Chrysin Attenuates Gentamicin-Induced Renal Injury in Rats Through Modulation of Oxidative Damage and Inflammation via Regulation of Nrf2/AKT and NF-kB/KIM-1 Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Experimental Design
2.3. Types of Samples Collected
2.4. Assay of Renal Function Markers
2.5. Assay of Proinflammatory, Antioxidant, and Oxidative Markers
2.6. Quantitative qRT-PCR of Pathogenic Molecules
2.7. Histopathology Study
2.8. Histopathological Semi-Quantitative Analysis
2.9. Statistical Analysis
3. Results
3.1. Serum and Urine Kidney Function Tests
3.2. Concentrations of Inflammatory and Antioxidant Markers in Kidney Tissues
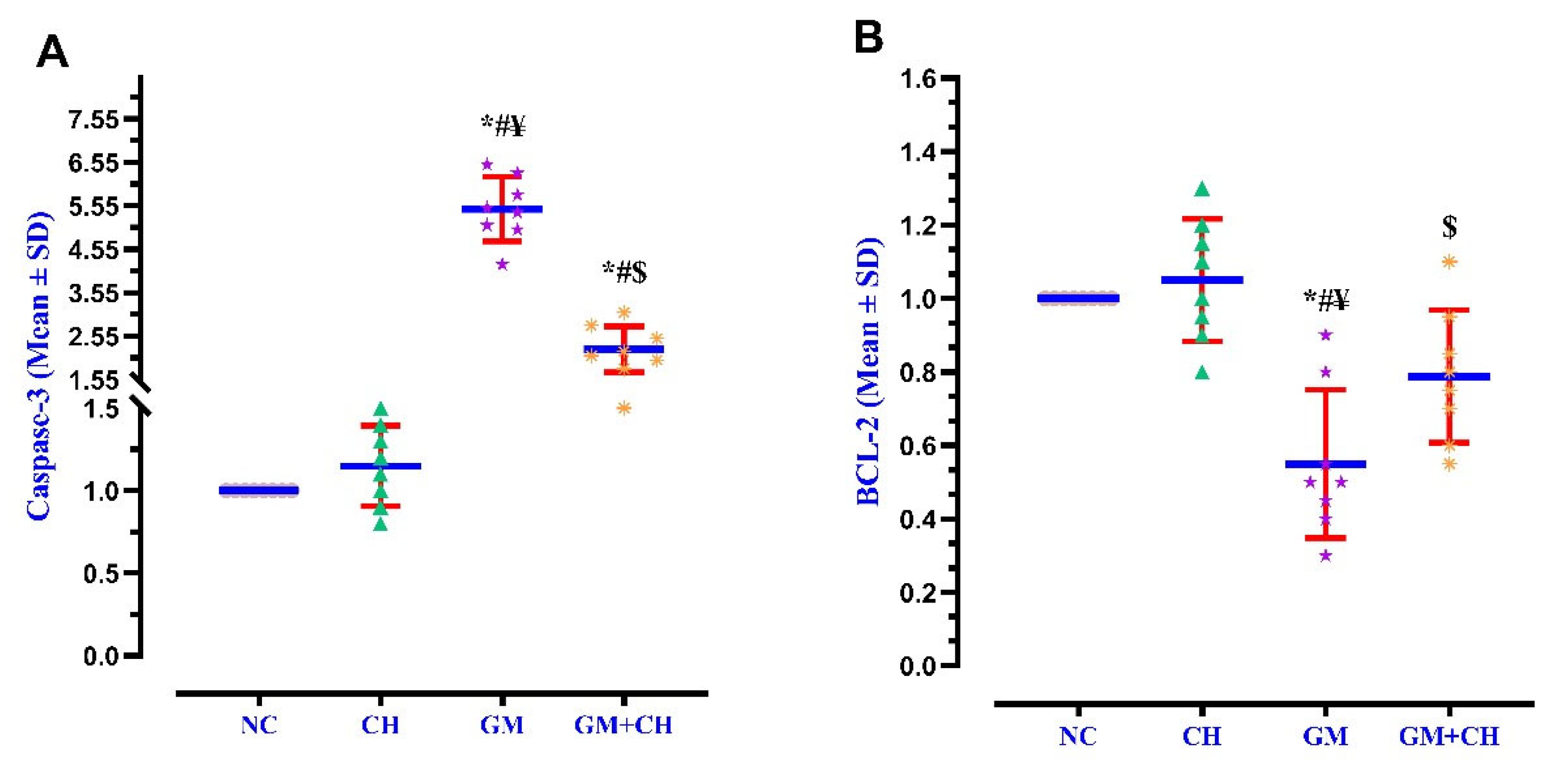
3.3. Expression of Markers Indicative of Kidney Pathogenesis
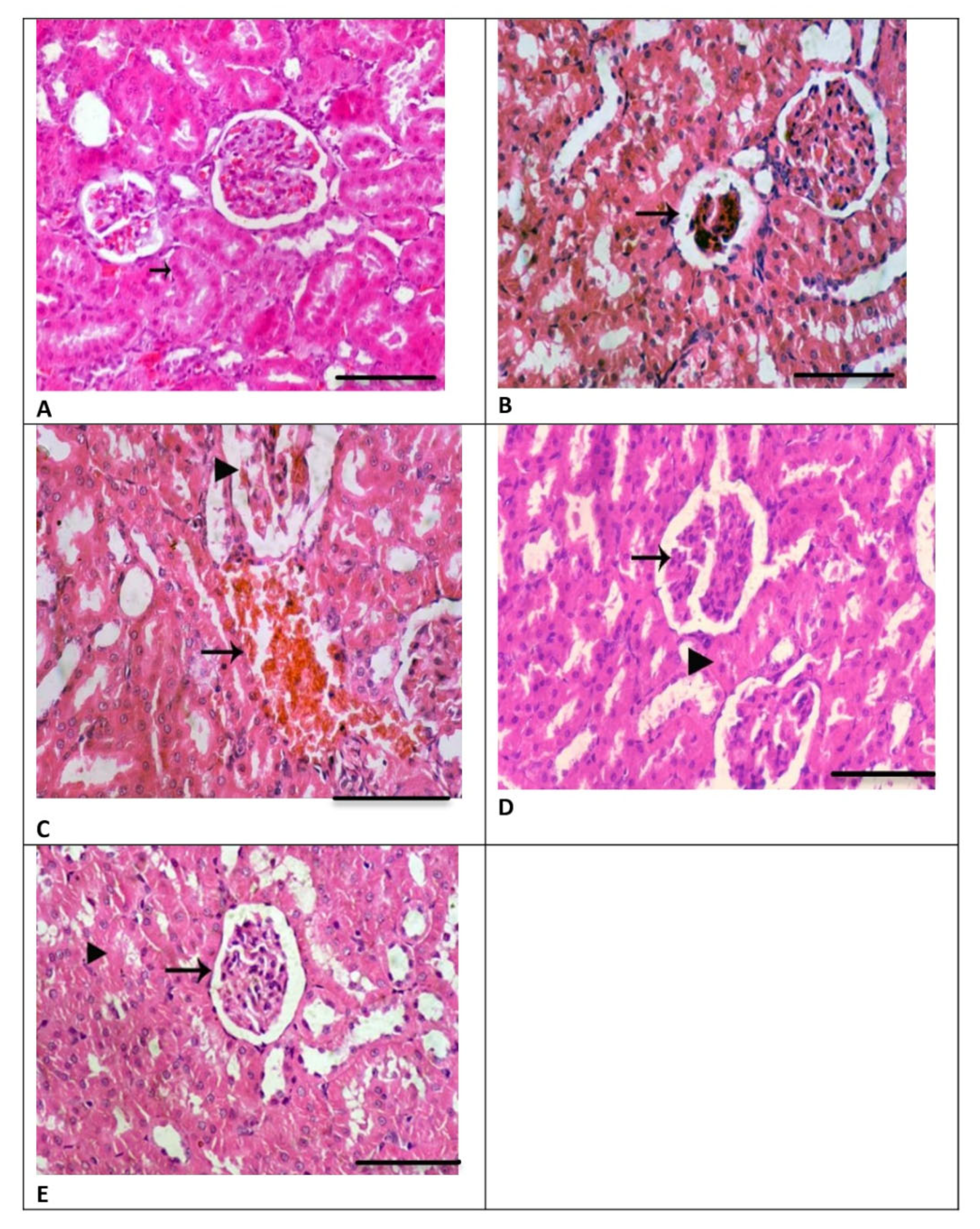
3.4. Renal Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassanein, E.H.M.; Ali, F.E.M.; Kozman, M.R.; Abd El-Ghafar, O.A.M. Umbelliferone attenuates gentamicin-induced renal toxicity by suppression of TLR-4/NF-κB-p65/NLRP-3 and JAK1/STAT-3 signaling pathways. Environ. Sci. Pollut. Res. Int. 2021, 28, 11558–11571. [Google Scholar] [CrossRef]
- Mestry, S.N.; Gawali, N.B.; Pai, S.A.; Gursahani, M.S.; Dhodi, J.B.; Munshi, R.; Juvekar, A.R. Punica granatum improves renal function in gentamicin-induced nephropathy in rats via attenuation of oxidative stress. J. Ayurveda Integr. Med. 2020, 11, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.M.; Mosaed, M.M.; Elshafey, S.H.; Bayomy, N.A. 6-gingerol ameliorates gentamicin induced renal cortex oxidative stress and apoptosis in adult male albino rats. Tissue Cell 2016, 48, 208–216. [Google Scholar] [CrossRef]
- Laorodphun, P.; Cherngwelling, R.; Panya, A.; Arjinajarn, P. Curcumin protects rats against gentamicin-induced nephrotoxicity by amelioration of oxidative stress, endoplasmic reticulum stress and apoptosis. Pharm. Biol. 2022, 60, 491–500. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, C.H.; Hsu, Y.H.; Chen, T.H.; Sue, Y.M.; Cheng, C.Y.; Chen, T.W. Leptin reduces gentamicin-induced apoptosis in rat renal tubular cells via the PI3K-Akt signaling pathway. Eur. J. Pharmacol. 2011, 658, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, G.; Shen, B.; Yao, Y.Y.; Hagiwara, M.; Mizell, B.; Teuton, M.; Grass, D.; Chao, L.; Chao, J. Role of tissue kallikrein in prevention and recovery of gentamicin-induced renal injury. Toxicol. Sci. 2008, 102, 433–443. [Google Scholar] [CrossRef]
