Montero, C.M.; Smith, J.D. Optimized Method to Generate Well-Characterized Macrophages from Induced Pluripotent Stem Cells. Biomedicines 2025, 13, 99. https://doi.org/10.3390/biomedicines13010099
Hai Q, Bazeley P, Han J, Brubaker G, Powers J, Diaz-Montero CM, Smith JD. Optimized Method to Generate Well-Characterized Macrophages from Induced Pluripotent Stem Cells. Biomedicines. 2025; 13(1):99. https://doi.org/10.3390/biomedicines13010099
Chicago/Turabian StyleHai, Qimin, Peter Bazeley, Juying Han, Gregory Brubaker, Jennifer Powers, Claudia M. Diaz-Montero, and Jonathan D. Smith. 2025. "Optimized Method to Generate Well-Characterized Macrophages from Induced Pluripotent Stem Cells" Biomedicines 13, no. 1: 99. https://doi.org/10.3390/biomedicines13010099
APA StyleHai, Q., Bazeley, P., Han, J., Brubaker, G., Powers, J., Diaz-Montero, C. M., & Smith, J. D. (2025). Optimized Method to Generate Well-Characterized Macrophages from Induced Pluripotent Stem Cells. Biomedicines, 13(1), 99. https://doi.org/10.3390/biomedicines13010099






