Phenotypes and Endotypes in Sarcoidosis: Unraveling Prognosis and Disease Course
Abstract
1. Introduction
2. Etiology
2.1. Genetic Background
2.2. Pathogenic Agents
2.3. Occupational Exposure
2.4. Lifestyle Factors
2.5. Seasonal Exposure
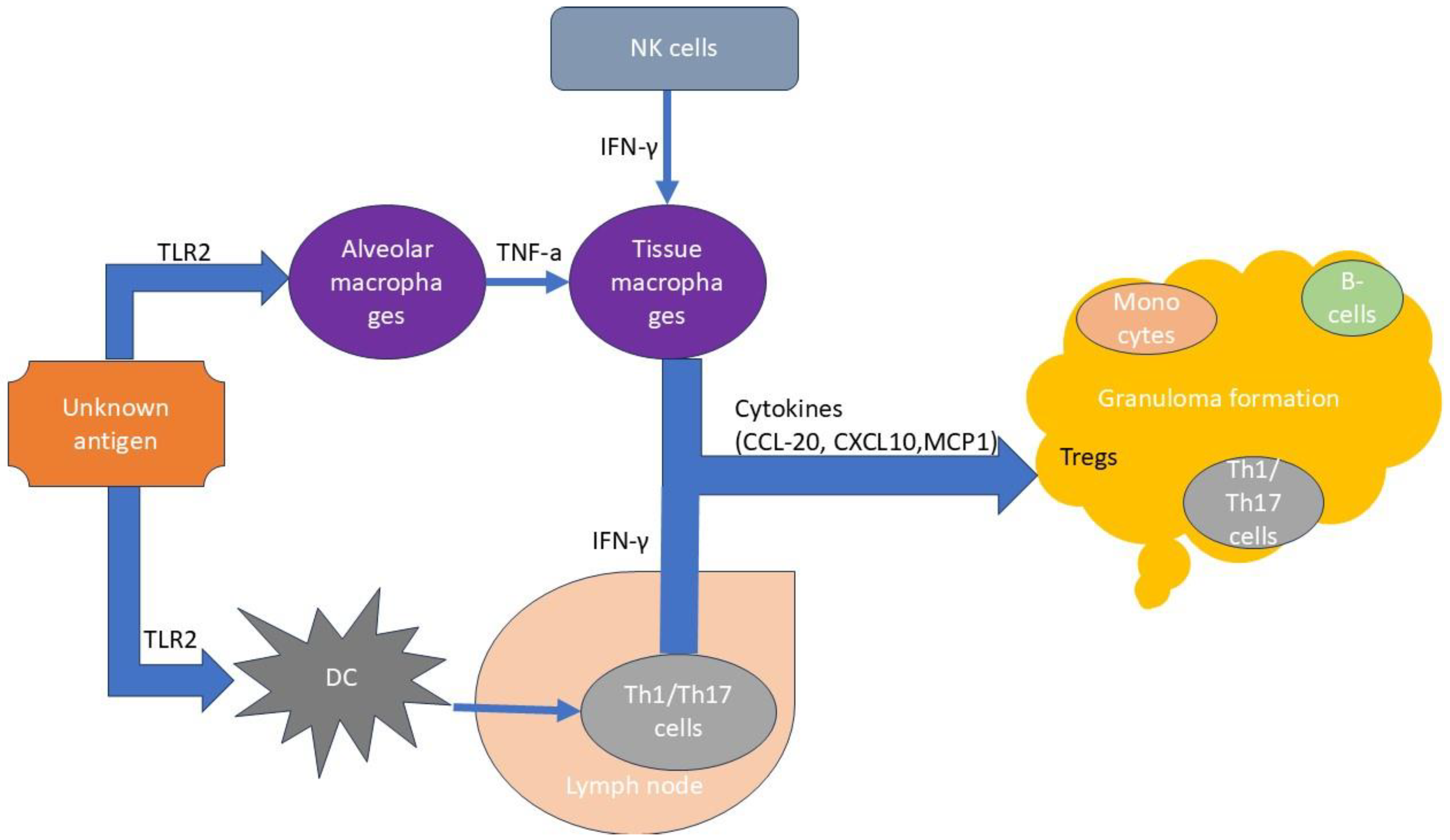
3. Immunopathogenesis
T Cells in Sarcoidosis Immunopathogenesis
4. Prognostic Factors in Sarcoidosis
4.1. Demographic Factors
4.2. Clinical Factors
4.3. Biomarkers
4.4. COVID-19
5. Phenotypes in Sarcoidosis
5.1. Radiology Phenotyping
5.2. Pulmonary Function Phenotyping
5.3. Clinical Tools for Phenotyping
5.4. Nuclear Imaging Phenotyping
5.5. Genetic Phenotyping
6. Endotypes in Sarcoidosis
6.1. Genotype Analysis
6.2. Autophagy
6.3. Implications for Treatment
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grunewald, J.; Grutters, J.C.; Arkema, E.V.; Saketkoo, L.A.; Moller, D.R.; Müller-Quernheim, J. Sarcoidosis. Nat. Rev. Dis. Primers 2019, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Cozier, Y.C.; Arkema, E.V.; Rodriguez, J.V.; Berman, J.S.; Govender, P. Epidemiology of Sarcoidosis: Solving the Jigsaw Puzzle. In Sarcoidosis (ERS Monograph); Bonella, F., Culver, D.A., Israël-Biet, D., Eds.; European Respiratory Society: Sheffield, UK, 2022; pp. 8–24. [Google Scholar]
- Rybicki, B.A.; Iannuzzi, M.C.; Frederick, M.M.; Thompson, B.W.; Rossman, M.D.; Bresnitz, E.A.; Terrin, M.L.; Moller, D.R.; Barnard, J.; Baughman, R.P. Familial Aggregation of Sarcoidosis. A Case-Control Etiologic Study of Sarcoidosis (ACCESS). Am. J. Respir. Crit. Care Med. 2001, 164, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Crouser, E.D.; Maier, L.A.; Wilson, K.C.; Bonham, C.A.; Morgenthau, A.S.; Patterson, K.C.; Abston, E.; Bernstein, R.C.; Blankstein, R.; Chen, E.S.; et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 201, e26–e51. [Google Scholar] [CrossRef]
- Sverrild, A.; Backer, V.; Kyvik, K.O.; Kaprio, J.; Milman, N.; Svendsen, C.B.; Thomsen, S.F. Heredity in Sarcoidosis: A Registry-Based Twin Study. Thorax 2008, 63, 894–896. [Google Scholar] [CrossRef]
- Grunewald, J.; Brynedal, B.; Darlington, P.; Nisell, M.; Cederlund, K.; Hillert, J.; Eklund, A. Different HLA-DRB1 Allele Distributions in Distinct Clinical Subgroups of Sarcoidosis Patients. Respir. Res. 2010, 11, 25. [Google Scholar] [CrossRef]
- Spagnolo, P.; Renzoni, E.A.; Wells, A.U.; Copley, S.J.; Desai, S.R.; Sato, H.; Grutters, J.C.; Abdallah, A.; Taegtmeyer, A.; Bois, R.M. C-C Chemokine Receptor 5 Gene Variants in Relation to Lung Disease in Sarcoidosis. Am. J. Respir. Crit. Care Med. 2005, 172, 721–728. [Google Scholar] [CrossRef]
- Spagnolo, P.; Sato, H.; Grunewald, J.; Brynedal, B.; Hillert, J.; Mañá, J.; Wells, A.U.; Eklund, A.; Welsh, K.I.; Bois, R.M. A Common Haplotype of the C-C Chemokine Receptor 2 Gene and HLA-DRB1*0301 Are Independent Genetic Risk Factors for Löfgren’s Syndrome. J. Intern. Med. 2008, 264, 433–441. [Google Scholar] [CrossRef]
- Wennerström, A.; Pietinalho, A.; Vauhkonen, H.; Lahtela, L.; Palikhe, A.; Hedman, J.; Purokivi, M.; Varkki, E.; Seppänen, M.; Lokki, M.-L. HLA-DRB1 Allele Frequencies and C4 Copy Number Variation in Finnish Sarcoidosis Patients and Associations with Disease Prognosis. Hum. Immunol. 2012, 73, 93–100. [Google Scholar] [CrossRef]
- Morais, A.; Lima, B.; Peixoto, M.J.; Alves, H.; Marques, A.; Delgado, L. BTNL2 Gene Polymorphism Associations with Susceptibility and Phenotype Expression in Sarcoidosis. Respir. Med. 2012, 106, 1771–1777. [Google Scholar] [CrossRef]
- Iannuzzi, M.C.; Maliarik, M.J.; Poisson, L.M.; Rybicki, B.A. Sarcoidosis Susceptibility and Resistance HLA-DQB1 Alleles in African Americans. Am. J. Respir. Crit. Care Med. 2003, 167, 1225–1231. [Google Scholar] [CrossRef]
- Grutters, J.C.; Sato, H.; Pantelidis, P.; Lagan, A.L.; McGrath, D.S.; Lammers, J.-W.J.; Bosch, J.M.M.; Wells, A.U.; Bois, R.M.; Welsh, K.I. Increased Frequency of the Uncommon Tumor Necrosis Factor -857T Allele in British and Dutch Patients with Sarcoidosis. Am. J. Respir. Crit. Care Med. 2002, 165, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Kieszko, R.; Krawczyk, P.; Chocholska, S.; Dmoszyńska, A.; Milanowski, J. TNF-Alpha and TNF-Beta Gene Polymorphisms in Polish Patients with Sarcoidosis: Connection with the Susceptibility and Prognosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2010, 27, 131–137. [Google Scholar] [PubMed]
- McDougal, K.E.; Fallin, M.D.; Moller, D.R.; Song, Z.; Cutler, D.J.; Steiner, L.L.; Cutting, G.R.; Group, A.C.C.E.S.S.R. Variation in the Lymphotoxin-Alpha/Tumor Necrosis Factor Locus Modifies Risk of Erythema Nodosum in Sarcoidosis. J. Investig. Dermatol. 2009, 129, 1921–1926. [Google Scholar] [CrossRef]
- Seitzer, U.; Swider, C.; Stüber, F.; Suchnicki, K.; Lange, A.; Richter, E.; Zabel, P.; Müller-Quernheim, J.; Flad, H.D.; Gerdes, J. Tumour Necrosis Factor Alpha Promoter Gene Polymorphism in Sarcoidosis. Cytokine 1997, 9, 787–790. [Google Scholar] [CrossRef]
- Fischer, A.; Ellinghaus, D.; Nutsua, M.; Hofmann, S.; Montgomery, C.G.; Iannuzzi, M.C.; Rybicki, B.A.; Petrek, M.; Mrazek, F.; Pabst, S. Identification of Immune-Relevant Factors Conferring Sarcoidosis Genetic Risk. Am. J. Respir. Crit. Care Med. 2015, 192, 727–736. [Google Scholar] [CrossRef]
- Fischer, A.; Nothnagel, M.; Franke, A.; Jacobs, G.; Saadati, H.R.; Gaede, K.I.; Rosenstiel, P.; Schürmann, M.; Müller-Quernheim, J.; Schreiber, S. Association of Inflammatory Bowel Disease Risk Loci with Sarcoidosis and Its Acute and Chronic Subphenotypes. Eur. Respir. J. 2011, 37, 610–616. [Google Scholar] [CrossRef]
- Spagnolo, P.; Renzoni, E.A.; Wells, A.U.; Sato, H.; Grutters, J.C.; Sestini, P.; Abdallah, A.; Gramiccioni, E.; Ruven, H.J.T.; Bois, R.M. C-C Chemokine Receptor 2 and Sarcoidosis: Association with Löfgren’s Syndrome. Am. J. Respir. Crit. Care Med. 2003, 168, 1162–1166. [Google Scholar] [CrossRef]
- Veltkamp, M.; Moorsel, C.H.M.; Rijkers, G.T.; Ruven, H.J.T.; Grutters, J.C. Genetic Variation in the Toll-Like Receptor Gene Cluster (TLR10-TLR1-TLR6) Influences Disease Course in Sarcoidosis. Tissue Antigens 2012, 79, 25–32. [Google Scholar] [CrossRef]
- Cooke, G.; Kamal, I.; Strengert, M.; Hams, E.; Mawhinney, L.; Tynan, A.; O’Reilly, C.; O’Dwyer, D.N.; Kunkel, S.L.; Knaus, U.G. Toll-Like Receptor 3 L412F Polymorphism Promotes a Persistent Clinical Phenotype in Pulmonary Sarcoidosis. QJM 2018, 111, 217–224. [Google Scholar] [CrossRef]