- Nassan, M.A.; Soliman, M.M.; Aldhahrani, A.; Althobaiti, F.; Alkhedaide, A.Q. Ameliorative impacts of Glycyrrhiza glabra root extract against nephrotoxicity induced by gentamicin in mice. Food Sci. Nutr. 2021, 9, 3405–3413. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Mehrad-Majd, H.; Mirhafez, S.R. Thymoquinone Ameliorates Acute Renal Failure in Gentamicin-Treated Adult Male Rats. Pharmacology 2015, 96, 112–117. [Google Scholar] [CrossRef]
- Taher, R.F.; Raslan, M.A.; Masoud, M.A.; Nassar, M.I.; Aboutabl, M.E. HPLC-ESI/MS profiling, phytoconstituent isolation and evaluation of renal function, oxidative stress and inflammation in gentamicin-induced nephrotoxicity in rats of Ficus spragueana Mildbr. & Burret. Biomed. Chromatogr. 2021, 35, e5135. [Google Scholar] [CrossRef]
- Jado, J.C.; Humanes, B.; González-Nicolás, M.; Camaño, S.; Lara, J.M.; López, B.; Cercenado, E.; García-Bordas, J.; Tejedor, A.; Lázaro, A. Nephroprotective Effect of Cilastatin against Gentamicin-Induced Renal Injury In Vitro and In Vivo without Altering Its Bactericidal Efficiency. Antioxidants 2020, 9, 821. [Google Scholar] [CrossRef]
- Salama, S.A.; Arab, H.H.; Maghrabi, I.A. Troxerutin down-regulates KIM-1, modulates p38 MAPK signaling, and enhances renal regenerative capacity in a rat model of gentamycin-induced acute kidney injury. Food Funct. 2018, 9, 6632–6642. [Google Scholar] [CrossRef]
- Ahmed, H.I.; Mohamed, E.A. Candesartan and epigallocatechin-3-gallate ameliorate gentamicin-induced renal damage in rats through p38-MAPK and NF-κB pathways. J. Biochem. Mol. Toxicol. 2019, 33, e22254. [Google Scholar] [CrossRef] [PubMed]
- Akcakavak, G.; Kazak, F.; Karatas, O.; Alakus, H.; Alakus, I.; Kirgiz, O.; Celik, Z.; Yilmaz Deveci, M.Z.; Ozdemir, O.; Tuzcu, M. Eucalyptol regulates Nrf2 and NF-kB signaling and alleviates gentamicin-induced kidney injury in rats by downregulating oxidative stress, oxidative DNA damage, inflammation, and apoptosis. Toxicol. Mech. Methods 2024, 34, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.E.M.; Sayed, A.M.; El-Bahrawy, A.H.; Omar, Z.M.M.; Hassanein, E.H.M. Targeting KEAP1/Nrf2, AKT, and PPAR-γ signals as a potential protective mechanism of diosmin against gentamicin-induced nephrotoxicity. Life Sci. 2021, 275, 119349. [Google Scholar] [CrossRef]
- Tomşa, A.M.; Răchişan, A.L.; Pandrea, S.L.; Benea, A.; Uifălean, A.; Toma, C.; Popa, R.; Pârvu, A.E.; Junie, L.M. Curcumin and Vitamin C Attenuate Gentamicin-Induced Nephrotoxicity by Modulating Distinctive Reactive Species. Metabolites 2022, 13, 49. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Ferreira-Santos, P.; Genisheva, Z.; El Ghouizi, A.; Aboulghazi, A.; Teixeira, J.A.; Lyoussi, B. Protective Effect of Honey and Propolis against Gentamicin-Induced Oxidative Stress and Hepatorenal Damages. Oxid. Med. Cell Longev. 2021, 2021, 9719906. [Google Scholar] [CrossRef]
- Rehman, M.U.; Tahir, M.; Khan, A.Q.; Khan, R.; Lateef, A.; Oday, O.H.; Qamar, W.; Ali, F.; Sultana, S. Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: Plausible role of NF-κB. Toxicol. Lett. 2013, 216, 146–158. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Iahtisham Ul, H.; Iqra, Y.; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235, 116797. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Xu, W.; Lu, H.; Yuan, Y.; Deng, Z.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-κB Pathways. Foods 2022, 11, 2439. [Google Scholar] [CrossRef]
- Temel, Y.; Kucukler, S.; Yıldırım, S.; Caglayan, C.; Kandemir, F.M. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 325–337. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Samarghandian, S.; Bafandeh, F. The Cardiovascular Protective Effects of Chrysin: A Narrative Review on Experimental Researches. Cardiovasc. Hematol. Agents Med. Chem. 2019, 17, 17–27. [Google Scholar] [CrossRef]
- Xu, M.; Shi, H.; Liu, D. Chrysin protects against renal ischemia reperfusion induced tubular cell apoptosis and inflammation in mice. Exp. Ther. Med. 2019, 17, 2256–2262. [Google Scholar] [CrossRef]
- Baykalir, B.G.; Arslan, A.S.; Mutlu, S.I.; Parlak Ak, T.; Seven, I.; Seven, P.T.; Yaman, M.; Gul, H.F. The protective effect of chrysin against carbon tetrachloride-induced kidney and liver tissue damage in rats. Int. J. Vitam. Nutr. Res. 2021, 91, 427–438. [Google Scholar] [CrossRef]
- Çomaklı, S.; Özdemir, S.; Güloğlu, M. Chrysin attenuates paclitaxel-induced hepatorenal toxicity in rats by suppressing oxidative damage, inflammation, and apoptosis. Life Sci. 2023, 332, 122096. [Google Scholar] [CrossRef]
- Rashid, S.; Ali, N.; Nafees, S.; Hasan, S.K.; Sultana, S. Mitigation of 5-Fluorouracil induced renal toxicity by chrysin via targeting oxidative stress and apoptosis in wistar rats. Food Chem. Toxicol. 2014, 66, 185–193. [Google Scholar] [CrossRef]
- Sultana, S.; Verma, K.; Khan, R. Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress. J. Pharm. Pharmacol. 2012, 64, 872–881. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Goudarzi, M.; Fatemi, I.; Basir, Z.; Malayeri, A.; Khalili, H. Chrysin attenuates sodium arsenite-induced nephrotoxicity in rats by suppressing oxidative stress and inflammation. Tissue Cell 2021, 73, 101657. [Google Scholar] [CrossRef]
- Kucukler, S.; Benzer, F.; Yildirim, S.; Gur, C.; Kandemir, F.M.; Bengu, A.S.; Ayna, A.; Caglayan, C.; Dortbudak, M.B. Protective Effects of Chrysin Against Oxidative Stress and Inflammation Induced by Lead Acetate in Rat Kidneys: A Biochemical and Histopathological Approach. Biol. Trace Elem. Res. 2021, 199, 1501–1514. [Google Scholar] [CrossRef]