- Daniil, Z.; Mollaki, V.; Malli, F.; Koutsokera, A.; Antoniou, K.M.; Rodopoulou, P.; Gourgoulianis, K.; Zintzaras, E.; Vassilopoulos, G. Polymorphisms and haplotypes in MyD88 are associated with the development of sarcoidosis: A candidate-gene association study. Mol. Biol. Rep. 2013, 40, 4281–4286. [Google Scholar] [CrossRef]
- Nguyen, T.; Liu, X.K.; Zhang, Y.; Dong, C. BTNL2, a Butyrophilin-Like Molecule That Functions to Inhibit T Cell Activation. J. Immunol. 2006, 176, 7354–7360. [Google Scholar] [CrossRef] [PubMed]
- Maliarik, M.J.; Chen, K.M.; Sheffer, R.G.; Rybicki, B.A.; Major, M.L.; Popovich, J.; Iannuzzi, M.C. The Natural Resistance-Associated Macrophage Protein Gene in African Americans with Sarcoidosis. Am. J. Respir. Cell Mol. Biol. 2000, 22, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Franke, A.; Fischer, A.; Jacobs, G.; Nothnagel, M.; Gaede, K.I.; Schürmann, M.; Müller-Quernheim, J.; Krawczak, M.; Rosenstiel, P. Genome-Wide Association Study Identifies ANXA11 as a New Susceptibility Locus for Sarcoidosis. Nat. Genet. 2008, 40, 1103–1106. [Google Scholar] [CrossRef]
- Kinloch, A.J.; Kaiser, Y.; Wolfgeher, D.; Ai, J.; Eklund, A.; Clark, M.R.; Grunewald, J. In Situ Humoral Immunity to Vimentin in HLA-DRB1*03+ Patients with Pulmonary Sarcoidosis. Front. Immunol. 2018, 9, 1516. [Google Scholar] [CrossRef] [PubMed]
- Dubaniewicz, A.; Dubaniewicz-Wybieralska, M.; Sternau, A.; Zwolska, Z.; Izycka-Swieszewska, E.; Augustynowicz-Kopec, E.; Skokowski, J.; Singh, M.; Zimnoch, L. Mycobacterium tuberculosis Complex and Mycobacterial Heat Shock Proteins in Lymph Node Tissue from Patients with Pulmonary Sarcoidosis. J. Clin. Microbiol. 2006, 44, 3448–3451. [Google Scholar] [CrossRef]
- Masoud, S.; Mihan, P.; Hamed, M.; Mehdi, M.; Mohamad, R.M. The Presence of Mycobacterial Antigens in Sarcoidosis-Associated Granulomas. Sarcoidosis Vasc. Diffuse Lung Dis. 2017, 34, 236–241. [Google Scholar] [CrossRef]
- Carlisle, J.; Evans, W.; Hajizadeh, R.; Nadaf, M.; Shepherd, B.; Ott, R.D.; Richter, K.; Drake, W. Multiple Mycobacterium Antigens Induce Interferon-Gamma Production from Sarcoidosis Peripheral Blood Mononuclear Cells. Clin. Exp. Immunol. 2007, 150, 460–468. [Google Scholar] [CrossRef]
- Oswald-Richter, K.A.; Beachboard, D.C.; Zhan, X.; Gaskill, C.F.; Abraham, S.; Jenkins, C.; Culver, D.A.; Drake, W. Multiple Mycobacterial Antigens Are Targets of the Adaptive Immune Response in Pulmonary Sarcoidosis. Respir. Res. 2010, 11, 161. [Google Scholar] [CrossRef]
- Ishige, I.; Eishi, Y.; Takemura, T.; Kobayashi, I.; Nakata, K.; Tanaka, I.; Nagaoka, S.; Iwai, K.; Watanabe, K.; Takizawa, T. Propionibacterium acnes is the Most Common Bacterium Commensal in Peripheral Lung Tissue and Mediastinal Lymph Nodes from Subjects Without Sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2005, 22, 33–42. [Google Scholar]
- Beijer, E.; Seldenrijk, K.; Meek, B.; Damen, J.; Quanjel, M.J.R.; Grutters, J.C.; Veltkamp, M. Detection of Cutibacterium acnes in Granulomas of Patients with Either Hypersensitivity Pneumonitis or Vasculitis Reveals That Its Presence is Not Unique for Sarcoidosis. ERJ Open Res. 2021, 7, 00930–02020. [Google Scholar] [CrossRef]
- Drake, W.P.; Culver, D.A.; Baughman, R.P.; Judson, M.A.; Crouser, E.D.; James, W.E.; Ayers, G.D.; Ding, T.; Abel, K.; Green, A. Phase II Investigation of the Efficacy of Antimycobacterial Therapy in Chronic Pulmonary Sarcoidosis. Chest 2021, 159, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Newman, L.S.; Rose, C.S.; Bresnitz, E.A.; Rossman, M.D.; Barnard, J.; Frederick, M.; Terrin, M.L.; Weinberger, S.E.; Moller, D.R.; McLennan, G. A Case Control Etiologic Study of Sarcoidosis: Environmental and Occupational Risk Factors. Am. J. Respir. Crit. Care Med. 2004, 170, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.C.; Zarnke, A.M. Sarcoidosis: An Occupational Disease? Chest 2021, 160, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Izbicki, G.; Chavko, R.; Banauch, G.I.; Weiden, M.D.; Berger, K.I.; Aldrich, T.K.; Hall, C.; Kelly, K.J.; Prezant, D.J. World Trade Center “Sarcoid-Like” Granulomatous Pulmonary Disease in New York City Fire Department Rescue Workers. Chest 2007, 131, 1414–1423. [Google Scholar] [CrossRef]
- Graff, P.; Larsson, J.; Bryngelsson, I.-L.; Wiebert, P.; Vihlborg, P. Sarcoidosis and Silica Dust Exposure Among Men in Sweden: A Case-Control Study. BMJ Open 2020, 10, 038926. [Google Scholar] [CrossRef]
- Rafnsson, V.; Ingimarsson, O.; Hjalmarsson, I.; Gunnarsdottir, H. Association Between Exposure to Crystalline Silica and Risk of Sarcoidosis. Occup. Environ. Med. 1998, 55, 657–660. [Google Scholar] [CrossRef]
- Lu, B.; Hiraki, L.T.; Sparks, J.A.; Malspeis, S.; Chen, C.-Y.; Awosogba, J.A.; Arkema, E.V.; Costenbader, K.H.; Karlson, E.W. Being Overweight or Obese and Risk of Developing Rheumatoid Arthritis Among Women: A Prospective Cohort Study. Ann. Rheum. Dis. 2014, 73, 1914–1922. [Google Scholar] [CrossRef]
- Tedeschi, S.K.; Barbhaiya, M.; Malspeis, S.; Lu, B.; Sparks, J.A.; Karlson, E.W.; Willett, W.; Costenbader, K.H. Obesity and the Risk of Systemic Lupus Erythematosus Among Women in the Nurses’ Health Studies. Semin. Arthritis Rheum. 2017, 47, 376–383. [Google Scholar] [CrossRef]
- Cozier, Y.C.; Barbhaiya, M.; Castro-Webb, N.; Conte, C.; Tedeschi, S.; Leatherwood, C.; Costenbader, K.H.; Rosenberg, L. A Prospective Study of Obesity and Risk of Systemic Lupus Erythematosus (SLE) Among Black Women. Semin. Arthritis Rheum. 2019, 48, 1030–1034. [Google Scholar] [CrossRef]
- Cozier, Y.C.; Coogan, P.F.; Govender, P.; Berman, J.S.; Palmer, J.R.; Rosenberg, L.O.; Gain, W. Relation to Incidence of Sarcoidosis in US Black Women: Data from the Black Women’s Health Study. Chest 2015, 147, 1086–1093. [Google Scholar] [CrossRef]
- Dumas, O.; Boggs, K.M.; Cozier, Y.C.; Stampfer, M.J.; Camargo, C.A. Prospective Study of Body Mass Index and Risk of Sarcoidosis in US Women. Eur. Respir. J. 2017, 50, 1701397. [Google Scholar] [CrossRef] [PubMed]
- Ungprasert, P.; Crowson, C.S.; Matteson, E.L. Smoking, obesity and risk of sarcoidosis: A population-based nested case-control study. Respir. Med. 2016, 120, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Valeyre, D.; Soler, P.; Clerici, C.; Pré, J.; Battesti, J.P.; Georges, R.; Hance, A.J. Smoking and Pulmonary Sarcoidosis: Effect of Cigarette Smoking on Prevalence, Clinical Manifestations, Alveolitis, and Evolution of the Disease. Thorax 1988, 43, 516–524. [Google Scholar] [CrossRef]