- Nagavally, R.R.; Sunilkumar, S.; Akhtar, M.; Trombetta, L.D.; Ford, S.M. Chrysin Ameliorates Cyclosporine-A-Induced Renal Fibrosis by Inhibiting TGF-β1-Induced Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2021, 22, 10252. [Google Scholar] [CrossRef]
- Pai, S.A.; Munshi, R.P.; Panchal, F.H.; Gaur, I.S.; Juvekar, A.R. Chrysin ameliorates nonalcoholic fatty liver disease in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, E.; Cekmen, M.; Ilbey, Y.O.; Simsek, A.; Polat, E.C.; Somay, A. Atorvastatin prevents gentamicin-induced renal damage in rats through the inhibition of p38-MAPK and NF-κB pathways. Ren. Fail. 2009, 31, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Refaat, B.; El-Boshy, M. Protective antioxidative and anti-inflammatory actions of β-caryophyllene against sulfasalazine-induced nephrotoxicity in rat. Exp. Biol. Med. 2022, 247, 691–699. [Google Scholar] [CrossRef]
- Crcek, M.; Zdovc, J.; Kerec Kos, M. A review of population pharmacokinetic models of gentamicin in paediatric patients. J. Clin. Pharm. Ther. 2019, 44, 659–674. [Google Scholar] [CrossRef]
- Kang, S.; Chen, T.; Hao, Z.; Yang, X.; Wang, M.; Zhang, Z.; Hao, S.; Lang, F.; Hao, H. Oxymatrine Alleviates Gentamicin-Induced Renal Injury in Rats. Molecules 2022, 27, 6209. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, K.; Cui, H.; Li, J.; Yao, D.; Wang, S.; Tian, X.; Kou, W.; Huang, J.; Wang, H.; et al. Baicalin-2-ethoxyethyl ester alleviates gentamicin-induced acute kidney injury via NF-κB signaling pathway. Biomed. Pharmacother. 2024, 172, 116276. [Google Scholar] [CrossRef]
- Avedissian, S.N.; Rohani, R.; Bradley, J.; Le, J.; Rhodes, N.J. Optimizing Aminoglycoside Dosing Regimens for Critically Ill Pediatric Patients with Augmented Renal Clearance: A Convergence of Parametric and Nonparametric Population Approaches. Antimicrob. Agents Chemother. 2021, 65, e02629-20. [Google Scholar] [CrossRef]
- Mousavinasab, S.R.; Akhoundi-Meybodi, Z.; Mahmoudi, L.; Karimzadeh, I. A randomized double-blinded placebo-controlled clinical trial on protective effects of pentoxifylline on gentamicin nephrotoxicity in infectious patients. Clin. Exp. Nephrol. 2021, 25, 844–853. [Google Scholar] [CrossRef]
- Da Silveira, A.R.; Rosa, É.V.F.; Sari, M.H.M.; Sampaio, T.B.; Dos Santos, J.T.; Jardim, N.S.; Müller, S.G.; Oliveira, M.S.; Nogueira, C.W.; Furian, A.F. Therapeutic potential of beta-caryophyllene against aflatoxin B1-Induced liver toxicity: Biochemical and molecular insights in rats. Chem. Biol. Interact. 2021, 348, 109635. [Google Scholar] [CrossRef]
- Farombi, E.O.; Ekor, M. Curcumin attenuates gentamicin-induced renal oxidative damage in rats. Food Chem. Toxicol. 2006, 44, 1443–1448. [Google Scholar] [CrossRef]
- Ghosh, S.; Ghosh, R.; Sawoo, R.; Dutta, P.; Bishayi, B. Impact of dual neutralization of TNF-α and IL-1β along with Gentamicin treatment on the functions of blood and splenic neutrophils and its role on improvement of S. aureus induced septic arthritis. Int. Immunopharmacol. 2023, 123, 110766. [Google Scholar] [CrossRef] [PubMed]
- Aurori, M.; Andrei, S.; Dreanca, A.I.; Morohoschi, A.G.; Cotul, M.; Niculae, M.; Nan, M.I.; Codea, A.R.; Gal, A.F. The Nephroprotective Effect of Cornelian Cherry (Cornus mas L.) and Rowanberry (Sorbus aucuparia L.) in Gentamicin-Induced Nephrotoxicity on Wistar Rats with Emphasis on the Evaluation of Novel Renal Biomarkers and the Antioxidant Capacity in Correlation with Nitro-Oxidative Stress. Nutrients 2023, 15, 4392. [Google Scholar] [CrossRef]
- Elgazzar, D.; Aboubakr, M.; Bayoumi, H.; Ibrahim, A.N.; Sorour, S.M.; El-Hewaity, M.; Elsayed, A.M.; Shehata, S.A.; Bayoumi, K.A.; Alsieni, M.; et al. Tigecycline and Gentamicin-Combined Treatment Enhances Renal Damage: Oxidative Stress, Inflammatory Reaction, and Apoptosis Interplay. Pharmaceuticals 2022, 15, 736. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Aboulhoda, B.E.; Mahmoud, R.H. Vitamin D attenuates gentamicin-induced acute renal damage via prevention of oxidative stress and DNA damage. Hum. Exp. Toxicol. 2019, 38, 321–335. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, X.; Zhang, Y.; George, J.; Cobbs, A.; Wang, G.; Li, L.; Emmett, N. Kidney Injury Molecule-1 Is Upregulated in Renal Lipotoxicity and Mediates Palmitate-Induced Tubular Cell Injury and Inflammatory Response. Int. J. Mol. Sci. 2019, 20, 3406. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Arjinajarn, P.; Pongchaidecha, A.; Chueakula, N.; Jaikumkao, K.; Chatsudthipong, V.; Mahatheeranont, S.; Norkaew, O.; Chattipakorn, N.; Lungkaphin, A. Riceberry bran extract prevents renal dysfunction and impaired renal organic anion transporter 3 (Oat3) function by modulating the PKC/Nrf2 pathway in gentamicin-induced nephrotoxicity in rats. Phytomedicine 2016, 23, 1753–1763. [Google Scholar] [CrossRef]
- Hanedan, B.; Ozkaraca, M.; Kirbas, A.; Kandemir, F.M.; Aktas, M.S.; Kilic, K.; Comakli, S.; Kucukler, S.; Bilgili, A. Investigation of the effects of hesperidin and chrysin on renal injury induced by colistin in rats. Biomed. Pharmacother. 2018, 108, 1607–1616. [Google Scholar] [CrossRef]