- Crouser, E.D.; Smith, R.M.; Culver, D.A.; Julian, M.W.; Martin, K.; Baran, J.; Diaz, C.; Erdal, B.S.; Hade, E.M. A Pilot Randomized Trial of Transdermal Nicotine for Pulmonary Sarcoidosis. Chest 2021, 160, 1340–1349. [Google Scholar] [CrossRef]
- Kajdasz, D.K.; Lackland, D.T.; Mohr, L.C.; Judson, M.A. A current assessment of rurally linked exposures as potential risk factors for sarcoidosis. Ann. Epidemiol. 2001, 11, 111–117. [Google Scholar] [CrossRef]
- Chioma, O.S.; Hesse, L.E.; Chapman, A.; Drake, W.P. Role of the Microbiome in Interstitial Lung Diseases. Front. Med. (Lausanne) 2021, 8, 595522. [Google Scholar] [CrossRef]
- James, D.G.; Lipman, M.C. Whipple’s disease: A granulomatous masquerader. Clin. Chest Med. 2002, 23, 513–519, xi-xii. [Google Scholar] [CrossRef]
- Ungprasert, P.; Crowson, C.S.; Matteson, E.L. Seasonal Variation in Incidence of Sarcoidosis: A Population-Based Study, 1976–2013. Thorax 2016, 71, 1164–1166. [Google Scholar] [CrossRef]
- Gerke, A.K.; Tangh, F.; Yang, M.; Cavanaugh, J.E.; Polgreen, P.M. An analysis of seasonality of sarcoidosis in the United States veteran population: 2000–2007. Sarcoidosis Vasc. Diffuse Lung Dis. 2012, 29, 155–158. [Google Scholar]
- Cozier, Y.C.; Berman, J.S.; Palmer, J.R.; Boggs, D.A.; Wise, L.A.; Rosenberg, L. Reproductive and Hormonal Factors in Relation to Incidence of Sarcoidosis in US Black Women: The Black Women’s Health Study. Am. J. Epidemiol. 2012, 176, 635–641. [Google Scholar] [CrossRef]
- Rossi, G.; Cavazza, A.; Colby, T.V. Pathology of Sarcoidosis. Clin. Rev. Allergy Immunol. 2015, 49, 36–44. [Google Scholar] [CrossRef]
- Damsky, W.; Wang, A.; Kim, D.J.; Young, B.D.; Singh, K.; Murphy, M.J.; Daccache, J.; Clark, A.; Ayasun, R.; Ryu, C. Inhibition of Type 1 Immunity with Tofacitinib is Associated with Marked Improvement in Longstanding Sarcoidosis. Nat. Commun. 2022, 13, 3140. [Google Scholar] [CrossRef] [PubMed]
- Co, D.O.; Hogan, L.H.; Il-Kim, S.; Sandor, M. T Cell Contributions to the Different Phases of Granuloma Formation. Immunol. Lett. 2004, 92, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, P.; Bruder, D. Mechanism of Granuloma Formation in Sarcoidosis. Curr. Opin. Hematol. 2017, 24, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, T.; Yoneyama, H.; Eishi, Y.; Matsuo, N.; Tatsumi, K.; Kimura, H.; Kuriyama, T.; Matsushima, K. Indigenous pulmonary Propionibacterium acnes primes the host in the development of sarcoid-like pulmonary granulomatosis in mice. Am. J. Pathol. 2004, 165, 631–639. [Google Scholar] [CrossRef]
- Kraaijvanger, R.; Veltkamp, M. The Role of Cutibacterium acnes in Sarcoidosis: From Antigen to Treatable Trait? Microorganisms 2022, 10, 1649. [Google Scholar] [CrossRef]
- Mortaz, E.; Adcock, I.M.; Abedini, A.; Kiani, A.; Kazempour-Dizaji, M.; Movassaghi, M.; Garssen, J. The role of pattern recognition receptors in lung sarcoidosis. Eur. J. Pharmacol. 2017, 808, 44–48. [Google Scholar] [CrossRef]
- Werner, J.L.; Escolero, S.G.; Hewlett, J.T.; Mak, T.N.; Williams, B.P.; Eishi, Y.; Nunez, G. Induction of Pulmonary Granuloma Formation by Propionibacterium acnes Is Regulated by MyD88 and Nox2. Am. J. Respir. Cell Mol. Biol. 2017, 56, 121–130. [Google Scholar] [CrossRef]
- Pabst, S.; Baumgarten, G.; Stremmel, A.; Lennarz, M.; Knufermann, P.; Gillissen, A.; Vetter, H.; Grohe, C. Toll-like receptor (TLR) 4 polymorphisms are associated with a chronic course of sarcoidosis. Clin. Exp. Immunol. 2006, 143, 420–426. [Google Scholar] [CrossRef]
- Robert, M.; Yatim, N.; Sacré, K.; Duffy, D. Sarcoidosis Immunopathogenesis—A New Concept of Maladaptive Trained Immunity. Trends Immunol. 2024, 45, 406–418. [Google Scholar] [CrossRef]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Moorlag, S.J.C.F.M.; Novakovic, B.; Li, Y.; Wang, S.-Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B. BCG Vaccination Protects Against Experimental Viral Infection in Humans Through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100 105. [Google Scholar] [CrossRef]
- Grunewald, J.; Spagnolo, P.; Wahlström, J.; Eklund, A. Immunogenetics of Disease-Causing Inflammation in Sarcoidosis. Clin. Rev. Allergy Immunol. 2015, 49, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Zissel, G.; Homolka, J.; Schlaak, J.; Schlaak, M.; Muller-Quernheim, J. Anti-inflammatory cytokine release by alveolar macrophages in pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 1996, 154, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Pabst, S.; Franken, T.; Schonau, J.; Stier, S.; Nickenig, G.; Meyer, R.; Skowasch, D.; Grohe, C. Transforming growth factor-beta gene polymorphisms in different phenotypes of sarcoidosis. Eur. Respir. J. 2011, 38, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.; Lim, C.X.; Weichhart, T.; Valeyre, D.; Bentaher, A.; Calender, A. Sarcoidosis and the mTOR, Rac1, and Autophagy Triad. Trends Immunol. 2020, 41, 286–299. [Google Scholar] [CrossRef]
- Pacheco, Y.; Valeyre, D.; El Jammal, T.; Vallee, M.; Chevalier, F.; Lamartine, J.; Sigaudo-Roussel, D.; Verrier, B.; Israel-Biet, D.; Freymond, N.; et al. Autophagy and Mitophagy-Related Pathways at the Crossroads of Genetic Pathways Involved in Familial Sarcoidosis and Host-Pathogen Interactions Induced by Coronaviruses. Cells 2021, 10, 1995. [Google Scholar] [CrossRef]
- Linke, M.; Pham, H.T.; Katholnig, K.; Schnoller, T.; Miller, A.; Demel, F.; Schutz, B.; Rosner, M.; Kovacic, B.; Sukhbaatar, N.; et al. Chronic signaling via the metabolic checkpoint kinase mTORC1 induces macrophage granuloma formation and marks sarcoidosis progression. Nat. Immunol. 2017, 18, 293–302. [Google Scholar] [CrossRef]
- Calender, A.; Lim, C.X.; Weichhart, T.; Buisson, A.; Besnard, V.; Rollat-Farnier, P.A.; Bardel, C.; Roy, P.; Cottin, V.; Devouassoux, G.; et al. Exome sequencing and pathogenicity-network analysis of five French families implicate mTOR signalling and autophagy in familial sarcoidosis. Eur. Respir. J. 2019, 54, 1900430. [Google Scholar] [CrossRef]
- Zhou, T.; Casanova, N.; Pouladi, N.; Wang, T.; Lussier, Y.; Knox, K.S.; Garcia, J.G.N. Identification of Jak-STAT signaling involvement in sarcoidosis severity via a novel microRNA-regulated peripheral blood mononuclear cell gene signature. Sci. Rep. 2017, 7, 4237. [Google Scholar] [CrossRef]