- Yao, W.; Cheng, J.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L.; Lu, G. Toxicological evaluation of a flavonoid, chrysin: Morphological, behavioral, biochemical and histopathological assessments in rats. Drug Chem. Toxicol. 2021, 44, 601–612. [Google Scholar] [CrossRef]
- Campos, H.M.; da Costa, M.; da Silva Moreira, L.K.; da Silva Neri, H.F.; Branco da Silva, C.R.; Pruccoli, L.; Dos Santos, F.C.A.; Costa, E.A.; Tarozzi, A.; Ghedini, P.C. Protective effects of chrysin against the neurotoxicity induced by aluminium: In vitro and in vivo studies. Toxicology 2022, 465, 153033. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.; Syed, R.; Rathi, A.K.; Lee, Y.J.; Sung, J.S.; Shinf, H.S.; Keum, Y.S. Chrysin-piperazine conjugates as antioxidant and anticancer agents. Eur. J. Pharm. Sci. 2016, 88, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Ileriturk, M.; Benzer, F.; Aksu, E.H.; Yildirim, S.; Kandemir, F.M.; Dogan, T.; Dortbudak, M.B.; Genc, A. Chrysin protects against testicular toxicity caused by lead acetate in rats with its antioxidant, anti-inflammatory, and antiapoptotic properties. J. Food Biochem. 2021, 45, e13593. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; Ma, J.; Han, X.W.; Jia, Y.X.; Yuan, H.F.; Shui, S.F.; Guo, D.; Yan, L. Chrysin ameliorates cerebral ischemia/reperfusion (I/R) injury in rats by regulating the PI3K/Akt/mTOR pathway. Neurochem. Int. 2019, 129, 104496. [Google Scholar] [CrossRef]
- Kattla, J.J.; Carew, R.M.; Heljic, M.; Godson, C.; Brazil, D.P. Protein kinase B/Akt activity is involved in renal TGF-β1-driven epithelial-mesenchymal transition in vitro and in vivo. Am. J. Physiol. Ren. Physiol. 2008, 295, F215–F225. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, S.; Li, K.; Wu, G.; Zheng, X.; Zheng, J.; Lu, X.; Zhang, L.; Li, J.; Su, Z.; et al. Coptisine protects against hyperuricemic nephropathy through alleviating inflammation, oxidative stress and mitochondrial apoptosis via PI3K/Akt signaling pathway. Biomed. Pharmacother. 2022, 156, 113941. [Google Scholar] [CrossRef]
- Xingyue, L.; Shuang, L.; Qiang, W.; Jinjuan, F.; Yongjian, Y. Chrysin Ameliorates Sepsis-Induced Cardiac Dysfunction Through Upregulating Nfr2/Heme Oxygenase 1 Pathway. J. Cardiovasc. Pharmacol. 2021, 77, 491–500. [Google Scholar] [CrossRef]
- Mantawy, E.M.; Esmat, A.; El-Bakly, W.M.; Salah ElDin, R.A.; El-Demerdash, E. Mechanistic clues to the protective effect of chrysin against doxorubicin-induced cardiomyopathy: Plausible roles of p53, MAPK and AKT pathways. Sci. Rep. 2017, 7, 4795. [Google Scholar] [CrossRef]