- Drent, M.; Crouser, E.D.; Grunewald, J. Challenges of Sarcoidosis and Its Management. N. Engl. J. Med. 2021, 385, 1018–1032. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L. Transcriptome-Based Network Analysis Reveals a Spectrum Model of Human Macrophage Activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Heufler, C. Interleukin-12 is Produced by Dendritic Cells and Mediates T Helper 1 Development as well as Interferon-γ Production by T Helper 1 Cells. Eur. J. Immunol. 1996, 26, 659–668. [Google Scholar] [CrossRef]
- Minshall, E.M.; Tsicopoulos, A.; Yasruel, Z.; Wallaert, B.; Akoum, H.; Vorng, H.; Tonnel, A.B.; Hamid, Q. Cytokine mRNA gene expression in active and nonactive pulmonary sarcoidosis. Eur. Respir. J. 1997, 10, 2034–2039. [Google Scholar] [CrossRef]
- Volpe, E.; Servant, N.; Zollinger, R.; Bogiatzi, S.I.; Hupé, P.; Barillot, E.; Soumelis, V. A Critical Function for Transforming Growth Factor-β, Interleukin 23, and Proinflammatory Cytokines in Driving and Modulating Human TH-17 Responses. Nat. Immunol. 2008, 9, 650–657. [Google Scholar] [CrossRef]
- Lepzien, R.; Rankin, G.; Pourazar, J.; Muala, A.; Eklund, A.; Grunewald, J.; Blomberg, A.; Smed-Sörensen, A. Mapping Mononuclear Phagocytes in Blood, Lungs, and Lymph Nodes of Sarcoidosis Patients. J. Leukoc. Biol. 2019, 105, 797–807. [Google Scholar] [CrossRef]
- Broos, C.E.; Nimwegen, M.; Kleinjan, A. Impaired Survival of Regulatory T Cells in Pulmonary Sarcoidosis. Respir. Res. 2015, 16, 108. [Google Scholar] [CrossRef]
- Hawkins, C.; Shaginurova, G.; Shelton, D.A.; Herazo-Maya, J.D.; Oswald-Richter, K.A.; Rotsinger, J.E.; Young, A.; Celada, L.J.; Kaminski, N.; Sevin, C. Local and Systemic CD4+ T Cell Exhaustion Reverses with Clinical Resolution of Pulmonary Sarcoidosis. J. Immunol. Res. 2017, 2017, 3642832. [Google Scholar] [CrossRef]
- Miedema, J.R.; Kaiser, Y.; Broos, C.E.; Wijsenbeek, M.S.; Grunewald, J.; Kool, M. Th17-Lineage Cells in Pulmonary Sarcoidosis and Löfgren’s Syndrome: Friend or Foe? J. Autoimmun. 2018, 87, 82–96. [Google Scholar] [CrossRef]
- Miedema, J.; Cinetto, F.; Smed-Sörensen, A.; Spagnolo, P. The Immunopathogenesis of Sarcoidosis. J. Autoimmun. 2024, 149, 103247. [Google Scholar] [CrossRef]
- Broos, C.E.; Koth, L.L.; van Nimwegen, M.; In ‘t Veen, J.; Paulissen, S.M.J.; van Hamburg, J.P.; Annema, J.T.; Heller-Baan, R.; Kleinjan, A.; Hoogsteden, H.C.; et al. Increased T-helper 17.1 cells in sarcoidosis mediastinal lymph nodes. Eur. Respir. J. 2018, 51, 1701124. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, Y.; Lepzien, R.; Kullberg, S.; Eklund, A.; Smed-Sörensen, A.; Grunewald, J. Expanded Lung T-bet+RORγT+ CD4+ T-Cells in Sarcoidosis Patients with a Favourable Disease Phenotype. Eur. Respir. J. 2016, 48, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Idali, F.; Wiken, M.; Wahlstrom, J.; Mellstedt, H.; Eklund, A.; Rabbani, H.; Grunewald, J. Reduced Th1 response in the lungs of HLA-DRB1*0301 patients with pulmonary sarcoidosis. Eur. Respir. J. 2006, 27, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Kozhaya, L.; McKevitt, K.; Djuretic, I.M.; Carlson, T.J.; Quintero, M.A.; McCauley, J.L.; Abreu, M.T.; Unutmaz, D.; Sundrud, M.S. Pro-Inflammatory Human Th17 Cells Selectively Express P-Glycoprotein and Are Refractory to Glucocorticoids. J. Exp. Med. 2014, 211, 89–104. [Google Scholar] [CrossRef]
- Sakthivel, P.; Grunewald, J.; Eklund, A.; Bruder, D.; Wahlström, J. Pulmonary Sarcoidosis is Associated with High-Level Inducible Co-Stimulator (ICOS) Expression on Lung Regulatory T Cells—Possible Implications for the ICOS/ICOS-Ligand Axis in Disease Course and Resolution. Clin. Exp. Immunol. 2016, 183, 294–306. [Google Scholar] [CrossRef]
- Oswald-Richter, K.A.; Richmond, B.W.; Braun, N.A.; Isom, J.; Abraham, S.; Taylor, T.R.; Drake, J.M.; Culver, D.A.; Wilkes, D.S.; Drake, W.P. Reversal of Global CD4+ Subset Dysfunction is Associated with Spontaneous Clinical Resolution of Pulmonary Sarcoidosis. J. Immunol. 2013, 190, 5446–5453. [Google Scholar] [CrossRef]
- Miyara, M.; Amoura, Z.; Parizot, C.; Badoual, C.; Dorgham, K.; Trad, S.; Kambouchner, M.; Valeyre, D.; Chapelon-Abric, C.; Debré, P. The Immune Paradox of Sarcoidosis and Regulatory T Cells. J. Exp. Med. 2006, 203, 359–370. [Google Scholar] [CrossRef]
- Miedema, J.R.; de Jong, L.J.; van Uden, D.; Bergen, I.M.; Kool, M.; Broos, C.E.; Kahlmann, V.; Wijsenbeek, M.S.; Hendriks, R.W.; Corneth, O.B.J. Circulating T cells in sarcoidosis have an aberrantly activated phenotype that correlates with disease outcome. J. Autoimmun. 2024, 149, 103120. [Google Scholar] [CrossRef]
- Bergantini, L.; d’Alessandro, M.; Del Zotto, G.; Marcenaro, E.; Bargagli, E. Characterization of Natural Killer and T Cells in Bronchoalveolar Lavage and Peripheral Blood of Sarcoidosis Patients. Front. Immunol. 2023, 13, 1080556. [Google Scholar] [CrossRef]
- Katchar, K.; Söderström, K.; Wahlstrom, J.; Eklund, A.; Grunewald, J. Characterisation of Natural Killer Cells and CD56+ T-Cells in Sarcoidosis Patients. Eur. Respir. J. 2005, 26, 77–85. [Google Scholar] [CrossRef]
- Saussine, A.; Tazi, A.; Feuillet, S.; Rybojad, M.; Juillard, C.; Bergeron, A.; Dessirier, V.; Bouhidel, F.; Janin, A.; Bensussan, A. Active Chronic Sarcoidosis is Characterized by Increased Transitional Blood B Cells, Increased IL-10-Producing Regulatory B Cells and High BAFF Levels. PLoS ONE 2012, 7, 43588. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-S.; Barber, L.; Akula, S.M.; Sigounas, G.; Kataria, Y.P.; Arce, S. Disturbed Homeostasis and Multiple Signaling Defects in the Peripheral Blood B-Cell Compartment of Patients with Severe Chronic Sarcoidosis. Clin. Vaccine Immunol. 2011, 18, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Elwazir, M.; Krause, M.L.; Bois, J.P.; Christopoulos, G.; Kendi, A.T.; Cooper, J.R.L.T.; Jouni, H.; Abouezzeddine, O.F.; Chareonthaitawee, P.; Abdelshafee, M. Rituximab for the Treatment of Refractory Cardiac Sarcoidosis: A Single-Center Experience. J. Card. Fail 2022, 28, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Tutor-Ureta, P.; Citores, M.J.; Castejón, R.; Mellor-Pita, S.; Yebra-Bango, M.; Romero, Y. Prognostic Value of Neutrophils and NK Cells in Bronchoalveolar Lavage of Sarcoidosis. Cytom. B Clin. Cytom. 2006, 70, 416–422. [Google Scholar] [CrossRef]
- Kamphuis, L.S.; Zelm, M.C.; Lam, K.H.; Rimmelzwaan, G.F.; Baarsma, G.S.; Dik, W.A.; Thio, H.B.; Daele, P.L.; Velthoven, M.E.; Batstra, M.R. Perigranuloma Localization and Abnormal Maturation of B Cells Emerging Key Players in Sarcoidosis? Am. J. Respir. Crit. Care Med. 2013, 187, 406–416. [Google Scholar] [CrossRef]
- Hunninghake, G.; Costabel, U. Statement on Sarcoidosis. Am. J. Respir. Crit. Care Med. 1999, 160, 20. [Google Scholar]
- Rybicki, B.A.; Major, M.; Popovich, J.; Maliank, M.J.; Lannuzzi, M.C. Racial Differences in Sarcoidosis Incidence: A 5-Year Study in a Health Maintenance Organization. Am. J. Epidemiol. 1997, 145, 234–241. [Google Scholar] [CrossRef]
- Swigris, J.J.; Olson, A.L.; Huie, T.J. Sarcoidosis-Related Mortality in the United States from 1988 to 2007. Am. J. Respir. Crit. Care Med. 2011, 183, 1524–1530. [Google Scholar] [CrossRef]
- Rabin, D.L.; Thompson, B.; Brown, K.M. Sarcoidosis: Social Predictors of Severity at Presentation. Eur. Respir. J. 2004, 24, 601–608. [Google Scholar] [CrossRef]
- Morimoto, T.; Azuma, A.; Abe, S.; Usuki, J.; Kudoh, S.; Sugisaki, K.; Oritsu, M.; Nukiwa, T. Epidemiology of Sarcoidosis in Japan. Eur. Respir. J. 2008, 31, 372–379. [Google Scholar] [CrossRef]
- Mañá, J.; Rubio-Rivas, M.; Villalba, N.; Marcoval, J.; Iriarte, A.; Molina-Molina, M. Multidisciplinary Approach and Long-Term Follow-Up in a Series of 640 Consecutive Patients with Sarcoidosis: Cohort Study of a 40-Year Clinical Experience at a Tertiary Referral Center in Barcelona, Spain. Medicine 2017, 96, e7595. [Google Scholar] [CrossRef] [PubMed]
- Judson, M.A.; Baughman, R.P.; Thompson, B.W. Two-Year Prognosis of Sarcoidosis: The ACCESS Experience. Sarcoidosis Vasc. Diffuse Lung Dis. 2003, 20, 204–211. [Google Scholar] [PubMed]
- Judson, M.A.; Boan, A.D.; Lackland, D.T. The Clinical Course of Sarcoidosis: Presentation, Diagnosis, and Treatment in a Large White and Black Cohort in the United States. Sarcoidosis Vasc. Diffuse Lung Dis. 2012, 29, 119–127. [Google Scholar] [PubMed]
- Inoue, Y.; Inui, N.; Hashimoto, D.; Enomoto, N.; Fujisawa, T.; Nakamura, Y. Cumulative Incidence and Predictors of Progression in Corticosteroid-Naive Patients with Sarcoidosis. PLoS ONE 2015, 10, 0143371. [Google Scholar] [CrossRef]
- Neville, E.; Walker, A.N.; James, D.G. Prognostic Factors Predicting the Outcome of Sarcoidosis: An Analysis of 818 Patients. Q. J. Med. 1983, 52, 525–533. [Google Scholar]
- Rossides, M.; Kullberg, S.; Askling, J.; Eklund, A.; Grunewald, J.; Arkema, E.V. Sarcoidosis Mortality in Sweden: A Population-Based Cohort Study. Eur. Respir. J. 2018, 51, 1701815. [Google Scholar] [CrossRef]
- Caplan, A.; Rosenbach, M.; Imadojemu, S. Cutaneous Sarcoidosis. Semin. Respir. Crit. Care Med. 2020, 41, 689–699. [Google Scholar] [CrossRef]
- Haimovic, A.; Sanchez, M.; Judson, M.A.; Prystowsky, S. Sarcoidosis: A Comprehensive Review and Update for the Dermatologist. J. Am. Acad. Dermatol. 2012, 66, 699.e1–699.e18. [Google Scholar] [CrossRef]
- Birnie, D.H.; Sauer, W.H.; Bogun, F.; Cooper, J.M.; Culver, D.A.; Duvernoy, C.S. HRS Expert Consensus Statement on the Diagnosis and Management of Arrhythmias Associated with Cardiac Sarcoidosis. Heart Rhythm. 2014, 11, 1304–1323. [Google Scholar] [CrossRef]
- Kouranos, V.; Tzelepis, G.E.; Rapti, A.; Mavrogeni, S.; Aggeli, K.; Douskou, M. Complementary Role of CMR to Conventional Screening in the Diagnosis and Prognosis of Cardiac Sarcoidosis. JACC Cardiovasc. Imaging 2017, 10, 1437–1447. [Google Scholar] [CrossRef]
- Murtagh, G.; Laffin, L.J.; Beshai, J.F.; Maffessanti, F.; Bonham, C.A.; Patel, V.A. Prognosis of Myocardial Damage in Sarcoidosis Patients with Preserved Left Ventricular Ejection Fraction: Risk Stratification Using Cardiovascular Magnetic Resonance. Circ. Cardiovasc. Imaging 2016, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hulten, E.; Agarwal, V.; Cahill, M.; Cole, G.; Vita, T.; Parrish, S. Presence of Late Gadolinium Enhancement by Cardiac Magnetic Resonance Among Patients with Suspected Cardiac Sarcoidosis Is Associated with Adverse Cardiovascular Prognosis. Circ. Cardiovasc. Imaging 2016, 9, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Nadel, J.; Lancefield, T.; Voskoboinik, A.; Taylor, A.J. Late Gadolinium Enhancement Identified with Cardiac Magnetic Resonance Imaging in Sarcoidosis Patients Is Associated with Long-Term Ventricular Arrhythmia and Sudden Cardiac Death. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 634–641. [Google Scholar] [PubMed]
- Shafee, M.A.; Fukuda, K.; Wakayama, Y.; Nakano, M.; Kondo, M.; Hasebe, Y. Delayed Enhancement on Cardiac Magnetic Resonance Imaging Is a Poor Prognostic Factor in Patients with Cardiac Sarcoidosis. J. Cardiol. 2012, 60, 448–453. [Google Scholar] [CrossRef]
- Bargagli, E.; Bennett, D.; Maggiorelli, C.; Sipio, P.; Margollicci, M.; Bianchi, N. Human Chitotriosidase: A Sensitive Biomarker of Sarcoidosis. J. Clin. Immunol. 2013, 33, 264–270. [Google Scholar] [CrossRef]
- Bergantini, L.; Bianchi, F.; Cameli, P.; Mazzei, M.A.; Fui, A.; Sestini, P. Prognostic Biomarkers of Sarcoidosis: A Comparative Study of Serum Chitotriosidase, ACE, Lysozyme, and KL-6. Dis. Markers 2019, 2019, 8565423. [Google Scholar] [CrossRef]
- Paone, G.; Leone, A.; Batzella, S.; Conti, V.; Belli, F.; Marchis, L. Use of Discriminant Analysis in Assessing Pulmonary Function Worsening in Patients with Sarcoidosis by a Panel of Inflammatory Biomarkers. Inflamm. Res. 2013, 62, 325–332. [Google Scholar] [CrossRef]
- Kobayashi, S.; Myoren, T.; Oda, S.; Inari, M.; Ishiguchi, H.; Murakami, W. Urinary 8-Hydroxy-2’-Deoxyguanosine as a Novel Biomarker of Inflammatory Activity in Patients with Cardiac Sarcoidosis. Int. J. Cardiol. 2015, 190, 319–328. [Google Scholar] [CrossRef]
- Myoren, T.; Kobayashi, S.; Oda, S.; Nanno, T.; Ishiguchi, H.; Murakami, W. An Oxidative Stress Biomarker, Urinary 8-Hydroxy-2’-Deoxyguanosine, Predicts Cardiovascular-Related Death After Steroid Therapy for Patients with Active Cardiac Sarcoidosis. Int. J. Cardiol. 2016, 212, 206–213. [Google Scholar] [CrossRef]
- Kiko, T.; Yoshihisa, A.; Kanno, Y.; Yokokawa, T.; Abe, S.; Miyata-Tatsumi, M. A Multiple Biomarker Approach in Patients with Cardiac Sarcoidosis. Int. Heart J. 2018, 59, 996–1001. [Google Scholar] [CrossRef]