- Mishra, A.; Mishra, P.S.; Bandopadhyay, R.; Khurana, N.; Angelopoulou, E.; Paudel, Y.N.; Piperi, C. Neuroprotective Potential of Chrysin: Mechanistic Insights and Therapeutic Potential for Neurological Disorders. Molecules 2021, 26, 6456. [Google Scholar] [CrossRef]
- Ragab, E.M.; El Gamal, D.M.; Mohamed, T.M.; Khamis, A.A. Therapeutic potential of chrysin nanoparticle-mediation inhibition of succinate dehydrogenase and ubiquinone oxidoreductase in pancreatic and lung adenocarcinoma. Eur. J. Med. Res. 2022, 27, 172. [Google Scholar] [CrossRef]


| Protein-Encoding Gene | 5′-3′ Sequence | |
|---|---|---|
| Forward | Reverse | |
| AKT | 5′-TCT ATG GCG CTG AGA TTG TG-3′ | 5′-CTT AAT GTG CCC GTC CTT GT-3′ |
| Nrf2 | 5′GAG GAU GGG AAA CCU UAC UTT-3′ | 5’AGU AAG GUU UCC CAU CCU CTT-3′ |
| NF-κB | 5′-AAG CAC TCG GAT ACA GCA GC-3′ | 5′-AGT CGT CAT AGG GCA GCT CA-3′ |
| KIM-1 | 5′TTC AGG AAG CTG AGC AAA CAT-3′ | 5′CCC CAA CAT GTC GTT GTG ATT-3′ |
| Caspase-3 | AGTTGGACCCACCTTGTGAG | AGTCTGCAGCTCCTCCACAT |
| BCL-2 | CACCCCTGGCATCTTCTCCTT | AGCGTCTTCAGAGACAGCCAG |
| Actin | 5′-CAC GAT GGA GGG GCC GGA CTC ATC-3′ | 5′-TAA AGA CCT CTA TGC CAA CAC AGT-3′ |
| Score | Tubular Damage | Glomerular Changes | Vascular Changes |
|---|---|---|---|
| 0 | None | None | None |
| 1 | Mild tubular degeneration or necrosis | Mild glomerular shrinkage with minimal increase in uriniferous space | Mild hemorrhage |
| 2 | Moderate tubular degeneration or necrosis | Moderate glomerular shrinkage with mild increase in uriniferous space | Moderate interstitial hemorrhage |
| 3 | Severe tubular necrosis | Severe glomerular shrinkage with high uriniferous space | Severe interstitial hemorrhage |
| NC Group | CH Group | GM Group | CH + GM Group | ||
|---|---|---|---|---|---|
| Serum | Creatinine (mg/dL) | 0.32 ± 0.10 | 0.37 ± 0.08 | 0.63 ± 0.15 *#¥ | 0.42 ± 0.09 $ |
| Urea (mg/dL) | 35.62 ± 5.3 | 34.87 ± 5.1 | 61.9 ± 10.1 *#¥ | 39.62 ± 6.4 $ | |
| Total protein (g/dL) | 7.05 ± 0.89 | 7.43 ± 0.92 | 5.91 ± 0.22 *#¥ | 6.90 ± 0.78 | |
| Albumin (g/dL) | 4.37 ± 0.52 | 4.55 ± 0.36 | 3.57 ± 0.52 *#¥ | 4.16 ± 0.44 | |
| 24 h Urine | Urine volume (mL) | 8.45 ± 0.88 | 8.50 ± 1.01 | 5.72 ± 0.48 *#¥ | 7.47 ± 0.90 $ |
| Urine flow (µL/min) | 5.86 ± 0.61 | 5.90 ± 070 | 3.97 ± 0.34 *#¥ | 5.19 ± 0.63 | |
| Creatinine (mg/dL) | 41.1 ± 6.02 | 42.5 ± 5.55 | 25.4 ± 3.20 *#¥ | 38.3 ± 2.50 $ | |
| Creatinine clearance (mL/min) | 0.55 ± 0.13 | 0.49 ± 0.11 | 0.12 ± 0.05 *#¥ | 0.35 ± 0.10 *#$ | |
| Total protein (mg/dL) | 3.80 ± 0.39 | 3.97 ± 0.50 | 11.71 ± 1.98 *#¥ | 6.41 ± 1.06 *#$ | |
| NC Group | CH Group | GM Group | CH + GM Group | |