- Schimmelpennink, M.C.; Quanjel, M.; Vorselaars, A.; Wiertz, I.; Veltkamp, M.; Moorsel, C. Value of Serum Soluble Interleukin-2 Receptor as a Diagnostic and Predictive Biomarker in Sarcoidosis. Expert Rev. Respir. Med. 2020, 14, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Popevic, S.; Sumarac, Z.; Jovanovic, D.; Babic, D.; Stjepanovic, M.; Jovicic, S.; Sobic-Saranovic, D.; Filipovic, S.; Gvozdenovic, B.; Omcikus, M.; et al. Verifying Sarcoidosis Activity: Chitotriosidase versus ACE in Sarcoidosis—A Case-control Study. J. Med. Biochem. 2016, 35, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, X.; Zhao, M.; Lower, E.E.; Baughman, R.P. SACE and IL-2R as serum biomarkers for evaluation of multi-organ involvement and prognosis of sarcoidosis. Respir. Res. 2023, 24, 219. [Google Scholar] [CrossRef] [PubMed]
- Bergantini, L.; Spalletti, M.; d’Alessandro, M.; Genovese, M.; Masotto, E.; Cameli, P. Predictive Role of Natural Killer Cells in Bronchoalveolar Lavage Fluid of Patients with Sarcoidosis. Pulmonology 2024, 31, 2416867. [Google Scholar] [CrossRef]
- Drent, M.; Mansour, K.; Linssen, C. Bronchoalveolar lavage in sarcoidosis. Semin. Respir. Crit. Care Med. 2007, 28, 486–495. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. Br. J. Pharmacol. 2020, 177, 4825–4844. [Google Scholar] [CrossRef]
- Gassen, N.C.; Papies, J.; Bajaj, T.; Emanuel, J.; Dethloff, F.; Chua, R.L.; Trimpert, J.; Heinemann, N.; Niemeyer, C.; Weege, F.; et al. SARS-CoV-2-mediated dysregulation of metabolism and autophagy uncovers host-targeting antivirals. Nat. Commun. 2021, 12, 3818. [Google Scholar] [CrossRef]
- Aveyard, P.; Gao, M.; Lindson, N.; Hartmann-Boyce, J.; Watkinson, P.; Young, D.; Coupland, C.A.C.; Tan, P.S.; Clift, A.K.; Harrison, D.; et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: A population cohort study. Lancet Respir. Med. 2021, 9, 909–923. [Google Scholar] [CrossRef]
- Hadi, Y.B.; Lakhani, D.A.; Naqvi, S.F.Z.; Singh, S.; Kupec, J.T. Outcomes of SARS-CoV-2 infection in patients with pulmonary sarcoidosis: A multicenter retrospective research network study. Respir. Med. 2021, 187, 106538. [Google Scholar] [CrossRef]
- Al-Omoush, O.; Khalil, L.; Ramadan, A.; Tarakhan, H.; Alzoubi, A.; Nabil, A.; Hajali, M.; Abdelazeem, B.; Saleh, O. Sarcoidosis and COVID-19 Vaccines: A Systematic Review of Case Reports and Case Series. Rev. Med. Virol. 2025, 35, e70011. [Google Scholar] [CrossRef]
- Nagai, S.; Handa, T.; Ito, Y.; Ohta, K.; Tamaya, M.; Izumi, T. Outcome of sarcoidosis. Clin. Chest Med. 2008, 29, 565–574, x. [Google Scholar] [CrossRef] [PubMed]
- Culver, D.A.; Baughman, R.P. It’s time to evolve from Scadding: Phenotyping sarcoidosis. Eur. Respir. J. 2018, 51, 1800050. [Google Scholar] [CrossRef] [PubMed]
- Scadding, J.G. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br. Med. J. 1961, 2, 1165–1172. [Google Scholar] [CrossRef]
- Kirkil, G.; Lower, E.E.; Baughman, R.P. Predictors of Mortality in Pulmonary Sarcoidosis. Chest 2018, 153, 105–113. [Google Scholar] [CrossRef]
- Ungprasert, P.; Carmona, E.M.; Utz, J.P.; Ryu, J.H.; Crowson, C.S.; Matteson, E.L. Epidemiology of Sarcoidosis 1946–2013: A Population-Based Study. Mayo Clin. Proc. 2016, 91, 183–188. [Google Scholar] [CrossRef]
- Hillerdal, G.; Nou, E.; Osterman, K.; Schmekel, B. Sarcoidosis: Epidemiology and Prognosis: A 15-Year European Study. Am. Rev. Respir. Dis. 1984, 130, 29–32. [Google Scholar]
- Boros, P.W.; Enright, P.L.; Quanjer, P.H.; Borsboom, G.J.; Wesolowski, S.P.; Hyatt, R.E. Impaired Lung Compliance and DL,CO but No Restrictive Ventilatory Defect in Sarcoidosis. Eur. Respir. J. 2010, 36, 1315–1322. [Google Scholar] [CrossRef]
- Siltzbach, L.E. Sarcoidosis: Clinical Features and Management. Med. Clin. N. Am. 1967, 51, 483–502. [Google Scholar] [CrossRef]
- Rømer, F.K. Presentation of Sarcoidosis and Outcome of Pulmonary Changes. Dan. Med. Bull. 1982, 29, 27–32. [Google Scholar]
- Zhang, Y.; Du, S.S.; Zhao, M.M.; Li, Q.H.; Zhou, Y.; Song, J.C.; Chen, T.; Shi, J.Y.; Jie, B.; Li, W.; et al. Chest high-resolution computed tomography can make higher accurate stages for thoracic sarcoidosis than X-ray. BMC Pulm. Med. 2022, 22, 146. [Google Scholar] [CrossRef]
- Mostard, R.L.; Van Kuijk, S.M.; Verschakelen, J.A.; van Kroonenburgh, M.J.; Nelemans, P.J.; Wijnen, P.A.; Drent, M. A predictive tool for an effective use of (18)F-FDG PET in assessing activity of sarcoidosis. BMC Pulm. Med. 2012, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.R.; Sivarasan, N.; Johannson, K.A.; George, P.M.; Culver, D.A.; Devaraj, A.; Lynch, D.A.; Milne, D.; Renzoni, E.; Nunes, H.; et al. High-resolution CT phenotypes in pulmonary sarcoidosis: A multinational Delphi consensus study. Lancet Respir. Med. 2024, 12, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.C.; Strek, M.E. Pulmonary fibrosis in sarcoidosis. Clinical features and outcomes. Ann. Am. Thorac. Soc. 2013, 10, 362–370. [Google Scholar] [CrossRef]
- Kouranos, V.; Ward, S.; Kokosi, M.A.; Castillo, D.; Chua, F.; Judge, E.P.; Thomas, S.; Van Tonder, F.; Devaraj, A.; Nicholson, A.G.; et al. Mixed Ventilatory Defects in Pulmonary Sarcoidosis: Prevalence and Clinical Features. Chest 2020, 158, 2007–2014. [Google Scholar] [CrossRef]
- Sharp, M.; Psoter, K.J.; Balasubramanian, A.; Pulapaka, A.V.; Chen, E.S.; Brown, S.W.; Mathai, S.C.; Gilotra, N.A.; Chrispin, J.; Bascom, R.; et al. Heterogeneity of Lung Function Phenotypes in Sarcoidosis: Role of Race and Sex Differences. Ann. Am. Thorac. Soc. 2023, 20, 30–37. [Google Scholar] [CrossRef]
- Judson, M.A.; Baughman, R.P.; Teirstein, A.S.; Terrin, M.L.; Yeager, H., Jr. Defining organ involvement in sarcoidosis: The ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 1999, 16, 75–86. [Google Scholar]
- Judson, M.A.; Costabel, U.; Drent, M.; Wells, A.; Maier, L.; Koth, L.; Shigemitsu, H.; Culver, D.A.; Gelfand, J.; Valeyre, D.; et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc. Diffuse Lung Dis. 2014, 31, 19–27. [Google Scholar]
- Judson, M.A.; Mack, M.; Beaumont, J.L.; Watt, R.; Barnathan, E.S.; Victorson, D.E. Validation and important differences for the Sarcoidosis Assessment Tool. A new patient-reported outcome measure. Am. J. Respir. Crit. Care Med. 2015, 191, 786–795. [Google Scholar] [CrossRef]
- Patel, A.S.; Siegert, R.J.; Creamer, D.; Larkin, G.; Maher, T.M.; Renzoni, E.A.; Wells, A.U.; Higginson, I.J.; Birring, S.S. The development and validation of the King’s Sarcoidosis Questionnaire for the assessment of health status. Thorax 2013, 68, 57–65. [Google Scholar] [CrossRef]
- Vorselaars, A.D.; Crommelin, H.A.; Deneer, V.H.; Meek, B.; Claessen, A.M.; Keijsers, R.G.; van Moorsel, C.H.; Grutters, J.C. Effectiveness of infliximab in refractory FDG PET-positive sarcoidosis. Eur. Respir J. 2015, 46, 175–185. [Google Scholar] [CrossRef]
- Korenkomp, I.H.E.; Maier, L.A.; Grutters, J.C. Serum and Imaging Biomarkers. In Sarcoidosis (ERS Monograph); Bonella, F., Culver, D.A., Israël-Biet, D., Eds.; European Respiratory Society: Sheffield, UK, 2022; pp. 107–121. [Google Scholar]
- Treglia, G.; Annunziata, S.; Sobic-Saranovic, D.; Bertagna, F.; Caldarella, C.; Giovanella, L. The role of 18F-FDG-PET and PET/CT in patients with sarcoidosis: An updated evidence-based review. Acad. Radiol. 2014, 21, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Papiris, S.A.; Georgakopoulos, A.; Papaioannou, A.I.; Pianou, N.; Kallergi, M.; Kelekis, N.L.; Gialafos, H.; Manali, E.D.; Chatziioannou, S. Emerging phenotypes of sarcoidosis based on 18F-FDG PET/CT: A hierarchical cluster analysis. Expert Rev. Respir. Med. 2020, 14, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Schupp, J.C.; Freitag-Wolf, S.; Bargagli, E.; Mihailovic-Vucinic, V.; Rottoli, P.; Grubanovic, A.; Muller, A.; Jochens, A.; Tittmann, L.; Schnerch, J.; et al. Phenotypes of organ involvement in sarcoidosis. Eur. Respir. J. 2018, 51, 1700991. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, B.A.; Sinha, R.; Iyengar, S.; Gray-McGuire, C.; Elston, R.C.; Iannuzzi, M.C.; Consortium, S.S. Genetic linkage analysis of sarcoidosis phenotypes: The sarcoidosis genetic analysis (SAGA) study. Genes Immun. 2007, 8, 379–386. [Google Scholar] [CrossRef][Green Version]
- Freitag-Wolf, S.; Schupp, J.C.; Frye, B.C.; Fischer, A.; Anwar, R.; Kieszko, R.; Mihailovic-Vucinic, V.; Milanowski, J.; Jovanovic, D.; Zissel, G.; et al. Genetic and geographic influence on phenotypic variation in European sarcoidosis patients. Front. Med. (Lausanne) 2023, 10, 1218106. [Google Scholar] [CrossRef]
- Konigsberg, I.R.; Lin, N.W.; Liao, S.Y.; Liu, C.; MacPhail, K.; Mroz, M.M. Multi-Omic Signatures of Sarcoidosis and Progression in Bronchoalveolar Lavage Cells. Respir. Res. 2023, 25, 289. [Google Scholar] [CrossRef]
- Dyskova, T.; Fillerova, R.; Novosad, T.; Kudelka, M.; Zurkova, M.; Gajdos, P. Correlation Network Analysis Reveals Relationships Between MicroRNAs, Transcription Factor T-Bet, and Deregulated Cytokine/Chemokine-Receptor Network in Pulmonary Sarcoidosis. Mediat. Inflamm. 2015, 2015, 121378. [Google Scholar] [CrossRef]
- Novosadova, E.; Chabronova, A.; Kolek, V.; Petrek, M.; Navratilova, Z. The Serum Expression of Selected miRNAs in Pulmonary Sarcoidosis With/Without Löfgren’s Syndrome. Mediat. Inflamm. 2016, 2016, 1246129. [Google Scholar] [CrossRef]
- Vukmirovic, M.; Yan, X.; Gibson, K.F.; Gulati, M.; Schupp, J.C.; DeIuliis, G. Transcriptomics of Bronchoalveolar Lavage Cells Identifies New Molecular Endotypes of Sarcoidosis. Eur. Respir. J. 2021, 58, 2002950. [Google Scholar] [CrossRef]
- Van Moorsel, C.H.; Petrek, M.; Rivera, N.V. Unravelling the Genetic Basis of Sarcoidosis. In Sarcoidosis; ERS Monograph; Bonella, F., Culver, D.A., Israël-Biet, D., Eds.; European Respiratory Society: Sheffield, UK, 2022; pp. 41–56. [Google Scholar] [CrossRef]
- Casanova, N.G.; Gonzalez-Garay, M.L.; Sun, B.; Bime, C.; Sun, X.; Knox, K.S.; Crouser, E.D.; Sammani, N.; Gonzales, T.; Natt, B.; et al. Differential transcriptomics in sarcoidosis lung and lymph node granulomas with comparisons to pathogen-specific granulomas. Respir. Res. 2020, 21, 321. [Google Scholar] [CrossRef]
- Lin, Y.; Wei, J.; Fan, L. BTNL2 Gene Polymorphism and Sarcoidosis Susceptibility: A Meta-Analysis. PLoS ONE 2015, 10, 0122639. [Google Scholar] [CrossRef] [PubMed]
- Morais, A.; Lima, B.; Alves, H. Associations Between Sarcoidosis Clinical Course and ANXA11 rs1049550 C/T, BTNL2 rs2076530 G/A, and HLA Class I and II Alleles. Clin. Respir. J. 2018, 12, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pabst, S.; Kubisch, C. First Independent Replication Study Confirms the Strong Genetic Association of ANXA11 with Sarcoidosis. Thorax 2010, 65, 939–940. [Google Scholar] [CrossRef]
- Mrazek, F.; Stahelova, A.; Kriegova, E. Functional Variant ANXA11 R230C: True Marker of Protection and Candidate Disease Modifier in Sarcoidosis. Genes Immun. 2011, 12, 490–494. [Google Scholar] [CrossRef][Green Version]
- Morais, A.; Lima, B.; Peixoto, M. Annexin A11 Gene Polymorphism (R230C Variant) and Sarcoidosis in a Portuguese Population. Tissue Antigens 2013, 82, 186–191. [Google Scholar] [CrossRef]
- Sikorova, K.; Kishore, A.; Rapti, A. Association of TGF-β3 and ANXA11 with Pulmonary Sarcoidosis in a Greek Population. Expert Rev. Respir. Med. 2020, 14, 1065–1069. [Google Scholar] [CrossRef]
- Levin, A.M.; Iannuzzi, M.C.; Montgomery, C.G. Association of ANXA11 Genetic Variation with Sarcoidosis in African Americans and European Americans. Genes Immun. 2013, 14, 13–18. [Google Scholar] [CrossRef][Green Version]
- Mirsaeidi, M.; Vu, A.; Zhang, W. Annexin A11 Is Associated with Pulmonary Fibrosis in African American Patients with Sarcoidosis. Sarcoidosis Vasc. Diffuse Lung Dis. 2016, 33, 418–422. [Google Scholar]
- Xiong, Y.; Institutet, K.; Kullberg, S. Sex Differences in the Genetics of Sarcoidosis Across European and African Ancestry Populations. Res. Sq. 2021, 10, 1132799. [Google Scholar] [CrossRef]
- Goljan-Geremek, A.; Radziński, P.; Puścińska, E.; Demkow, U. Defining Serum Tumor Necrosis Factor-α Concentration-Related Endotype of Sarcoidosis: A Real-Life, Retrospective, Observational Polish Study. Pol. Arch. Intern. Med. 2024, 134, 16718. [Google Scholar]
- Crouser, E.D.; Locke, L.W.; Julian, M.W.; Bicer, S.; Sadee, W.; White, P.; Schlesinger, L.S. Phagosome-regulated mTOR signalling during sarcoidosis granuloma biogenesis. Eur. Respir. J. 2021, 57, 2002695. [Google Scholar] [CrossRef] [PubMed]
- Wijsenbeek, M.S.; Culver, D.A. Treatment of Sarcoidosis. Clin. Chest Med. 2015, 36, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Redl, A.; Doberer, K.; Unterluggauer, L.; Kleissl, L.; Krall, C.; Mayerhofer, C.; Reininger, B.; Stary, V.; Zila, N.; Weninger, W.; et al. Efficacy and safety of mTOR inhibition in cutaneous sarcoidosis: A single-centre trial. Lancet Rheumatol. 2024, 6, e81–e91. [Google Scholar] [CrossRef] [PubMed]

| Genetic factors |
| HLA-DRB1*1101 [6] |
| HLA-DRB1*03, *0301 or *1501 (associated with Löfgren syndrome) [8] |
| HLA-DRB1*07, *14, *15, *01 and *03 and DQB1*0602 (higher likelihood of progressive pulmonary sarcoidosis) [6] |
| TNF-α polymorphisms (308AA and rs1800629) [12,13,14,15] |