|---|---|---|---|---|
| TNF-α (pg/mL) | 58.1 ± 6.72 | 56.1 ± 5.42 | 190.1 ± 13.61 *#¥ | 109.1 ± 18.18 *#$ |
| IL1β (pg/mL) | 37.6 ± 13.11 | 33.1 ± 6.64 | 187.2 ± 17.90 *#¥ | 89.2 ± 13.12 *#$ |
| IL6 (pg/mL) | 63.1 ± 7.37 | 62.8 ± 8.01 | 248.8 ± 20.21 *#¥ | 103.6 ± 18.78 *#$ |
| IL18 (pg/mL) | 13.6 ± 3.11 | 12.50 ± 2.92 | 47.25 ± 6.71 *#¥ | 32.01 ± 4.78 *#$ |
| IL10 (pg/mL) | 37.6 ± 7.63 | 39.1 ± 4.92 | 19.8 ± 4.05 *#¥ | 35.1 ± 5.28 |
| HSP25 (ng/mL) | 1.73 ± 0.66 | 1.80 ± 0.40 | 5.98 ± 1.07 *#¥ | 2.15 ± 0.74 |
| GSH (mg/g) | 33.8 ± 5.44 | 34.4 ± 5.15 | 20.2 ± 5.07 *#¥ | 29.6 ± 5.26 |
| SOD (U/g) | 50.1 ± 6.68 | 52.6 ± 6.16 | 28.5 ± 5.47 *#¥ | 46.5 ± 5.70 |
| GPx1 (µg/mg) | 5.2 ± 0.1.1 | 5.5 ± 0.73 | 3.1 ± 0.74 *#¥ | 4.5 ± 0.44 # |
| CAT (U/mg) | 236 ± 29.8 | 239 ± 35.3 | 170 ± 18.70 *#¥ | 223 ± 22.6 |
| MDA (nmol/g) | 26.7 ± 7.12 | 25.6 ± 5.09 | 55.6 ± 7.85 *#¥ | 31.1 ± 7.60 |
| H2O2 (μM/g) | 1.70 ± 0.34 | 1.62 ± 0.29 | 9.5 ± 1.17 *#¥ | 3.1 ± 0.76 *# |
| Protein Carbonyl (nmol/g) | 0.56 ± 0.20 | 0.53 ± 0.10 | 5.4 ± 0.92 *#¥ | 0.73 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albukhari, T.A.; Bagadood, R.M.; Bokhari, B.T.; Filimban, W.A.; Sembawa, H.; Nasreldin, N.; Gadalla, H.E.; El-Boshy, M.E. Chrysin Attenuates Gentamicin-Induced Renal Injury in Rats Through Modulation of Oxidative Damage and Inflammation via Regulation of Nrf2/AKT and NF-kB/KIM-1 Pathways. Biomedicines 2025, 13, 271. https://doi.org/10.3390/biomedicines13020271
Albukhari TA, Bagadood RM, Bokhari BT, Filimban WA, Sembawa H, Nasreldin N, Gadalla HE, El-Boshy ME. Chrysin Attenuates Gentamicin-Induced Renal Injury in Rats Through Modulation of Oxidative Damage and Inflammation via Regulation of Nrf2/AKT and NF-kB/KIM-1 Pathways. Biomedicines. 2025; 13(2):271. https://doi.org/10.3390/biomedicines13020271
Chicago/Turabian StyleAlbukhari, Talat A., Rehab M. Bagadood, Bayan T. Bokhari, Waheed A. Filimban, Hatem Sembawa, Nani Nasreldin, Hossam E. Gadalla, and Mohamed E. El-Boshy. 2025. "Chrysin Attenuates Gentamicin-Induced Renal Injury in Rats Through Modulation of Oxidative Damage and Inflammation via Regulation of Nrf2/AKT and NF-kB/KIM-1 Pathways" Biomedicines 13, no. 2: 271. https://doi.org/10.3390/biomedicines13020271
APA StyleAlbukhari, T. A., Bagadood, R. M., Bokhari, B. T., Filimban, W. A., Sembawa, H., Nasreldin, N., Gadalla, H. E., & El-Boshy, M. E. (2025). Chrysin Attenuates Gentamicin-Induced Renal Injury in Rats Through Modulation of Oxidative Damage and Inflammation via Regulation of Nrf2/AKT and NF-kB/KIM-1 Pathways. Biomedicines, 13(2), 271. https://doi.org/10.3390/biomedicines13020271