| Polymorphisms in Toll-like receptors (TLRs) [19,20] |
| Butyrophilin-like-2 gene [22] |
| Annexin A11 gene [24] |
| Vimentin gene [25] |
| Infectious agents |
| Mycobacterium species [26,28] |
| Propionibacterium (Cutibacterium) acnes [30,31] |
| Occupational exposure |
| Silica and metal dust [36,37] |
| Inhalation of organic bioaerosols (livestock, mold and industrial organic dusts) [33] |
| Insecticides [33] |
| Lifestyle factors |
| Obesity [38,51] |
| Diet [46] |
| Seasonal exposure |
| Increased incidence in winter and summer [49,50] |
| Parenchymal Disease | Airway Disease |
| Pulmonary fibrosis | Bullous emphysema |
| Bronchiectasis | |
| Bronchial stenosis | |
| Pulmonary Vascular Disease | Pleural Disease |
| Pulmonary hypertension | Pneumothorax |
| Pulmonary embolism | Pleural effusion |
| Coronary heart disease | Pleural thickening |
| Lung Cancer | Infections |
| Aspergilloma | |
| Invasive aspergillosis | |
| Bacterial pneumonia | |
| COVID-19 |
| Clinical parameters Löfgren syndrome (erythema nodosum, bilateral hilar lymphadenopathy and acute-onset fever) [1,4] Heerfordt syndrome (anterior uveitis, bilateral parotid gland enlargement, facial nerve palsy and fever) [4] Clinical tools for phenotyping: Sarcoidosis Assessment Tool [147] WASOG organ assessment tool [148] | Pathognomonic of sarcoidosis. Young females, patients of Scandinavian origin and good prognosis Pathognomonic of sarcoidosis Likelihood of 15 organs involved in sarcoidosis (definite, probable and possible); histological confirmation of sarcoidosis required Update of the previous instrument and probability of organ involvement |
| Ethnicity Asian [101] Black [99] | Increased incidence of cardiac, muscular, renal and ocular manifestations Increased incidence of extra-pulmonary disease, progressive pulmonary disease, and liver and bone manifestations |
| Radiological parameters Scadding chest X-ray [134] HRCT phenotyping [143] | Five stages associated with resolution and prognosis: normal (0), bilateral hilar lymphadenopathy without pulmonary infiltrates (1), bilateral hilar lymphadenopathy with pulmonary infiltrates (2), pulmonary infiltrates without bilateral hilar lymphadenopathy (3) and extensive fibrosis with distortion or bullae (4). Non-fibrotic subtypes: multiple peri-bronchovascular, peri-fissural or subpleural micronodules; multiple larger peri-bronchovascular nodules; scattered larger nodules; consolidation as the predominant or sole abnormality Likely-to-be-fibrotic subtypes: bronchocentric reticulation with or without dense parenchymal opacification without cavitation, bronchocentric reticulation and dense parenchymal opacification with cavitation and large bronchocentric masses (i.e., PMF lookalike) |
| Pulmonary function phenotyping [145,146] | Normal, restrictive, obstructive or mixed pattern. Mixed disease associated with lower DLCO, stage 4 disease and higher mortality than seen in a purely obstructive defect; Restrictive is the most common pattern among Black individuals, while White individuals most commonly present normal PFTs; males frequently show obstruction and females restriction |
| Nuclear imaging phenotyping [154] | Four phenotypes: |
| |
| Genetic phenotyping [156] | Five phenotypes: |
|
| HLADRB1*03, *0301 or *1501 HLA polymorphism (rs4143332) [6,7,8,9,165] | Associated with Löfgren syndrome |
| HLADRB1*07, *14, *15, *01 and *03 and DQB1*0602 [6,11] | Higher likelihood of progressive pulmonary sarcoidosis |
| TNF-α polymorphisms (308AA and rs1800629) [12,13,173] | Pulmonary disease progression and acute-onset disease |
| ANXA11 locus rs1049550 [169] | Protective effect by the minor T allele |
| Polymorphisms in TLRs (absence of the common haplotype in the TLR10–TLR1–TLR6 gene cluster, TLR3 L412F, MyD88 and CybB/Nox2) | Chronic, persistent disease |
| SEPP1 [158] | Worse lung function |
| IL20RB, ABCC11, SFSWAP, AGBL4, miR-146a-3p and miR-378b in a multi-omics model [158] | Associated with progressive sarcoidosis |
| miR-21-5p, miR-340-5p and miR-212-3p [160] | Differentiate patients with Löfgren syndrome |
| miR-155, let-7c and transcription factor T-bet [159] | Progressive disease |
| Th1, Th17, IFN-γ and NFAT signaling (CD28, STAT1, CXCR3 and CCR4 genes) [161] | Hilar lymphadenopathy |
| IL-2 and IL-7 pathways (MRC2, SLC40A1, F2R, IL7, PTPN7, ADORA2A, SPRY2, PLA2G7 and PTGS1 genes) [161] | More severe bronchial wall thickening |
| TGF-b1 and MTOR pathways (TGFBR1, COL3A1, TLR3, ID1, TCF4, IGFBP6, PLA2G7, FADS1, ARGHAP12 and MMP10, SC5D, HIF1A and PPAR-α) [161] | Parenchymal involvement—pulmonary fibrosis |
| Four gene modules [161] | 4 novel endotypes: chronic sarcoidosis, hilar lymphadenopathy and acute lymphocytic inflammation, multi-organ involvement with increased immune response and extra-ocular involvement with PI3K activation |
| mTOR pathway activation [67,69,174] | Sarcoidosis progression |
| JAK/STAT pathway activation (17-gene signature) [71] | Sarcoidosis progression |
| Increased Th1 and Th17.1 cells [1,82] | Chronic sarcoidosis |
| Increased Th17 cells [80] | Löffler syndrome |
| High expression of CD25, CTLA4, CD69, PD-1 and CD95 in blood Tregs [89] | Chronic sarcoidosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papanikolaou, I.C.; Chytopoulos, K.; Kaitatzis, D.; Kostakis, N.; Bogiatzis, A.; Steiropoulos, P.; Drakopanagiotakis, F. Phenotypes and Endotypes in Sarcoidosis: Unraveling Prognosis and Disease Course. Biomedicines 2025, 13, 287. https://doi.org/10.3390/biomedicines13020287
Papanikolaou IC, Chytopoulos K, Kaitatzis D, Kostakis N, Bogiatzis A, Steiropoulos P, Drakopanagiotakis F. Phenotypes and Endotypes in Sarcoidosis: Unraveling Prognosis and Disease Course. Biomedicines. 2025; 13(2):287. https://doi.org/10.3390/biomedicines13020287
Chicago/Turabian StylePapanikolaou, Ilias C., Konstantinos Chytopoulos, Dimitrios Kaitatzis, Nikolaos Kostakis, Anastasios Bogiatzis, Paschalis Steiropoulos, and Fotios Drakopanagiotakis. 2025. "Phenotypes and Endotypes in Sarcoidosis: Unraveling Prognosis and Disease Course" Biomedicines 13, no. 2: 287. https://doi.org/10.3390/biomedicines13020287
APA StylePapanikolaou, I. C., Chytopoulos, K., Kaitatzis, D., Kostakis, N., Bogiatzis, A., Steiropoulos, P., & Drakopanagiotakis, F. (2025). Phenotypes and Endotypes in Sarcoidosis: Unraveling Prognosis and Disease Course. Biomedicines, 13(2), 287. https://doi.org/10.3390/biomedicines13020287






